Abstract
Surface soil (0–20 cm) samples (n = 143) were collected from vegetable, maize, and paddy farmland used for commercial crops in Liaoning, China. Sixteen priority polycyclic aromatic hydrocarbons (PAHs) listed in US Environmental Protection Agency were analyzed by high-performance liquid chromatography using a fluorescence detector. The soil concentrations of the 16 PAH ranged from 50 to 3,309 ng/g with a mean of 388 ng/g. The highest concentration of total PAHs found in soil of the vegetable farmland was 448 ng/g in average, followed by maize and paddy with total PAHs of 391 and 331 ng/g, respectively. Generally, the low molecular weight PAHs were more predominant than the high molecular weight PAHs in most of the soils. The evaluation of soil PAH contamination based on the Canadian criterion indicated that only naphthalene, phenanthrene, and pyrene were over the target values in several sampling sites. Isomer pair ratios and principal component analysis indicated that biomass and coal combustion were the main sources of PAHs in this area. And the average value of total B[a]Peq concentration in vegetable soils was higher than paddy and maize soils. We suggest that biomass burning should be abolished and commercial farming should be carried out far from the highways to ensure the safety of food products derived from commercial farming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is a vital natural resource on which human lives depend (Maliszewska-Kordybach et al. 2009). However, with the rapid development of economies, modernization of industry, and urbanization, pollutants such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), pesticides, veterinary drugs, feed additives, and other emerging pollutants have been released into the soils through many paths, each of which contributes to the contaminated environmental matrix (Wang et al. 2008).
Farm-based commercial vegetable cropping is an open system in which soil plays a key role in ensuring the safety of the food product (Wang et al. 2008). In recent years, the eco-environment of the commercial farm has been suffering from serious impacts of pollution due to the unprecedented level of human activity. This has resulted in incidents where agricultural products have been contaminated due to soil pollution. The quality and safety of the agricultural products has thus become one of the key issues of common concern within the agriculture industry. Ensuring the safety of farm produce and maintenance of a high quality of production is critical for sustainable development (Liu et al. 2009). Central to this issue is the control of toxic and harmful pollutants.
Information on pollutants in soils of commercial farmland has mainly focused on the heavy metals (Cu, Zn, Pb, Cd, Hg, As, Cr) in top soil in order to evaluate whether soils could meet the production standard for green products (Li et al. 2006; Pandey and Pandey 2009; Song et al. 2006). Studies on persistent organic pollutants in agricultural soils have also been reported, but most of the investigations were focused on the historical aspects of wastewater irrigation and the impact of economic development in the agricultural soil (Nam et al. 2003; Tao et al. 2004; Cai et al. 2007; Maliszewska-Kordybach et al. 2009; Agarwal et al. 2009; Hu et al. 2009). Few studies have been undertaken to investigate persistent organic pollutants in farm produce up to the current time because it was usually assumed to be a green and uncontaminated clean area.
PAHs are persistent organic pollutants some of which are considered to be human carcinogens (IARC 1983). Sixteen PAHs have been listed as priority pollutants by the US Environmental Protection Agency. PAHs can enter the soil through endogenetic and anthropogenic processes (Wilcke 2000; Cai et al. 2007). The latter process contributes the most to the accumulation of these chemicals through solid waste discharge, automobile exhaust, atmospheric emission, and wastewater irrigation. Pollution of PAHs in soils may be linked with adverse effects on the ecosystem and also have influence on the quality of products from commercial agriculture (Song et al. 2006; Yin et al. 2008). The quality and safety of farm produce is closely related to the environment in which they were produced and may restrict efficient operation of modern agricultural production (Ciganek et al. 2002).
Tieling is located in the mid-region of Songliao plain, Liaoning, in northeastern of China. It is a region of high-quality crop production, with an area of 530,000 ha of maize, paddy rice, soybean, and vegetables being the main species that contribute to an annual yield up to 500,000 tons, accounting for its reputation as the golden zone for crop planting. Identification of pollutants and pollution control of PAHs is essential to ensure the product quality and food safety (Agarwal et al. 2009).
