Abstract
Macro-porous resins are widely used as adsorbents for the treatment of organic contaminants in wastewater and for the pre-concentration of organic solutes from water. However, the sorption mechanisms for organic contaminants on such adsorbents have not been systematically investigated so far. Therefore, in this study, the sorption capacities and affinities of 24 organic chemicals by XAD-7 were investigated and the experimentally obtained sorption isotherms were fitted to the Dubinin-Ashtakhov model. Linear positive correlations were observed between the sorption capacities and the solubilities (S W) of the chemicals in water or octanol and between the sorption affinities and the solvatochromic parameters of the chemicals, indicating that the sorption of various organic compounds by XAD-7 occurred by non-linear partitioning into XAD-7, rather than by adsorption on XAD-7 surfaces. Both specific interactions (i.e., hydrogen-bonding interactions) as well as nonspecific interactions were considered to be responsible for the non-linear partitioning. The correlation equations obtained in this study allow the prediction of non-linear partitioning using well-known chemical parameters, namely S W, octanol-water partition coefficients (K OW), and the hydrogen-bonding donor parameter (α m). The effect of pH on the sorption of ionizable organic compounds (IOCs) could also be predicted by combining the correlation equations with additional equations developed from the estimation of IOC dissociation rates. The prediction equations developed in this study and the proposed non-linear partition mechanism shed new light on the selective removal and pre-concentration of organic solutes from water and on the regeneration of exhausted XAD-7 using solvent extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since their discovery in the 1960s (Kunin et al. 1961), macro-reticular polymeric sorbents (resins) have been attracting increasing attention as one of the most promising alternatives to activated carbon for the removal of organic chemicals from wastewater (Xu et al. 2003; Pan et al. 2008a; Li and Chase 2010). The sorption of an organic chemical by sorbents such as resins may be described in terms of sorption capacity and sorption affinity (Yang and Xing 2010; Wu et al. 2012). Sorption capacity of a resin is the maximum amount of the chemicals adsorbed on the resin, whereas sorption affinity is a parameter that indicates the total strength of all the interactions between the chemical and the resin on which they are adsorbed (Yang and Xing 2010; Wu et al. 2012). Three main types of interactions, namely, hydrophobic effects (induced by interactions such as van der Waals force), π-π bonding interactions, and hydrogen-bonding interactions, have been identified and are thought to be responsible for the sorption of organic chemicals by resins (Thurman et al. 1978; Xu et al. 2003; Qiu et al. 2005; Feng et al. 2012; Ghatbandhe et al. 2012; Pan and Zhang 2012; Vincenza et al. 2013). Polanyi theory has been regarded as a powerful theory that explains the sorption of organic chemicals from aqueous solutions on resins (Pan and Zhang 2012, 2013). The sorption capacity and sorption affinity of organic chemicals including non-ionic compounds (e.g., nitrobenzene) and ionizable compounds (e.g., aniline, phenol, and their substitutes) by the resins can be determined using the fitted isotherm parameters (i.e., the saturated adsorbed solute capacity Q 0 and the correlating divisor E) of the Polanyi theory-based Dubinin-Ashtakhov (DA) model (Yang et al. 2008; Yang and Xing 2010; Wu et al. 2012; Pan and Zhang 2012, 2013).
Knowledge of the correlations between the sorption capacities and affinities of organic chemicals toward resins and the properties of the organic chemicals may be helpful in understanding the underlying sorption mechanisms as well as for the quantitative prediction of the sorption of an organic chemical by a polymeric sorbent (Thurman et al. 1978; Pan and Zhang 2012, 2013). These correlations can be used to theoretically identify the solutes that are capable of adsorbing toward a given sorbent and to predict their sorption capacities and affinities (Thurman et al. 1978). Understanding the underlying sorption mechanisms and quantitative predictions of the sorption of organic chemicals on resins are vital for choosing appropriate resins, synthesizing specific resins, and choosing optimal regeneration of resins for wastewater treatment applications (Thurman et al. 1978; Pan and Zhang 2012, 2013). In a previous study (Thurman et al. 1978), an inverse logarithmic relationship between the sorption coefficients (K, which is the ratio of the chemical concentration adsorbed on the resin to the equilibrium concentration of the chemical in the solution) at a given initial chemical concentration and aqueous molar solubility (S W) was observed for 20 organic chemicals on Amberlite XAD-8, which is a porous acrylic resin. The organic chemicals studied included aromatic, aliphatic, and alicyclic organic solutes with carboxyl, hydroxyl, amine, and methyl functional groups. The log K-log S W relationship was used to predict the value of K for a given organic chemical on XAD-8 using only S W (Thurman et al. 1978). In a recent study by Pan and Zhang (2012), important linear free energy relationships between the n-hexadecane-water partition coefficient—normalized parameter E (i.e., E m) of the DA model-fitted isotherms and the solvatochromic parameters (i.e., hydrogen-bonding donor parameter (α m) of phenols and nitrobenzene)—were observed for the sorption of phenols and nitrobenzene by resins including XAD-4 and XAD-7. This suggested that hydrophobic effects and hydrogen-bonding interactions were predominantly responsible for the sorption. A negative linear correlation between Q 0 (parameter from the DA isotherms) and the molecular size of the selected chemicals was also observed for XAD-4 and XAD-7 (Pan and Zhang 2012). However, this negative linear correlation was not applicable for other chemicals (i.e., 5 anilines and caffeine) on XAD-4 and XAD-7, as reported in a later study by Pan and Zhang (2013). Therefore, correlations between the Q 0 of the organic chemicals on the resins and the properties of the organic chemicals have not been developed yet, which limits the understanding of the underlying sorption mechanisms and the quantitative prediction of sorption.
