Abstract
The concentrations, composition profiles, and sources of polycyclic aromatic hydrocarbons (PAHs) were analyzed in 55 surface soil samples collected from four oil fields across China (Daqing, DQ; Shengli, SL; Xinjiang, XJ; and Huabei, HB). The total 16 priority PAHs concentrations of DQ, SL, XJ, and HB ranged from 857 to 27,816; 480 to 20,625; 497 to 43,210; and 12,112 to 45,325 ng/g, respectively, with means of 9160; 6394; 13,569; and 22,954 ng/g and the seven possible carcinogenic PAHs accounted for 8–25.7 % of the total PAHs. Almost all the samples were heavily contaminated, and phenanthrene, chrysene, and pyrene were the most dominant components. The PAH isomeric ratios indicated that PAHs in oil fields mainly originated from petroleum. The toxic assessment illustrated that people living and working in oil fields would suffer low carcinogenic risk, which was somehow coincided with the results of epidemiological survey on cancer incidence. It seems essential to pay more attention to the chronic human health effects of exposure to oil fields and to focus new studies on the public health field that involves a large number of people all over the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental pollutants originated mainly from anthropogenic sources such as combustion of fossil fuels and direct release of oil and its products (Jiang et al. 2009; Wang et al. 2010; Hussar et al. 2012; Kim et al. 2013). Because of the mutagenic and carcinogenic potential of some PAHs, the US Environmental Protection Agency (EPA) has classified 16 species of PAHs as priority pollutants. And seven of these PAHs are considered as probable human carcinogens, i.e., benzo[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene, dibenz[a,h]anthracene, and indeno[1,2,3-cd]pyrene (Ene et al. 2012; Tsibart and Gennadiev 2013). Their influences on human health have been caused great concern both by researchers and publics.
Oil exploration and production processes can greatly contribute to localized loadings of PAHs (Bojes and Pope 2007; Liang et al. 2012; Xia et al. 2012). In oil fields, PAHs enter into environment with crude oil through accidental spillage or leaks of oil from producing wells, storage tanks, gathering lines, transportation lines, and pits (Kuang et al. 2011; Sojinua et al. 2010; Hong and Luthy 2007). In the past several decades, there have been numerous petroleum leak accidents happened, and moreover, natural seepage of crude oil also contributes to the PAHs load in the environment (Farwell et al. 2009).
According to BP Statistical Review of World Energy June 2013, by 2012, China is the second largest oil consumption country and is the world’s fourth largest oil production country after Saudi Arabia, Russia, and USA, with 4155 thousand barrels per day. Along with increase demand for energy to support rapid economic growth at last and next decades in China, PAHs have been and will be continually entered into environment. Many researches have presented environmental behaviors, ecological effect, and remediation of PAHs in air and water (Henri 2011; Chaudhary et al. 2011; Hirano et al. 2011; Bin et al. 2009; Meckenstock and Mouttaki 2011). However, there are few reports about the degree of contamination in natural soil around certain oil fields and its risk for human health. Therefore, it is meaningful to assess the degree of risk level of PAHs in oil fields across China, which is both sink and source of pollutants for human and environment.
This study carried out four oil fields in different geographic regions, Daqing (DQ), Shengli (SL), Xinjiang (XJ), and Huabei (HB), which produce about 79 % of the total crude oil of China (Ma et al. 2012). By collecting and analyzing soil samples from the four oil fields, the primary objectives of this study were to understand the characterization of PAHs and to assess the degree of environment pollution and potential human cancer risk involving PAHs in soils of oil fields across China.
Materials and methods
Chemicals and materials
A mixture of 16 PAHs [naphthalene (NAP), acenaphthene (ACE), avenaphthylene (ACY), fluorene (FLO), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), benz[a]anthrancene (BaA), chrysene (CHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IcdP), dibenzo[a,h]anthracene (DahA), and benzo[ghi]perylene (BghiP) was purchased from J&K chemical Ltd., USA. The internal standards for these PAHs were deuterated PAHs (NAP-d 8 , ACE-d 10 , ANT-d 10 , CHR-d 12 , and Perelyne-d 12 ), and they were diluted to 4000 μg/L by dichloromethane. Neutral alumina (100–200 mesh) and silica gel (100–200 mesh) were obtained from Beijing Chemical Reagent Co. and heated at 450 °C for 4 h, kept in a sealed desiccator. Sodium sulfate was baked at 450 °C for 6 h and stored in a sealed desiccator. All organic solvents of HPLC grade were obtained from Fisher Co..