In this study, we investigated the distribution of polycyclic aromatic hydrocarbons in soils from farm crops in Tieling region, Liaoning Province. Isomer pair ratios (IPR) and principal component analysis (PCA) were used to analyze the origins of PAHs. The aim of the present work was to contribute to improved protection and pollution control in commercial farms.
Materials and methods
Reagents
Hexane, acetone, and dichloromethane were chromatographic grade or residue analysis grade and purchased from Fluka (Switzerland). Acetonitrile (as high-performance liquid chromatography (HPLC) mobile phase) was HPLC purity grade and purchased from Sigma Ltd (USA), and ultrapure water (as HPLC mobile phase) was collected from the Mili-Q system. Silica gel (70–230 mesh), sodium sulfate, and sodium chloride were residue analysis grade and obtained from Sigma Ltd (USA). Mixed PAH standards (SS EPA 610 polycyclic aromatic hydrocarbon mix) were procured from state standard bureau (Gaithersburg, Germany). All glassware were immersed in acid, rinsed out with water and acetone, heated, and stored at 120 °C.
Soils sampling
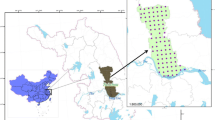
Samples were collected from paddy, maize, and vegetable fields in the Tieling farm region, Liaoning Province in September after the harvesting season. Surface soil samples (0–20 cm) were taken with a stainless steel soil auger after removal of the uppermost plant cover. Five samples were collected over an area of several hundred square meters, pooled, and homogenized to provide a composite sample. All samples were transported to a laboratory, air-dried at room temperature, sieved through a 2-mm sieve mesh after removing twigs and stones and stored in the dark before further characterization. A total of 143 soil samples were collected, 46 of which were collected from the paddy field, 56 samples were obtained from maize land, and 41 were from vegetable farmland. The location and detail information for sampling sites is shown in Fig. 1.
Soil extraction and cleanup
Extraction of PAHs was performed according to an ultrasonic extraction method (Song et al. 2002). Dried soil (5 g) was weighed into a 25-ml brown centrifuge tube prior to the addition of 25 ml of dichloromethane and was extracted using an ultrasonic cleaner (KQ-250DB, Kunshan, China) in a water bath at 30–35 °C for 2 h. The supernatant was collected and centrifuged at 2,500 rpm for 5 min and then reduced to dryness in a rotary evaporator and made up to 1.0 ml with acetone and transported into a pipette column equipped with 1.0 g of silica gel (70–230 mesh, Sigma) for cleanup. The column was eluted with dichloromethane and n-hexane (1:1), and the eluate was reduced to dryness again in a nitrogen stream and was made up to 1.0 ml with acetonitrile for analysis of PAHs. All extractions were carried out in triplicate.
Determination of PAHs in soils
Twenty microliters of extract was injected and separated on into a 250 × 4.6-mm, I.D. 5-μm RP-C18 octadecylsilane column following an acetonitrile/water gradient running from 50:50 to 100 acetonitrile over 30 min at a flow rate of 1.0 ml/min. A two model high-pressure LC pump (Agilent 1100, USA) equipped with fluorescence detector (Agilent 1100, USA) produced the gradient. Further selectivity was obtained by monitoring the chromatographic effluent fluorometrically with excitation and emission wavelength optimized for the detection of individual PAHs. An external standard (SS EPA 610 polycyclic aromatic hydrocarbons mix) was used for quantification of 16 PAHs. Fluorescence data were qualified on an Agilent 1100 minichroma data handling system by manual integration. Identification of PAHs was carried out on the basis of retention time with further confirmation on a photodiode array detector (Agilent 1100, USA) if necessary.
Statistical analysis
Experimental results were expressed on a dry weight basis. Statistical analysis of the results including PCA was carried out with the software package SPSS 17.0 for windows. Arithmetic mean was used to present the average PAH concentrations.