Pan and Zhang (2013) observed that in addition to the hydrophobic effects and hydrogen-bonding interactions, π-π bonding interactions, which are identified by the polarity/polarizability (π*) of the chemicals, were also important for the sorption of chemicals such as nitrobenzene, caffeine, phenols, and anilines on resins such as XAD-4, XAD-7, and MN200. This observation is not in agreement with their previous observation that hydrophobic effects and hydrogen-bonding interactions are predominantly responsible for the sorption of organic chemicals on resins (Pan and Zhang 2012). A predictive model using a phase conversion approach (from aqueous phase to n-hexadecane and ideal gas phase) was developed to accurately estimate the sorption of organic chemicals on the resins (Pan and Zhang 2013). However, this model is very complex and is based on (i) a number of multiple linear regressions between the net free energy change of the chemicals during phase conversion and their solvatochromic parameters (i.e., the intrinsic molar volume V I, polarity/polarizability π*, and the hydrogen-bonding donor parameter α m) at a given sorption amount (q e) and (ii) a number of regression relationships describing the dependency of the multiple linear regression coefficients on q e, within a wide concentration-range (C e) (Pan and Zhang 2013). In addition to the solvatochromic parameter S W, n-hexadecane-water partition coefficients (K HW) and gas-water distribution coefficients (K GW) of the chemicals were required for making predictions using this model (Pan and Zhang 2013). Therefore, this model is not facile for predictive applications. Moreover, the regression relationships describing the dependence of the multiple linear regression coefficients on q e are physically meaningless from the point of view of understanding the underlying sorptive mechanisms.
In this study, the sorption isotherms of 24 organic chemicals by XAD-7 resin were obtained and fitted to the DA model, to investigate the sorption affinities and capacities. The organic chemicals studied include 2 polycyclic aromatic hydrocarbons (PAHs), 7 nitrobenzenes, 7 anilines, and 8 phenols. The selected chemicals are included in the list of priority pollutants by most national environmental protection agencies, owing to the potential risks posed by them to organisms and/or human health at low concentrations (Yang et al. 2005, 2006a, 2008; Zhang et al. 2007). XAD-7, which was developed by Rohm and Haas Company in the 1970s, was selected owing to its applications in the sorptive removal of organic chemicals (Xu et al. 2003; Kunin et al. 1961; Pan et al. 2008; Li and Chase 2010; Pan and Zhang 2012, 2013). We observed positive logarithmic relationships between the sorption capacity (i.e., Q 0 of the DA isotherms) and S W or octanol solubility (S octanol) for the 24 organic chemicals selected in this study and the 14 chemicals reported by Pan and Zhang (2013) on XAD-7. A significant linear-free energy relationship between the sorption affinities (i.e., E of the DA model-fitted isotherms) and the solvatochromic parameters (i.e., α m) was also observed for the 24 organic chemicals in this study and the 14 chemicals reported by Pan and Zhang (2013) on XAD-7. These relationships could be used to predict the sorption of organic chemicals on XAD-7 and help understand the sorption mechanisms.
Materials and methods
Chemicals and XAD-7 resin
The characteristics including the IUPAC name, CAS number, quality, provider, and structural information of the 24 investigated organic chemicals are listed in Table S1. All of the organic chemicals were used as received. S W, molecular weight, log K OW (octanol-water partition coefficients), pK a, and the various solvatochromic parameters of the organic chemicals are listed in Table 1.
Amberlite XAD-7, which is a macro-porous resin, was purchased from Rohm and Haas Company (Philadelphia, PA, USA). The chemical structure of XAD-7 is shown in Figure S1 in the Supporting Information. To remove possible residual impurities, XAD-7 was extracted with ethanol for 8 h using the Soxlet method and then dried in vacuum at 325 K. The Brunauer-Emmett-Teller (BET) surface area, pore volume, and average pore diameter of XAD-7 obtained from the nitrogen adsorption-desorption isotherms using Quantachrome Autosorb-1MP-VP (Boynton, USA) at 77 K were 419 m2/g, 0.795 cm3/g, and 5.4 nm, respectively, which is in agreement with the values reported previously by Pan and Zhang (2012; 2013) (i.e., 495 m2/g, 1.12 cm3/g, and 4.9 nm, respectively).