Site description and sampling
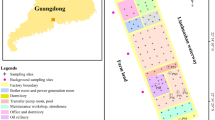
A total 55 soil samples were collected from four oil fields, which were mainly distributed in north of China: Xingjiang (XJ, 10 samples around Kelamayi and 10 samples around Korla) in northwest China, Daqing (DQ, 15 samples) in northeast China, Shengli (SL, 15 samples) in the Yellow River area, and Huabei (HB, 5 samples) in the Huabei Plain in north China (Fig. 1). Soils were collected around crude oil pumping wells where contamination occurred and kept in cold boxes until transported to the lab. All the soil samples were air dried at room temperature, sieved through a 40 mesh sieve, removed stones and residual roots, and stored in desiccators prior to analysis.
Sample extraction and cleanup
EPA method 3550C was modified to extract PAHs from the soils sample. Briefly, 5 g of soil was ultrasonic extracted using three 25 ml portions of n-hexane and acetone (3:1, v/v) for 5 min after 200 ng internal standards was spiked into the sample. The extract was concentrated to 2 ml by a rotary evaporator and then fractionated and cleaned up by an alumina/silica gel column. The column was eluted with 60 ml of CH2Cl2/n-hexane (1:1, v/v) to obtain PAHs. The PAH fraction was finally concentrated to 1 ml under nitrogen, and then analyzed by GC/MS.
GC/MS analysis and quantification
The PAHs were quantified by GC/MS (Agilent GC 6980 N/5973I MSD) using the internal standards. An HP-5 MS column (30 m × 0.25 mm × 0.25 μm) was equipped with helium as the carrier gas at a constant flow rate of 1.2 ml/min. The oven temperature was programmed from 60 °C (5 min) to 280 °C at 5 °C/min, and held at 280 °C for 20 min. The MSD was operated at 70 eV and the ion source temperature was 280 °C. All PAH concentrations were determined using selected ion monitoring (SIM).
Quality control and quality assurance
The procedural blanks, spiked blanks, and sample duplicates were routinely analyzed with soil samples. Limit of detection (LOD) was calculated as three times the noise level of the chromatogram in blank sample, and LODs of PAHs were 0.31 ng/g (ACE) and 1.32 ng/g (BghiP). The recovery efficiency was checked by analyzing soil samples spiked with known amount of PAH standard. The recoveries from NAP to BghiP were from 79 to 111 % for the 16 individual PAHs, and the recoveries for the deuterated PAHs were from 85 to 112 %. The average coefficients of variation for the samples were 17 % (3–37 %) for 16 PAHs.
Results and discussion
PAHs concentrations
The concentrations of the 16 individual PAHs and 7 carcinogenic PAHs (∑PAH7C) in soil samples from four oil fields are given in Table 1. The total PAHs concentrations of DQ, SL, XJ, and HB ranged from 857 to 27,816; 480 to 20,625; 497 to 43,210; and 12,112 to 45,325 ng/g, respectively, with average concentrations of 9160; 6394; 13,569; and 22,954 ng/g. The minimum PAHs levels were found in SL oil field and the maximum were found in SD oil field. The ∑PAH7C concentrations accounted for 8.0–25.7 % of the total PAHs, and the proportion of the ∑PAH7C was the highest in DQ oil field, and followed by SL and HB. In addition, BaP concentrations, one of the most potent carcinogenic PAHs, varied from 4.0 to 93.0, 12.7 to 235.6, 7.1 to 73.4, and 22.2 to 197.1 ng/g, respectively, in DQ, SL, XJ, and HB.