Results and discussion
Total PAHs in soils
Figures 2, 3, and 4 show the concentration of total PAHs (∑PAHs) in paddy, maize, and vegetable soils from farm produce production base, Tieling region. The results indicated that the average concentration of ∑PAHs in vegetable soils was 448 ng/g (Table 1) and the highest concentration of total PAHs found in the samples was 3,309 ng/g. PAH concentrations were over 500 ng/g in seven samples and over 1,000 ng/g in two samples. PAH concentrations were below 500 ng/g in 34 samples, which accounted for 83 % of the total vegetable samples (Fig. 2).
For soils used for maize crops, the PAH concentration varied from site to site and ranged from 69 to 1,950 ng/g with an arithmetic mean of 391 ng/g. Concentrations of PAHs in 77 % maize soils were below 500 ng/g, and PAHs above 1,000 ng/g were recorded in two samples (Fig. 3).
In paddy soils, the concentration of ∑PAHs ranged from 72 to 1448 ng/g with a mean of 331 ng/g (Table 1). Concentrations of PAHs under 500 ng/g were detected in 39 of 46 samples, which accounted for 85 % of the total number of samples. ∑PAHs was above 500 ng/g in seven of 46 samples and was over 1,000 ng/g in two of these (Fig. 4).
According to the above results, concentrations of ∑PAHs in most crop soils analyzed were below 500 ng/g. Among PAHs detected in the three types of soils, the highest was detected in vegetable soils, followed by maize soils and paddy soils. The highest concentration of ∑PAHs may be influenced by external environmental inputs including traffic, coal combustion, and wastewater irrigation. At the same time, agricultural practice can also contribute to the elevated content of PAHs, as a result of multi-cropping index, which could account for the higher PAHs in vegetable soils than that in paddy and maize farmland. Paddy soils are usually irrigated and saturated with water, and this may account for an increased degradation of PAHs into harmless chemicals (Nam et al. 2003), which may explain why paddy soils have the lowest ∑PAH concentration. The ∑PAH concentration in paddy soils in our study was a little higher than that of South Korea (PAHs 236 ng/g) (Nam et al. 2003) but was within the range as reported in the agricultural soils of Pearl River Delta, China (Li et al. 2007). Other studies indicate that the concentration of PAHs varies significantly between different agricultural soils (Holoubek et al. 2009; Placha et al. 2009; Song et al. 2006; Agarwal et al. 2009; Maliszewska-Kordybach 2000; Tao et al. 2004; Wang et al. 2010).
Patterns of individual PAHs in soils
Table 1 indicated that 13 of 16 individual PAHs tested were detected in paddy, maize, and vegetable soils. Some of the individual PAHs were under the detection limit, including acenaphthylene (ACY), benzo[a]anthracene (BaA), and indeno[1,2,3-cd]pyrene (IcdP) in paddy soils and dibenzo[a,h]anthracene (DahA), ACY, and BaA both in maize and vegetable soils.
The general profile of PAH in the three types of soils was similar (Table 1). Phenanthrene (PHE) was the predominant PAH with the average concentration of 193 ng/g in vegetable soil, 177 ng/g in maize soil, and 145 ng/g in paddy soils, respectively. The second-most prolific PAH was naphthalene (NAP) followed by fluoranthene (FTH) and fluorene (FLU). This was similar to other findings (Wilcke et al. 2005; Maliszewska-Kordybach et al. 2009; Wang et al. 2010; Bucheli et al. 2004). The contribution of individual PAH in paddy, maize, and vegetable soils followed in the order: PHE > FTH ≈ NAP > FLU > pyrene (PYR) > chrysene (CHR) > acenaphthene (ANT) ≈ benzo[b]fluoranthene (BbF) ≈ IcdP ≈ benzo[k]fluoranthene (BkF) > benzo[ghi]perylene (BghiP) ≈ benzo[a]pyrene (BaP) ≈ BaA ≈ DahA ≈ ACE ≈ ACY, indicating the lower molecular hydrocarbons more prevalent than the higher molecular ones. The concentrations of all individual PAH in vegetable soils were higher than that in maize and paddy soils except for CHR and DahA.