Sorption experiments
The sorption isotherms were determined by a batch equilibration technique at room temperature (25 ± 1 °C). Briefly, the organic chemicals, except for the PAHs (i.e., naphthalene and phenanthrene), 1,2-dinitrobenzene, and 1,4-dinitrobenzene, were dissolved in a background solution containing 0.01 mol/L of CaCl2 in deionized distilled water. Methanol solutions of naphthalene, phenanthrene, 1,2-dinitrobenzene, and 1,4-dinitrobenzene were added to the background solution for adsorption. The volume fraction of methanol in the solution of each vial was controlled to below 0.002 to avoid the cosolvent effect. Aqueous solutions of the chemicals (8 or 40 mL) were mixed with XAD-7 in 8 or 40 mL screw cap vials. The XAD-7 dose was adjusted such that over 20 % of the added organic compounds were adsorbed by XAD-7. The pH of the mixtures was adjusted using 0.1 mol/L HCl or 0.1 mol/L NaOH solution. To suppress the ionization effect on the isotherms, the final solution pH after sorption was maintained at 4.0 for 2-chlorophenol, 2-nitrophenol, 3-nitrophenol, and 4-nitrophenol and at 8.0 for aniline and 4-methylaniline. For the other compounds, the final solution pH after sorption was maintained at 7.0. After equilibration for 48 h by shaking at 150 rpm (preliminary tests indicated that the apparent sorption equilibrium was reached before 48 h), the mixtures in the vials were separated by centrifugation at 3500g for 20 min. The concentrations of the compounds (except for naphthalene and phenanthrene) in the supernatants were determined using UV-spectroscopy (Shimadzu, UV-2450, Tokyo, Japan) at their maximum adsorption wavelengths (λ max), which are listed in Table 1. The concentrations of naphthalene and phenanthrene were determined using a fluoro-spectrophotometer (Shimadzu, RF-5301PC, Tokyo, Japan) at the excitation and emission wavelengths listed in Table 1. Experimental uncertainties evaluated in vials using chemical solution were less than 4 % of the initial chemical concentrations. Therefore, the amounts of organic chemicals adsorbed by XAD-7 were calculated directly based on the mass difference of the organic chemicals in the initial and equilibrium solutions. The pH dependence of the adsorption of anilines and phenols for a given concentration of organic chemicals in the pH range of 1 to 12 were also determined using the same experimental procedure described above.
Sorption models and regression analyses
The DA model (Eq. 1), which has been successfully used for fitting the sorption isotherms of non-ionic compounds (e.g., nitrobenzene) and ionizable compounds (e.g., aniline, phenol, and their substitutes) by resins in previous studies (Pan and Zhang 2012, 2013) was employed in the present study to fit the experimental data.
In the above equation, ε [kJ/mol] = RTln(S W/C e) is the effective adsorption potential, R [=8.314 × 10−3 KJ/mol · K] is the universal gas constant, T [K] is the absolute temperature, and b is a fitting parameter. E can also be used to successfully identify the sorption affinity of organic chemicals (i.e., the strength of interactions for the sorption of organic chemicals) (Yang et al. 2008; Yang and Xing 2010; Wu et al. 2012; Pan and Zhang 2012, 2013). The isotherms were also fitted by the Freundlich model (Eq. 2).
In the above equation, K f [(mg/g)/(mg/L)n] is the affinity coefficient and 1/n is the exponential coefficient.
Results and discussion
Isotherms and their fitting to DA and Freundlich models
The sorption isotherms of the 24 organic chemicals by XAD-7 fit well to both the DA and Freundlich models (Fig. 1 and Table 2). The fits were non-linear, as indicated by the values of the fit parameters 1/n and E of the Freundlich and DA models, which were <1 and >5.71, respectively (Yang et al. 2008; Yang and Xing 2010; Wu et al. 2012). The DA model fit parameters (i.e., Q 0, E, and b) of the isotherms for the 24 chemicals are listed in Table 2. The isotherms fit well to the DA model, as indicated by the low MWSE (mean-weighted square error) values and r 2 values close to 1, as shown in Table 2. The b values of the organic chemicals by XAD-7 were nearly constant with an average value of 1.03 (Table 2), indicating that b was independent of the type of chemicals. Therefore, it is not surprising that the isotherms obtained from the sorption of the 24 organic chemicals by XAD-7 also fit well to the Freundlich model (Table 2), as observed in the previous studies (Li and Chase 2010), because the Freundlich model is a special form of the DA model, with the parameter b set to 1 (Yang et al. 2006a, 2006b, 2010; Yang and Xing 2010).
Correlations between sorption capacities and chemical properties
Positive logarithmic relationships between Q 0 (obtained from the DA model-fits) and S W (in Table 1) and S octanol (molar solubility in octanol) were observed for the 24 organic chemicals investigated in this study (Eqs. 3 and 4, Fig. 2).
S octanol was calculated from the values of K OW in Table 1 and S W (i.e., S octanol = K OW × S W). These positive logarithmic relationships are also applicable to the sorption of the 14 chemicals on XAD-7 previously reported (Pan and Zhang 2013), as shown in Fig. 2. Eqs. 5 and 6 are the new logarithmic relationships of Q 0 involving both S W and S octanol and includes the previously reported Q 0 data.