According to few investigations about PAHs distributions in oil fields, the concentrations of PAHs are significantly various. For example, Liang et al. (2012) reported 304,000–435,000 ng/g in soil from oil exploring areas, and 435–2409 ng/g of PAHs in soils around oily sludge plants in Zhong Yuan oil field in China, while in Texas it reached to 31,000 to 86,400 ng/g in soils from oil exploration areas (Kuang et al. 2011; Bojes and Pope 2007). The PAH concentrations in soils are significantly various in different fields around the world (Table S2 in the Supplementary data). Moreover, compared with other type of soil, the concentrations of PAHs in soil of oil field generally are much higher than them in agricultural soil and urban soil. For example, the agricultural soils typically contain lower total PAHs concentrations (397 ng/g, Ping et al. 2007; 830 ng/g, Agarwal et al. 2009; 4 ng/g, Maliszewska-Kordybach et al. 2008; 130 ng/g, Yang et al. 2012 ), while the urban soils are found to be more contaminated with PAHs concentrations ranged from 199 to 51,822 ng/g (Morillo et al. 2007; Rhind et al. 2013; Jiang et al. 2009; Ma and Zhou 2011). Soil often serves as a great sink storing pollutants from different sources of adjacent regions (Irene and Gerhard 2009; Collins et al. 2013). PAHs in soil of oil fields across China with concentrations between 480 to 45,325 ng/g indicated that the soil around oil fields stored great amount of PAHs (mean, 12,759 ng/g) and need to be regulated as its potential ecological and health risk.
The total PAHs concentrations of soils are regulated in few countries, and Maliszewska-kordybach (1996) suggested a soil contamination classification system based on total PAHs concentration as follows: non-contaminated soil (<200 ng/g), weekly contaminated soil (200–600 ng/g), contaminated soil (600–1000 ng/g), and heavily contaminated soil (>1000 ng/g). According to this classification system, only four sampling sites are weekly contaminated (1 for DQ, 1 for SL, and 2 for XJ), all of the last samples in this study were heavily contaminated. It is illustrated that serious PAH pollution existed in nature soil of oil fields across China, and strategy is needed to prevent the fields turning to be sources, which would transfer PAHs into groundwater or air in those regions.
PAHs profiles and sources
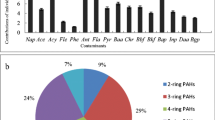
According to the number of aromatic rings, the 16 PAH compounds were divided into five groups. Higher proportions of individual PAHs with three rings (46.5–69.6 %) and four rings (15.7–40.9 %) were measured in the soil samples, followed by two rings (7.5–13.8 %), five rings (0.6–2.4 %), and six rings PAHs (0.3–2.6 %) (Fig. 2). Only in SL oil field, the proportion of six rings PAHs was higher than five rings. Among all 16 PAHs, PHE, CHR, and PYR were predominant species and the proportions were 37.1–63.6 %, 8.3–21.7 %, and 7.0–23.2 %, respectively, followed by NAP and FLA (3.2–8.1 % and 5.3–9.1 %, respectively).
It is recognized that biomass combustion and release of petroleum production are the two main sources of anthropogenic PAHs detected in the environment. PAH diagnostic ratios were used in many researches to determine the source of PAHs in soils, pyrogenic origin, or petrogenic source (Agarwal et al. 2009; Pies et al. 2008; Liu et al. 2010; Maliszewska-Kordybach et al. 2008). Two specific PAH ratios were calculated for the investigated soil samples: ANT/(ANT + PHE) and FLA/(FLA + PYR). Generally, the ratio of ANT/(ANT + PHE) < 0.1 indicates petroleum as a PAH source, while a ratio >0.1 suggests biomass combustion. In addition, the ratio of FLA/(FLA + PYR) <0.4 implies that PAHs are mainly from petrogenic source, 0.4–0.5 implies fossil fuel combustion, and >0.5 implies combustion of grass, wood, and coal (Tobiszewski and Namiesnik 2012).
The cross plot of ANT/(ANT + PHE) and FLA/(FLA + PYR) was showed in Fig. 3. For the ratio of ANT/(ANT + PHE), almost all soil samples had a ratio smaller than 0.1, except one sample in HB, one in DQ, one in SL, and three in XJ, which indicated that the main PAH sources are petrogenic. In addition, eight samples were found that the FLA/(FLA + PYR) ratios were larger than 0.4, which might imply that emission from biomass combustion still contributes to PAHs in soils in few oil fields in China.