Among 16 PAHs listed in US Environmental Protection Agency, seven have been classified as probable human carcinogens. They are BaA, CHR, BbF, BkF, BaP, DahA, and IcdP (∑PAH7c). Table 1 showed that the concentration of ∑PAH7c ranged from 11 to 99 ng/g with an arithmetic mean of 42 ng/g in vegetable soils and accounted for 9 % of ∑PAH16. In maize soils, it ranged from 7 to 133 ng/g with an arithmetic mean of 36 ng/g and accounted for 8 % of ∑PAH16, while in paddy soils, it was 27 ng/g, accounting for 8 % of ∑PAH16. The percentage of ∑PAH7c in vegetable, maize, and paddy soils of this study was much lower than that in other cities of China (Taizhou 46–57 %; Beijing 52 %) (Shen et al. 2009; Liu et al. 2010). As one of the most potential carcinogenic PAHs, the average concentrations of BaP were 3 ng/g in paddy soils, 4 ng/g in maize soils, and 6 ng/g in vegetable soils, respectively. The concentrations of BaP in our study were similar to those reported by Li (Li et al. 2008), but were lower than those of most reported both in China and the other countries for agricultural soils, where soil content of BaP was within 10–36 ng/g (Zhang et al. 2006; Maliszewska-Kordybach et al. 2009; Agarwal et al. 2009; Ping et al. 2007; Nam et al. 2003). Figure 5 shows that the distribution patterns of two- to six-ring PAHs in paddy, maize, and vegetable soils were similar with each other; 2 + 3 rings PAHs contributed higher, and it represented 84 % of the total PAHs for paddy soil, 85 % for maize soil, and 83 % for vegetable soils, respectively.
One of the reasons for the dominance of 2 + 3 rings PAHs in the regions investigated in this study may be that they originated from a distant source of pollution (Nam et al. 2008; Aamot et al. 1996). Meharg et al. (1998) noted that lower molecular weight of PAHs can travel longer distance than those of higher molecular weight. As a result, 2 + 3 rings PAHs may be transported more efficiently over a longer distance (Chung et al. 2007; Wilcke 2000; Agarwal et al. 2009). Another reason for the predominance of lower molecular weight PAHs may be attributed to the pyrolytic process of incomplete combustion such as biomass burning (Garcia-Falcon et al. 2006), which can result into the production of lower molecular weight PAHs (Agarwal et al. 2009). The similar profile of different PAHs in paddy, maize, and vegetable soils implies that PAHs in soils may originate from the same sources.
Classification of PAH contamination in soils
Since the PAH concentration of soils is not yet regulated in China, the Canadian soil criterion (CCME 2007) were applied to evaluate the level of soil contamination by PAHs in relation to acceptable target values of the PAH concentration for agricultural soils in this study. Table 2 indicated that the concentrations of BaA, BbF, BkF, BaP, DahA, and IcdP were all within the target values in all soils. Only NAP, PHE and PYR were over the target values in paddy, maize, and vegetable soils according to the recommended PAH concentrations in the regulation from Canada for agricultural soils. The contaminated area of PHE was the widest, with 30, 39, and 35 sites over the target values in paddy, maize, and vegetable soils respectively.
Source identification of PAHs in soils
To further distinguish the possible sources of PAHs in paddy, maize, and vegetable soils, PAH isomer pair ratios and principal component analysis were used.