The log K OW values of 2-naphthol, bisphenol A, and caffeine used in the regression analysis of Eq. 6, are 2.70 (Chen et al. 2008), 2.2 (Pan et al. 2008b), and −0.07 (Karnjanapiboonwong et al. 2010), respectively. There is no significant difference between Eqs. 3 and 5 or between Eqs. 4 and 6, when the standard errors of the regression coefficients are considered. Similar logarithmic relationships between Q 0 and S W and S octanol (Eqs. 7 and 8) can be obtained for the sorption of the previously reported 14 compounds on XAD-4 (Pan and Zhang 2013), as shown in Figure S2.
These positive logarithmic relationships (Eqs. 3–8) indicate that the sorption behavior of organic chemicals on XAD-7 and XAD-4 is similar to the dissolution of organic chemicals in water and their partition into organic solvents (e.g., octanol). A logarithmic relationship between S octanol and S w can also be observed (Eq. 9 and Fig. 3).
Correlations of log S octanol (mol/L) with log S W for the chemicals investigated in this study and reported by Pan and Zhang 2013. The dot line represents the values equal to log S W values
Therefore, it may be concluded that the sorption of organic chemicals on XAD-7 and XAD-4 resembles the partition of organic chemicals into organic polymers (i.e., XAD-7 and XAD-4) from water and is not caused by adsorption on the surface of XAD-7 and XAD-4. This is due to the premise of solute partition, i.e., organic compounds with high water solubility are usually also more compatible with organic solvents (Chiou and Kile 1998; Chiou 2002). It is well-known that solute partition is responsible for the sorption of organic chemicals onto polymers and natural organic matters from water (Chiou and Kile 1998), as well as the distribution of organic chemicals between organic solvents (e.g., octanol) and water (Chiou and Kile 1998; Chiou 2002).
In a previous study (Thurman et al. 1978), the sorption of organic chemicals was attributed to adsorption on the surface of XAD resins including XAD-1, XAD-2, XAD-4, XAD-7, and XAD-8. Hydrophobic effects were suggested to be responsible for the adsorption, owing to the negative relationships observed between log K and log S W (Karger et al. 1976; Thurman et al. 1978). If the sorption mechanism may be explained by the hydrophobic adsorption concept, in addition to the larger log K values, chemicals with low solubility should adsorb more on the hydrophobic surface of XAD resins with higher sorption capacities compared to the chemicals with high solubility, because low-solubility chemicals are more hydrophobic relative to the high-solubility chemicals. Therefore, the hydrophobic adsorption concept is inconsistent with the positive logarithm relationships between log Q 0 and log S W (Eqs. 3, 5, and 7) (i.e., low-solubility chemicals can adsorb more by XAD-7 and XAD-4 with high sorption capacities (Figs. 2 and S2). The partition of organic chemicals into polymers, natural organic matters, and organic solvents (e.g., octanol) from water is also promoted by the low solubilities (i.e., poor compatibilities) of the chemicals in water (Chiou and Kile 1998; Chiou 2002), i.e., the hydrophobic effect, which in fact explains the observed negative relationships between log K and log S W for XAD resins (Karger et al. 1976; Thurman et al. 1978). In another study, Pan and Zhang (2012) observed a negative linear correlation between Q 0 and the molecular size of the chemicals for sorption by XAD-4 and XAD-7. They suggested that the sorption capacity of organic chemicals on XAD-4, XAD-7, and MN200 was dependent on the pore volume of the resins (i.e., chemicals with larger molecular volumes have less sorption capacities). Further, they suggested that sorption could be explained by the pore-filling mechanism and the relative sorption potential of the resin surfaces (i.e., only part of resin surface can offer sufficient energy to overcome water-solute attraction for organic chemical sorption) (Pan and Zhang 2013). The latter (i.e., the relative sorption potential of the resin surfaces) implies that chemicals with high water solubilities will have less sorption capacity on the resin surfaces. For example, Pan and Zhang (2013) suggested that chemicals with higher β m and α m values exhibited a lower tendency for adsorption on the resin surfaces, because they could form stronger hydrogen bonds with water (α m = 0.82, β m = 0.35) than with the resins. However, the concept of relative sorption potential of the resin surfaces is also inconsistent with the positive logarithm relationships observed between log Q 0 and log S W (Eqs. 3, 5, and 7) for XAD-7 and XAD-4 (Figs. 2 and S2). 1,4-Dinitrobezene has the same molecular volume and hydrogen-bonding potential (α m = 0 and β m = 0.46) as 1,3-dinitrobezene and has a smaller molecular volume and weaker hydrogen-bonding potential compared to 1,3,5-trinitrobezene (α m = 0 and β m = 0.61) (Table 1). However, Q 0 of 1,4-dinitrobezene was far lower than that of 1,3-dinitrobezene and 1,3,5-trinitrobezene (Table 2), indicating that this case could not be explained by the combination of pore-filling and relative sorption potential of the resin surfaces. This combination of mechanisms also failed to explain the higher Q 0 values reported for 2-naphthol on XAD-4 and XAD-7 compared to the values reported for 3-nitroaniline and 4-nitroaniline, since the molecular volume and hydrogen-bonding potential of 2-naphthol are higher than that of 3-nitroaniline and 4-nitroaniline (Pan and Zhang 2013).