Toxicity and potential cancer risk assessment of PAHs
PAHs are greatly concerned because of their documented mutagenicity and carcinogenicity. Seven species of PAHs have been considered carcinogenic, i.e., BaA, CHR, BbF, BkF, BaP, IcdP, and DahA. Since BaP was the only PAH with sufficient toxicological data to derive a carcinogenic potency factor, US EPA supplied a first approach to the toxicity of the seven PAHs referring it to that presented for BaP, and in addition, several researchers have tried to establish the real value of every single PAH Toxic Equivalence Factor (TEF) (Nisbet and LaGoy 1992; Yang et al. 2012; Cao et al. 2013; Qiao et al. 2006). And in this study, it is calculated as BaP-equivalent concentrations (BaPeq), and the results were listed in Table 2.
The total toxic BaPeq values in four oil fields were 113.34, 168.03, 93.63, and 211.51 ng/g, and the BaPeq concentrations of the seven considered carcinogenic PAHs were also calculated (105.50 ng/g in DQ, 161.32 ng/g in SL, 80.50 ng/g in XJ, and 192.14 ng/g in ZY). The seven carcinogenic PAHs showed very high contribution rates on the BaPeq concentrations. In addition, compared with the total PAHs concentrations, although the average concentration of PAHs in SL oil field was the lowest, the BaPeq level was extremely high, and the carcinogenic potency of PAHs should be given more attention due to potential environmental risk in SL oil field. That might suggest that it is not enough to control the hazard of PAHs by monitoring their total concentrations in soil from oil fields.
According to Man et al. (2013), it is hypothesized that potential cancer risk imposed on people living or working around oil fields as a result of being in contact with contaminated soil occurred via three major exposure pathways, i.e., accidental ingestion of soil particles; dermal absorption of pollutants via soil particle contact; and inhalation of fugitive soil particle. The potential cancer risks in four oil fields were calculated according to Man et al.’s methods, which used BaPeq as the concentrations of pollutants to estimate human cancer risk. The calculation equations are as follows:
Where Cancer riskingest is the cancer risk via ingestion of soil particles; Csoil is the concentration of the pollutant in soil; IngR is the ingestion rate of soil; EF is the exposure frequency; ED is the exposure duration; BW is the average body weight; AT is the averaging time; CF is the conversion factor; SFO is the oral slope factor.
Where Cancer riskdermal is the cancer risk via dermal contact of soil particles; SA is the surface area of the skin that contacts soil; AFsoil is the skin adherence factor for soil; ABS is the dermal absorption factor for BaP; GIABS is the gastrointestinal absorption factor.
Where Cancer riskinhale is the cancer risk via inhalation of soil particles; ET is the exposure time; IUR is the inhalation unit risk; AT* is the averaging time; PEF is the particle emission factor. The values of parameters could be found in supplementary data (Table S3).
Following the method, qualitative descriptions of lifetime cancer risks are defined as: very low when the estimated value is ≤10−6; low from 10−6< to <10−4, moderate from 10−4≤ to <10−3, high from 10−3≤ to <10−1, and very high when value is ≥10−1.
Because people in oil fields would not only expose via single pathway, the integrate cancer risk index of PAHs was calculated (Table 3). The averaged risk index was 1.105 × 10−6 for the four oil fields in China, which might imply that people living and working in oil fields suffer low cancer risk according to the qualitative descriptions of lifetime cancer risks by Man et al. (2013). If considering on the max value from the four oil fields (Table 3), it is clearly that potential cancer risk exists for human working in oil fields in China after exposure to oil contaminated soil, which indicated the requirement for establishing a range of contaminated soil standards and protection policies.
The carcinogenic potential in the investigated oil fields was somehow coincided with the results of epidemiological survey on cancer incidence in China (Table 4). SL oil field with the highest potential cancer risk index demonstrated the highest incidence of malignancy and leukemia in the four investigated oil fields. Even though HB presented a lower incidence of malignancy, its incidence of leukemia (Moolgavkar et al. 2014), a cancer considered related with petrochemical pollution, was relative high which was consistent with its potential cancer risk index. Meanwhile, the incidences of cancer in the four investigated oil fields were higher than average of China, which evidenced the existence of carcinogenic potential in oil fields evaluated by cancer risk index. The similar results could be found in other oil fields around the world, such as in Koprivnica-Krizevci Country, Croatia (Gazdek et al. 2007) and in the Amazon basin of Ecuador (Hurtig and Sebastian 2002), which both concluded that proximity to oil fields represented an increased population risk of developing haematopoietic cancers. In view of these results, it seems essential to pay more attention to the human health effects of exposure to oil fields, and focuses new studies on such a relevant but overlooked aspect of public health, which involved a large number of people all over the world.