Isomer pair ratios
To distinguish between combustion and petroleum sources, several isomer pair ratios were commonly used, including (1) the ratios of anthracene to anthracene plus phenanthrene (ANT/ANT + PHE), (2) fluoranthene to fluoranthene plus pyrene (FTH/FTH + PYR), (3) benz[a]anthracene to benz[a]anthracene plus chrysene (BaA/BaA + CHR), and (4) indeno[1,2,3-cd]pyrene to indeno[1,2,3-cd]pyrene plus benzo[ghi]perylene (IcdP/IcdP + BghiP) (Ping et al. 2007; Yin et al. 2008; Maliszewska-Kordybach et al. 2008; Yunker et al. 2002). Since benz[a]anthracene and indeno[1,2,3-cd]pyrene were not detected in this study, we adopted the ratio of FTH/FTH + PYR and ANT/ANT + PHE to analyze the source of PAHs.
Figure 6 shows that the ratios of ANT/ANT + PHE varied from 0 to 0.328 for all soils and the ratios of ANT/ANT + PHE were below 0.1 for 91 % samples in paddy soils, for 95 % samples in maize soils and 98 % for vegetable soils. The ratios of FTH/FTH + PYR were above 0.5 in 98 % samples of paddy soil, and it was greater than 0.5 in all samples in maize and vegetable soils.
According to Yunker et al. (2002), if the ratio of ANT/ANT + PHE is greater than 0.1, it indicates that PAHs in soils came from a petroleum origin, while the ratio less than 0.1 implied that the combustion source contributed to the PAHs. In this study, the ratios of ANT/ANT + PHE were below 0.1 in most samples, indicating that the combustion source was one of the contributors to the elevated PAHs in paddy, maize, and vegetable soils. Data in Fig. 4 showed that the ratios of FTH/FTH + PYR were all above 0.5 except one in paddy, maize, and vegetable soils, suggesting that grass, wood, and coal combustion could be a main source of PAHs in our study (FTH/FTH + PYR less than 0.4 implied petroleum sources; between 0.4 and 0.5 indicated PAHs derived from petroleum combustion; if FTH/FTH + PYR was greater than 0.5, it implied grass, wood, and coal combustion contributed to the PAHs in soil). This agreed with the observations that biomass burning and coal combustion are common in the rural area of China, especially in winter.
Principal component analysis
Table 3 shows the individual PAHs in the principal component matrix in paddy, maize, and vegetable soils. In paddy soils, the majority of variance (67.58 %) was explained by three factors. Factor 1 explained 38.06 % of the total variance of the data and had a strong significant correlation with FLU (0.74), PHE (0.91), ANT (0.76), FTH (0.77), PYR (0.81), and BbF (0.63). The second component responsible for 19.47 % of the total variance was mainly loaded with NAP (0.64), ACE (0.85), and BkF (−0.74). The third component was dominated by CHR (−0.76).
The variance of 68.07 % was explained by three factors in maize soils where 38.06 % of the total variance of the data was explained by factor 1 and FLU (0.72), PHE (0.93), ANT (0.89), FTH (0.89), PYR (0.94), BkF (0.66), and BghiP (−0.62) were significantly correlated. Factor 2 was mainly loaded with ACE (0.71), BaP (0.61), and BghiP (0.62). Factor 3 was dominated by BbF (0.63).
In vegetable soils, there were also three factors to explain the majority of variance (73.31 %). Factor 1 explained 45.12 % of the total variance of the data, and it was dominated by NAP (0.90), FLU (0.91), PHE (0.81), ANT (0.96), FTH (0.89), PYR (0.97), and BbF (0.69). Factor 2 had a strong significant correlation with CHR (0.71), BaP (0.80), and IcdP (0.65). The third component was dominated by ACE (0.81) and BkF (−0.70).