As shown in Figs. 2, S2, and 3, log Q 0 for XAD-4 and XAD-7 and log S octanol values for the chemicals investigated in this and the previous study (Pan and Zhang 2013) are greater than the corresponding log S W values, as indicated by the values of log Q 0 and log S octanol above the dotted lines, which represents the log S W values. Therefore, XAD-4, XAD-7, and octanol are relatively non-polar for the dissolution of the organic chemicals compared to water. The log Q 0 values for the 14 chemicals considered in the previous study (Pan and Zhang 2013) on XAD-4 are greater than those on XAD-7 (Figure S2), indicating that XAD-4 is relatively non-polar compared to XAD-7. Moreover, XAD-4 and XAD-7 are more compatible with polar organic chemicals, whereas octanol is more compatible with non-polar organic chemicals, as indicated by the crossing of the dotted lines (representing the log S octanol values) and the solid lines fitted by Eqs. 6 and 8 (Figs. 2 and S2). This suggests that XAD-4 and XAD-7 are more polar compared to octanol.
Additionally, the previously reported log Q 0 values for the sorption of 11 chemicals on MN200 resin (Pan and Zhang 2013) are almost independent of the corresponding log S W/log S octanol values and are constant with an average value of 0.622 (Figure S3). This is different from the linear positive relationships between log Q 0 and log S W/log S octanol for the sorption of organic chemicals by XAD-7 and XAD-4 (Figs. 2 and S2), suggesting that the sorption mechanisms of organic chemicals on MN200 are different from those in the cases of XAD-7 and XAD-4. Sorption of the organic chemicals on MN200 occurs by pore-filling, as suggested by the previous studies, since the maximum sorption volumes of the 11 compounds on MN200 are comparable to the pore volume of MN200 (Pan and Zhang 2013). The difference between the sorption mechanism of the organic chemicals on MN200 and on XAD-7 and XAD-4 could be attributed to the differences in the structural rigidity of these polymers. Polymers are typically classified into two types based on their structural rigidity, namely the rubbery state (which is “soft”) and the glassy state (which is “hard”) (Pignatello and Xing 1996; Xing et al. 1996; Xing and Pignatello 1997). Adsorption on an external surface or an internal pore surface is responsible for the sorption of solutes on glassy polymers, whereas partition is responsible for the sorption of solutes on rubbery polymers (Pignatello and Xing 1996; Xing et al. 1996; Xing and Pignatello 1997). XAD-7 and XAD-4 are “soft” rubbery polymers, while MN200 has characteristics of a “hard” glassy polymer. XAD-7, which is polymethacrylate (Figure S1), is synthesized from methacrylate, which does not contain hard components (e.g., benzene ring) in its structure. Therefore, the polymerized materials are “soft” and can act as partition phases for organic chemicals. Although XAD-4 and MN200 (polystyrene) are both synthesized from styrene, which contains a hard benzene ring in its structure, the cross-linking in XAD-4 occurs to a lower degree compared to that in MN200 (Pan and Zhang 2013). As a result, XAD-4 is “soft”, rendering it suitable for the partition of organic chemicals, whereas MN200 is “hard” making it suitable for adsorption.
Correlations between sorption affinity and chemical properties
Solvatochromic parameters (i.e., V I, π*, β m, and α m) are useful for predicting the chemical properties, toxicity, mobility, and environmental behaviors (Kamlet et al. 1988; Marcus 1991; Hickey and Passino-Reader 1991; Nirmalakhandan et al. 1998; Crittenden et al. 1999) of organic chemicals. The linear solvation energy relationships (LSERs)-model based on solvatochromic parameters had been developed widely to predict the aqueous solubility of chemicals, octanol-water partition, solubility in blood and body organs, partition between blood and body organs, HPLC capacity factors, and toxicity of chemicals toward a variety of species (Kamlet et al. 1988). The LSER models have also been utilized to predict the sorption potential of aromatics, halogenated aliphatics, and halogenated aromatics from aqueous solutions on activated carbons and synthetic polymeric adsorbents by correlating the solvatochromic parameters with the sorption coefficients (Crittenden et al. 1999). The LSER model, which considers solvent-related effects, may advance the understanding of sorption mechanisms, particularly in the area of solute-sorbent interactions (Yang et al. 2008; Yang and Xing 2010; Wu et al. 2012; Pan and Zhang 2012, 2013).