References
Agarwal T, Khillare PS, Shridhar V, Ray S (2009) Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. J Hazard Mater 163:1033–1039
Bin C, Karthiga N, Loh KC (2009) Biodegradation of aromatic compounds: Current status and opportunities for biomolecular approaches. Appl Microbiol Biotechnol 85:207–228
Bojes HK, Pope PG (2007) Characterization of EPA’s 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul Toxicol Pharm 47:288–295
Cao XF, Liu M, Song YF, Ackland ML (2013) Composition, sources, and potential toxicology of polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in Liaoning, People’s Republic of China. Environ Monit Assess 185:2231–2241
Chaudhary P, Sharma R, Singh SB, Nain L (2011) Bioremediation of PAH by Streptomyces sp. Bull Environ Contam Toxicol 86:268–271
Chen WQ, Zheng RS, Zhang SW, Li N, Zhao P, Li GL, Wu LY, He J (2012) Report of incidence and mortality in China cancer registries, 2008. Chin J Cancer Res 24:171–180
Collins CD, Mosquera-Vazquez M, Gomez-Eyles JL, Mayer P, Gouliarmou V, Blum E (2013) Is there sufficient ‘sink’ in current bioaccessibility determinations of organic pollutants in soils? Environ Pollut 181:128–132
Ene A, Bogdevich O, Sion A (2012) Levels and distribution of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in topsoils from SE Romania. Sci Total Environ 439:76–89
Farwell C, Reddy CM, Peacock E, Nelson RK, Washburn L, Valentine DL (2009) Weathering and the fallout plume of heavy oil from strong petroleum seeps near coal oil point, CA. Environ Sci Technol 43:3542–3548
Gazdek D, Strnad M, Mustajbegovic J, Lojan Z (2007) Lymphohematopoietic malignancies and oil exploitation in Koprivnica-Krizevci County, Croatia. Int J Occup Environ Health 13:258–267
Henri S (2011) Developmental brain and behavior toxicity of air pollutants: a focus on the effects of polycyclic aromatic hydrocarbons (PAHs). Crit Rev Environ Sci Technol 41:2026–2047
Hirano M, Tanaka S, Asami O (2011) Classification of polycyclic aromatic hydrocarbons based on mutagenicity in lung tissue through DNA microarray. Environ Toxicol 28:652–659
Hong L, Luthy RG (2007) Availability of polycyclic aromatic hydrocarbons from lampblack-impacted soils at former oil-gas plant sites in California, USA. Environ Toxicol Chem 26:394–405
Hurtig AK, Sebastian MS (2002) Geographical differences in cancer incidence in the Amazon basin of Ecuador in relation to residence near oil fields. Int J Epidemiol 31:1021–1027
Hussar E, Richards S, Lin ZQ, Dixon RP, Johnson KA (2012) Human health risk assessment of 16 priority polycyclic aromatic hydrocarbons in soils of Chattanooga, Tennessee, USA. Water Air Soil Pollut 223:5535–5548
Irene S, Gerhard L (2009) Cycling of DDT in the global environment 1950–2002: World ocean returns the pollutant. Geophys Res Lett 36:L24602
Jiang YF, Wang XT, Wang F, Jia Y, Wua MH, Sheng GY, Fu JM (2009) Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere 75:1112–1118
Kim KH, Jahan SA, Kabir E, Brown RJC (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Kuang SP, Wu ZC, Zhao LS (2011) Accumulation and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soils around oil sludge in Zhongyuan oil field, China. Environ Earth Sci 64:1353–1362
Li XL, Wang XY, Wang R, Li XX, Li J, Chen XL (2010) The mortality rates and trend of leukemia during 1970–2005 in Shandong Province. Chin J Health Stat 27:271–274
Liang YT, Zhang X, Wang J, Li GH (2012) Spatial variations of hydrocarbon contamination and soil properties in oil exploring fields across China. J Hazard Mater 242:371–378
Liu Y, Chen L, Zhao J, Wei Y, Pan Z, Meng XZ, Huang Q, Li W (2010) Polycyclic aromatic hydrocarbons in the surface soil of Shanghai, China: Concentrations, distribution and sources. Org Geochem 41:355–362