The low molecular weight PAHs, including NAP, ACE, FLU, PHE, ANT, FTH, and PYR, are predominant emissions of biomass burning (Mastral et al. 1996; Jenkins et al. 1996). Factor 1 in paddy, maize, and vegetable soils showed a similar PAH profile (FLU, PHE, ANT, FTH, and PYR), which all suggested biomass burning as the main sources. BkF and BbF are largely released by both gasoline and diesel engines (Harrison et al. 1996; Liu et al. 2010), i.e., the traffic emission. So factor 2 in paddy soil represented traffic emission. BaP, IcdP, and BghiP are considered to be produced from incomplete combustion of the fossil fuels, and traffic emission (Harrison et al. 1996; Nielsen 1996; Khalili et al. 1995) and wood burning also have been suggested as the source of BaP (Kulkarni and Venkataraman 2000; Kakareka et al. 2005). BaP and CHR were markers of coal combustion (Agarwal et al. 2009; Khalili et al. 1995). As a result, the second component in maize and vegetable soils both represented fossil fuels combustion. Factor 3 in paddy soil suggested that coal combustion may be one of the sources. And traffic emission may be one of the PAH sources in maize and vegetable soils.
Soil toxicity assessment based on the total concentration of seven probable carcinogenic PAHs
Since benzo[a]pyrene was the most studied PAH and its toxicological data were sufficient for calculation of quantitative estimates of carcinogenic potency, toxicity equivalency factors (TEF) were suggested by US EPA to quantify the carcinogenicity of other PAHs relative to BaP and then to estimate benzo[a]pyrene-equivalent concentration (B[a]Peq) (Nadal et al. 2004; Rey-Salgueiro et al. 2008; Agarwal et al. 2009; Peters et al. 1999). Calculated TEF for BaA, BaP, BbF, BkF, IcdP, DahA, and CHR are 0.1, 1, 0.1, 0.01, 0.1, 1, and 0.01, respectively (USEPA 1993; Nisbet and Lagoy 1992). The total benzo[a]pyrene-equivalent concentration (B[a]Peq) of the seven probable carcinogenic PAHs was calculated as:
where C i is the concentration of individual carcinogenic PAH and TEF i is the corresponding toxic equivalency factor.
In paddy soils, calculated total B[a]Peq concentration at different sampling sites varied from 0.35 to 17.97 ng/g, with an average value of 4.51 ng/g. In maize soils, the average value of total B[a]Peq was 4.59 ng/g, which was similar to that in paddy soils. And it ranged from 0.07 to 11.16 ng/g. The average value of total B[a]Peq in vegetable soils (8.05 ng/g) was higher than that in paddy and maize soils, ranging from 0.70 to 20.67 ng/g. The results suggested that vegetable soils may have the highest carcinogenic potential. In comparison with other studies, total B[a]Peq concentrations in paddy, maize, and vegetable soils of Liaoning, China were much lower than those of other sites reported, such as the agricultural soil from Delhi, Indian (154.12 ng/g in average) (Agarwal et al. 2009). It is suggested that as a green agricultural area, Tieling region should be paid more attention on the environmental monitoring and assessment in the future.
Conclusion
In combination with the IPR and PCA analysis, biomass and coal combustion are the main sources of PAHs in paddy, maize, and vegetable soils from commercial farms in Liaoning, China. Vegetable soils may have higher carcinogenic potential than paddy and maize soils according to B[a]Peq concentration. We suggest that biomass burning should be abolished and commercial farming should be carried out far from the highways. Human activities should be undertaken to avoid significant combustion of biomass and coal. In addition, regular monitoring should be provided to ensure the safety of food products derived from commercial farming.
References
Aamot, E., Steinnes, E., & Schmid, R. (1996). Polycyclic aromatic hydrocarbons in Norwegian forest soils: impact of long range atmospheric transport. Environmental Pollution, 92(3), 275–280.
Agarwal, T., Khillare, P. S., Shridhar, V., & Ray, S. (2009). Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. Journal of Hazardous Materials, 163(2–3), 1033–1039. doi:10.1016/j.jhazmat.2008.07.058.
Bucheli, T. D., Blum, F., Desaules, A., & Gustafsson, O. (2004). Polycyclic aromatic hydrocarbons, black carbon, and molecular markers in soils of Switzerland. Chemosphere, 56(11), 1061–1076. doi:10.1016/j.chemosphere.2004.06.002.