Multiple linear regressions, with the stepwise method employed for variable selection, were conducted to establish the LSERs combining E with V I, π*, β m, and α m (Eqs. 10 and 11, Fig. 4),
The LSER Eqs. 10 and 11 are in agreement with previously reported results (12). α m was retained in Eqs. 10 and 11, while β m, π*, and V I were ruled out by the stepwise method. The retention of α m in the equations indicates the important role played by the hydrogen-bonding donor ability of the solute on sorption. The solute molecules act as hydrogen-bonding donors, whereas XAD-7 functions as the hydrogen-bonding acceptor. The exclusion of β m and π* from the equations suggests that XAD-7 was unable to act as a hydrogen-bonding donor or form π-π interactions with the solute molecules. The exclusion of V I from the equations suggests that the sorption of organic chemicals by XAD-7 could not be induced by the molecular volume (e.g., cavitation energy, as reported in the literature (Abraham and Mcgowan 1987)). The intercepts of Eqs. 10 and 11 may be attributed to non-specific interactions such as hydrophobic effects arising from van der Waals force (Crittenden et al. 1999; Yang et al. 2008, 2010; Pan and Zhang 2012). In contrast to the linear isotherms for the partition of organic chemicals between organic solvents (e.g., octanol) and water (Chiou and Kile 1998; Chiou 2002), the isotherms for the partition of organic chemicals into XAD-7 and XAD-4 are non-linear (Fig. 1 and Table 2). Non-linear isotherms were also observed for the partition of organic chemicals into natural organic matters (Spurlock and Biggar 1994). Specific interactions including hydrogen-bonding were believed to be responsible for the non-linearity of the isotherm (Spurlock and Biggar 1994). Therefore, the hydrogen-bonding interactions observed here and in the previous studies (Pan and Zhang 2012, 2013) could partially account for the non-linear partition isotherms for the sorption of chemicals on XAD-7 and XAD-4 (Spurlock and Biggar 1994).
Prediction of sorption using the correlations and the effect of pH
The correlations obtained (i.e., Eqs. 6 and 11) with constant b values (1.03) allow the prediction of the sorption behaviors of organic chemicals on XAD-7, by simply using the well-known parameters of the chemicals, namely S W, K OW, and α m . For example, Fig. 5 shows that the predicted q e values using these correlations are in good agreement with the experimental values for all the 24 chemicals investigated in this study, based on 450 data points. The largest deviation (overestimation) of the estimated values from the experimental values (i.e., the dotted reference line) was observed for phenanthrene, which was followed by 1,4-dinitrobenzene (Fig. 5). At present, it is difficult to explain the reasons for this deviation. One possible reason could be that the S W or K OW values of phenanthrene and 1,4-dinitrobenzene were overestimated, which would result in the overestimation of their sorption capacities and, consequently, the sorption amounts. Since only three simple parameters (i.e., S W, K OW, and α m ) and two linear equations (Eqs. 6 and 11) with clear physical meaning were used in the prediction; this method is simpler than the method previously reported (Pan and Zhang 2013), which needs six parameters (i.e., S W, K HW, K GW, V I, π*, and α m) and at least five equations that do not have a physical meaning. Moreover, the good estimation obtained by the reported method may be a result of over-parameterization (Crittenden et al. 1999) and over-regression analysis, while neglecting the physical basis of the equations.
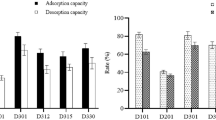
Phenols and anilines are ionizable organic compounds (IOCs). They can be dissociated by changing the solution pH, and the resulting dissociated species may have different sorption behavior compared to the non-dissociated species. As an example, as shown in Fig. 6, while the sorption of 2,4-dichlorophenol and 4-chloroaniline by XAD-7 was highly dependent on the solution pH in the region around the dissociation constant (pK a) of the compound, it was almost constant at pH values far away from the pK a (i.e., at|pH-pK a| > 2). The non-dissociated and dissociated species of phenols and anilines could both be sorbed on XAD-7 (Fig. 6). However, higher amounts of the non-dissociated species of either phenols or anilines were sorbed compared to the dissociated species (Fig. 6). Therefore, the prediction of the sorption of IOCs on XAD-7, using the correlations obtained in this study, should include pH-dependent effects, since the predictions using the correlations shown in Eqs. 6 and 11 were established for non-dissociated species of IOCs.
As shown in Fig. 6, for a given initial concentration, the pH dependence of the apparent K d (the sorption coefficient, K d = q e/C e) of 2,4-dichlorophenol and 4-chloroaniline could be estimated satisfactorily using Eqs. 12 and 13, respectively.
For phenols,
For anilines,
In the above equations, K d N is the sorption coefficient of the non-dissociated species and K d I is the sorption coefficient of the dissociated species. The K d N and K d I values used in Eqs. 12 and 13 for modeling and the initial concentrations of the compounds are also listed in Fig. 6. These two equations were established by estimating the non-dissociated and dissociated fractions of IOCs at various pH values. The equations for estimating the non-dissociated fractions of organic acids (f A N) and bases (f B N) are f A N = (1 + 10pH − pKa)−1 and f B N = (1 + 10pKa − pH)−1, respectively, whereas the equations for estimating the dissociated fraction of organic acids (f A I) and bases (f B I) are f A I = (1 + 10 pKa − pH)−1 and f B I = (1 + 10pH − pKa)−1, respectively. The values of either K d N or K d I in Eqs. 12 and 13 are assumed to be constant over the entire pH range, for a given initial concentration. The good fit of the equations for the pH-dependent sorption of 2,4-dichlorophenol and 4-chloroaniline on XAD-7 (Fig. 6) indicates that the pH dependence of sorption may be attributed to the dissociation of the IOCs and can be predicted using the correlations presented in Eqs. 12 and 13.