Ma J, Zhou YZ (2011) Soil pollution by polycyclic aromatic hydrocarbons: a comparison of two Chinese cities. J Environ Sci China 23:1518–1523
Ma LW, Fu F, Lin Z, Liu P (2012) Oil development in China: Current status and future trends. Energy Policy 45:43–53
Maliszewska-Kordybach B (1996) Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochem 11:121–127
Maliszewska-Kordybach B, Smreczak B, Klimkowicz-Pawlas A, Terelak H (2008) Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere 73:1284–1291
Man YB, Kang Y, Wang HS, Laub W, Li H, Sun XL, Giesye JP, Chowb KL, Wong MH (2013) Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. J Hazard Mater 261:770–776
Meckenstock RU, Mouttaki H (2011) Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr Opin Biotechnol 22:406–414
Moolgavkar SH, Chang ET, Watson H, Lau E (2014) Cancer mortality and quantitative oil production in the Amazon region of Ecuador, 1990–2010. Cancer Causes Control 25:59–72
Morillo E, Romero AS, Maqueda C, Madrid L, Ajmone-Marsan F, Grcman H, Dayidson CM, Hursthousee AS, Villaverde J (2007) Soil pollution by PAHs in urban soils: a comparison of three European cities. J Environ Monit 9:1001–1009
Nisbet IC, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72:1594–1601
Qiao M, Wang CX, Huang SB, Wang DH, Wang ZJ (2006) Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake. China Environ Int 32:28–33
Rhind SM, Kyle CE, Kerr C, Osprey M, Zhang ZL, Duff EI, Lilly A, Nolan A, Hudson G, Towers W, Bell J, Coul M, McKenzie C (2013) Concentrations and geographic distribution of selected organic pollutants in Scottish surface soils. Environ Pollut 182:15–27
Sojinua OSS, Wang JZ, Sonibared OO, Zeng ET (2010) Polycyclic aromatic hydrocarbons in sediments and soils from oil exploration areas of the Niger Delta, Nigeria. J Hazard Mater 174:641–647
Tang Y, Shen BH, Chen GX (2010) Analysis of incidence and dynamic epidemiology for malignancies from 1997 to 2006 in Kelamayi oilfield in Xinjiang. Chin J Cancer Prev Treat 17:125–129
Tobiszewski M, Namiesnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Tsibart AS, Gennadiev AN (2013) Polycyclic aromatic hydrocarbons in soils: sources, behavior, and indication significance (a review). Eurasian Soil Sci 46:728–741
Wang WT, MasseySimonich SL, Xue M, Zhao JY, Zhang N, Wang R, Cao J, Tao S (2010) Concentrations, sources and spatial distribution of polycyclic aromatic hydrocarbons in soils from Beijing, Tianjin and surrounding areas, North China. Environ Pollut 158:1245–1251
Wang Y, Wang DP, Wang YH (2012) Cancer incidence analysis of Daqing City during 2005–2009. Chin J Publ Health Manag 1:77–78
Xia K, Hagood G, Childers C, Atkins J, Rogers B, Ware L, Armbrust K, Jewell J, Gatian N, Diaz D, Folmer H (2012) Polycyclic aromatic hydrocarbons (PAHs) in Mississippi seafood from areas affected by the deepwater horizon oil spill. Environ Sci Technol 46:5310–5318
Yang B, Xue ND, Zhou LL, Li FS, Cong X, Han BL, Li HY, Yan YZ, Liu B (2012) Risk assessment and sources of polycyclic aromatic hydrocarbons in agricultural soils of Huanghuai plain, China. Ecotoxicol Environ Safe 84:304–310
Zhang SW, Chen WQ, Kong LZ (2007) An annual report: Cancer incidence in 35 cancer registries in China, 2003. Bull Chin Cancer 7:87–89
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Wang, J., Cao, X., Liao, J. et al. Carcinogenic potential of PAHs in oil-contaminated soils from the main oil fields across China. Environ Sci Pollut Res 22, 10902–10909 (2015). https://doi.org/10.1007/s11356-014-3954-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3954-9