Cai, Q. Y., Mo, C. H., Li, Y. H., Zeng, Q. Y., Katsoyiannis, A., Wu, Q. T., et al. (2007). Occurrence and assessment of polycyclic aromatic hydrocarbons in soils from vegetable fields of the Pearl River Delta, South China. Chemosphere, 68(1), 159–168. doi:10.1016/j.chemosphere.2006.12.015.
CCME (2007). Canadian Soil Quality Guidelines for the protection of environmental and human health, updated in 2007. http://www.ccme.ca/assets/pdf/rev_soil_summary_tbl_7.0_e.pdf.
Chung, M. K., Hu, R., Cheung, K. C., & Wong, M. H. (2007). Pollutants in Hong Kong soils: polycyclic aromatic hydrocarbons. Chemosphere, 67(3), 464–473. doi:10.1016/j.chemosphere.2006.09.092.
Ciganek, M., Ulrich, R., Neca, J., & Raszyk, J. (2002). Exposure of pig fatteners and dairy cows to polycyclic aromatic hydrocarbons. Veterinární Medicína, 47(5), 137–142.
Garcia-Falcon, M. S., Soto-Gonzalez, B., & Simal-Gandara, J. (2006). Evolution of the concentrations of polycyclic aromatic hydrocarbons in burnt woodland soils. Environmental Science & Technology, 40(3), 759–763. doi:10.1021/es051803v.
Harrison, R. M., Smith, D. J. T., & Luhana, L. (1996). Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environmental Science & Technology, 30(3), 825–832.
Holoubek, I., Dusek, L., Sanka, M., Hofman, J., Cupr, P., Jarkovsky, J., et al. (2009). Soil burdens of persistent organic pollutants—their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environmental Pollution, 157(12), 3207–3217. doi:10.1016/j.envpol.2009.05.031.
Hu, G. J., Chen, S. L., Zhao, Y. G., Sun, C., Li, J., & Wang, H. (2009). Persistent toxic substances in agricultural soils of Lishui County, Jiangsu Province, China. Bulletin of Environmental Contamination and Toxicology, 82(1), 48–54. doi:10.1007/s00128-008-9501-y.
IARC. (1983). IARC monographs on the evaluation of the carcinogenic risk of chemicals to human (polynuclear aromatic compounds, part I: chemical environmental and experimental data). Geneva: World Health Organization.
Jenkins, B. M., Jones, A. D., Turn, S. Q., & Williams, R. B. (1996). Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environmental Science & Technology, 30(8), 2462–2469.
Kakareka, S. V., Kukharchyk, T. I., & Khomich, V. S. (2005). Study of PAH emission from the solid fuels combustion in residential furnaces. Environmental Pollution, 133(2), 383–387. doi:10.1016/j.envpol.2004.01.009.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline-engines, highway tunnels, and wood combustion emissions. Atmospheric Environment, 29(4), 533–542.
Kulkarni, P., & Venkataraman, C. (2000). Atmospheric polycyclic aromatic hydrocarbons in Mumbai, India. Atmospheric Environment, 34, 2785–2790.
Li, P. J., Stagnitti, F., Allinson, G., Turoczy, N., Xiong, X., & Peterson, J. (2006). Sorption and fractionation of copper in soil at a sewage irrigation farm in Australia. Communications in Soil Science and Plant Analysis, 37(7–8), 1031–1042. doi:10.1080/00103620600584776.
Li, Y. T., Li, F. B., Zhang, T. B., Yang, G. Y., Chen, J. J., & Wan, H. F. (2007). Pollution assessment, distribution and sources of PAHs in agricultural soils of Pearl River Delta—the biggest manufacturing base in China. Journal of Environmental Science and Health Part A—Toxic/Hazardous Substances & Environmental Engineering, 42(13), 1979–1987. doi:10.1080/10934520701628890.