Conclusion
Experimental testing of the environmental behaviors such as sorption for thousands of chemicals on various materials is difficult, owing to the limitations posed by the costs involved in such testing. Therefore, a method for the estimation of these behaviors is usually essential in cases where direct experimental data is unavailable and will provide a convenient method to assess the potential applications of various materials in environmental technology. The correlations between sorption capacity and chemical solubility in water or octanol and those between sorption affinity and the solvatochromic parameters of chemicals obtained in this study indicate that the sorption of organic chemicals on XAD-7 exhibits a non-linear partition behavior into XAD-7, as opposed to adsorption on XAD-7 surfaces. Specific interactions (i.e., hydrogen-bonding interactions between organic molecules and XAD-7) and the non-specific interactions arising from the van der Waals forces were responsible for the non-linear partition into XAD-7. The strong hydrogen-bonding interactions between solutes (which act as hydrogen-bonding donors) and XAD-7 (which acts as the hydrogen-bonding acceptor) observed in this study indicate that the solutes with hydrogen-bonding donor ability have higher sorption affinities compared to the ones without hydrogen-bonding donor ability, at relatively low concentrations. The non-linear partition mechanism implies that extraction by using solvents having a large solubility for organic chemicals would be a superior way for the regeneration of exhausted XAD-7. Moreover, the correlations obtained in this study allow the quantitative prediction of the non-linear partition of organic chemicals by XAD-7 by simply using the well-known parameters of chemicals (i.e., S W, K OW, and a m). The effect of solution pH on the sorption of IOCs could also be predicted using the obtained correlations along with the equations developed based on the estimation of IOC dissociation rates. The predictions are useful as a guide for the selective removal of a given organic contaminant and for the determination of the proper ratio of sample to column volumes during the pre-concentration of organic solutes from water. It was also observed that the sorption mechanisms on other resins such as MN200 could be different from that on XAD-7. Therefore, more studies need to be conducted to investigate the sorption mechanisms on other resins and improve the prediction methods.
References
Abraham MH, Mcgowan JC (1987) The use of characteristic volumes to measure cavity terms in reversed phase liquid chromatography. Chromatographia 23:243–246
Chiou CT (2002) Partition and adsorption of organic contaminants in environmental systems. Wiley, New York
Chen W, Duan L, Wang LL, Zhu DQ (2008) Adsorption of hydroxyl- and amino-substituted aromatics to carbon nanotubes. Environ Sci Technol 42:6862–6868
Chiou CT, Kile DE (1998) Deviations from sorption linearity on soils of polar and nonpolar organic compounds at low relative concentrations. Environ Sci Technol 32:338–343
Crittenden JC, Sanongraj S, Bulloch JL, Hand DW, Rogers TN, Speth TF, Ulmer M (1999) Correlation of aqueous-phase adsorption isotherms. Environ Sci Technol 33:2926–2933
Chen DH, Yaws CL (1999) Solubility in water and octanol-water partition coefficient, In: Chemical Properties Handbooks, 1st ed.; Yaws, C.L. ed.; Mcgraw-Hill: New York
Feng X, Kerry JD, Sangyoo P, Michio N, Brian RP (2012) Batch and column study: sorption of perfluorinated surfactants from water and cosolvent systems by Amberlite XAD resins. J Colloid Interf Sci 368:505–511
Ghatbandhe AS, Jahagirdar HG, Yenkie MKN (2012) Adsorption equilibrium and kinetics of 4-chlorophenol: a comparative study. J Chem Bio Phy Sci A 2:1241–1248
Hickey JP, Passino-Reader DR (1991) Linear solvation energy relationships: “rules of thumb” for estimation of variable values. Environ Sci Technol 25:1753–1760
Haderlein SB, Schwarzenbach RP (1993) Adsorption of substituted nitrobenzenes and nitrophenols to mineral surfaces. Environ Sci Technol 27:316–326
Haderlein SB, Weissmahr KW, Schwarzenbach RP (1996) Specific adsorption of nitroaromatic explosives and pesticides to clay minerals. Environ Sci Technol 30:612–622
Kamlet MJ, Doherty RM, Abraham MH, Marcus Y, Taft RW (1988) Linear solvation energy relationships. 46. An improved equation for correlation and prediction of octanol-water partition coefficients of organic nonelectrolytes (including strong hydrogen bond donor solutes). J Phys Chem 92:5244–5255
Kunin R, Meitzner E, Bortnick N (1961) Macroreticular ion exchange resins. J Am Chem Soc 84(2):305–306
Karger BL, Gant JR, HartkopfA WPH (1976) Hydrophobic effects in reversed-phase liquid chromatography. J Chromatogr A 128:65–78
Karnjanapiboonwong A, Morse AN, Maul JD, Anderson TA (2010) Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J Soil Sediment 10:1300–1307
Li J, Chase HA (2010) Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat Prod Rep 27:1493–1510
Marcus Y (1991) Linear solvation energy relationships: correlation and prediction of the distribution of organic solutes between water and immiscible organic solvents. J Phys Chem 95:8886–8891
Nirmalakhandan N, Egemen E, Trevizo C, Xu S (1998) Structure- and property-activity relationship models for prediction of microbial toxicity of organic chemicals to activated sludge. Ecotox Environ Safe 39:112–119
Pan B, Lin DH, Mashayekhi H, Xing BS (2008a) Adsorption and hysteresis of bisphenol A and 17 α-ethinyl estradiol on carbon nanomaterials. Environ Sci Technol 42:5480–5485
Pignatello JJ, Xing B (1996) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30:1–11
Pan BJ, Zhang HC (2012) A modified Polanyi-based model for mechanistic understanding of adsorption of phenolic compounds onto polymeric adsorbents. Environ Sci Technol 46:6806–6814
Pan BJ, Zhang HC (2013) Interaction mechanisms and predictive model for the sorption of aromatic compounds onto nonionic resins. J Phys Chem C 117:17707–17715
Pan BJ, Zhang WM, Pan BC, Qiu H, Zhang QR, Zhang QX, Zheng SR (2008b) Efficient removal of aromatic sulfonates from wastewater by a recyclable polymer: 2-naphthalene sulfonate as a representative pollutant. Environ Sci Technol 42:7411–7416
Qiu YP, Chen JL, Li AM, Zhang QX, Huang MS (2005) Adsorption of 2,4-D on modified hypercrosslinked polystyrene (NDA-99) and XAD-4 resin. Chinese J Polym Sci 23:435–440
Spurlock FC, Biggar JW (1994) Thermodynamics of organic chemical partition in soils. 2. Nonlinear partition of substituted phenylureas from aqueous solution. Environ Sci Technol 28:996–1002
Thurman EM, Malcolm RL, Alken GR (1978) Prediction of capacity factors for aqueous organic solutes adsorbed on a porous acrylic resin. Anal Chem 50:775–779
Vincenza F, Isabel BC, Ruben FJ, Manuela EP, Paula MLC (2013) Effects of physical parameters onto adsorption of the borderline amino acids glycine, lysine, taurine, and tryptophan upon Amberlite XAD16 resin. J Chem Eng Data 8:707–717
Wu WH, Chen W, Lin DH, Yang K (2012) Influence of surface oxidation of multiwalled carbon nanotubes on the adsorption affinity and capacity of polar and nonpolar organic compounds in aqueous phase. Environ Sci Technol 46:5446–5454
Xing B, Pignatello JJ, Gigliotti B (1996) Competitive sorption between atrazine and other organic compounds in soils and model sorbents. Environ Sci Technol 30:2432–2440
Xing B, Pignatello JJ (1997) Dual-mode sorption of low-polarity compounds in glassy poly(vinylchloride) and soil organic matter. Environ Sci Technol 31:792–796
Xu ZY, Zhang QX, Fang HHP (2003) Applications of porous resin sorbents in industrial wastewater treatment and resource recovery. Crit Rev Environ Sci Technol 33:363–389
Yang K, Xing BS (2010) Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem Rev 110:5989–6008
Yang K, Wu WH, Jing QF, Zhu LZ (2008) Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ Sci Technol 42:7931–7936
Yang K, Wu WH, Jing QF, Jiang W, Xing BS (2010) Competitive adsorption of naphthalene with 2,4-dichlorophenol and 4-chloroaniline on multiwalled carbon nanotubes. Environ Sci Technol 44:3021–3027
Yang K, Wang XL, Zhu LZ, Xing BS (2006a) Competitive sorption of pyrene, phenanthrene, and naphthalene on multiwalled carbon nanotubes. Environ Sci Technol 40:5804–5810
Yang K, Zhu LZ, Lou BF, Chen BL (2005) Correlations of nonlinear sorption of organic solutes with soil/sediment physicochemical properties. Chemosphere 61:116–128
Yang K, Zhu LZ, Xing BS (2006b) Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 40:1855–1861
Zhang WM, Zhang QJ, Pan BC, Lv L, Pan BJ, Xu ZW, Zhang QX, Zhao XS, Du W, Zhang QR (2007) Modeling synergistic adsorption of phenol/aniline mixtures in the aqueous phase onto porous polymer adsorbents. J Colloid Interf Sci 306:216–221
Acknowledgments
This work was supported partly by NSFC (21322702, 21137003, and 41273125), the National Science and Technology Pillar Program of China (2013BAC01B01), the Zhejiang University K. P. Chaos High Technology Development Foundation (2013RC015), and the Fundamental Research Funds for the Central Universities (2014XZZX003-30).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Yang, K., Qi, L., Wei, W. et al. Prediction of the sorption capacities and affinities of organic chemicals by XAD-7. Environ Sci Pollut Res 23, 1060–1070 (2016). https://doi.org/10.1007/s11356-014-4012-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-4012-3