Li, Y. T., Li, F. B., Chen, J. J., Yang, G. Y., Wan, H. F., Bin Zhang, T., et al. (2008). The concentrations, distribution and sources of PAHs in agricultural soils and vegetables from Shunde, Guangdong, China. Environmental Monitoring and Assessment, 139(1–3), 61–76. doi:10.1007/s10661-007-9816-x.
Liu, G. L., Wang, J. F., Li, Z. Y., Liang, S. Z., Liu, J. H., & Wang, X. N. (2009). Development of direct competitive enzyme-linked immunosorbent assay for the determination cadmium residue in farm produce. Applied Biochemistry and Biotechnology, 159(3), 708–717. doi:10.1007/s12010-009-8539-6.
Liu, S. D., Xia, X. H., Yang, L. Y., Shen, M. H., & Liu, R. M. (2010). Polycyclic aromatic hydrocarbons in urban soils of different land uses in Beijing, China: distribution, sources and their correlation with the city's urbanization history. Journal of Hazardous Materials, 177(1–3), 1085–1092. doi:10.1016/j.jhazmat.2010.01.032.
Maliszewska-Kordybach, B. (2000). Polycyclic aromatic hydrocarbons in agroecosystems—example of Poland. Polycyclic Aromatic Compounds, 21(1–4), 287–295.
Maliszewska-Kordybach, B., Smreczak, B., Klimkowicz-Pawlas, A., & Terelak, H. (2008). Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere, 73(8), 1284–1291. doi:10.1016/j.chemosphere.2008.07.009.
Maliszewska-Kordybach, B., Smreczak, B., & Klimkowicz-Pawlas, A. (2009). Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Science of the Total Environment, 407(12), 3746–3753. doi:10.1016/j.scitotenv.2009.01.010.
Mastral, A. M., Callen, M., & Murillo, R. (1996). Assessment of PAH emissions as a function of coal combustion variables. Fuel, 75, 1533–1536.
Meharg, A. A., Wright, J., Dyke, H., & Osborn, D. (1998). Polycyclic aromatic hydrocarbon (PAH) dispersion and deposition to vegetation and soil following a large scale chemical fire. Environmental Pollution, 99(1), 29–36.
Nadal, M., Schuhmacher, M., & Domingo, J. L. (2004). Levels of PAHs in soil and vegetation samples from Tarragona County, Spain. Environmental Pollution, 132(1), 1–11. doi:10.1016/j.envpol.2004.04.003.
Nam, J. J., Song, B. H., Eom, K. C., Lee, S. H., & Smith, A. (2003). Distribution of polycyclic aromatic hydrocarbons in agricultural soils in South Korea. Chemosphere, 50(10), 1281–1289.
Nam, J. J., Thomas, G. O., Jaward, F. M., Steinnes, E., Gustafsson, O., & Jones, K. C. (2008). PAHs in background soils from Western Europe: influence of atmospheric deposition and soil organic matter. Chemosphere, 70(9), 1596–1602. doi:10.1016/j.chemosphere.2007.08.010.
Nielsen, T. (1996). Traffic contribution of polycyclic aromatic hydrocarbons in the center of a large city. Atmospheric Environment, 30(20), 3481–3490.
Nisbet, I. C. T., & Lagoy, P. K. (1992). Toxic equivalency factors (TEFS) for polycyclic aromatic-hydrocarbons (PAHS). Regulatory Toxicology and Pharmacology, 16(3), 290–300. doi:10.1016/0273-2300(92)90009-x.
Pandey, J., & Pandey, U. (2009). Atmospheric deposition and heavy metal contamination in an organic farming system in a seasonally dry tropical region of India. Journal of Sustainable Agriculture, 33(4), 361–378. doi:10.1080/10440040902834954.
Peters, C. A., Knightes, C. D., & Brown, D. G. (1999). Long-term composition dynamics of PAH-containing NAPLs and implications for risk assessment. Environmental Science & Technology, 33(24), 4499–4507. doi:10.1021/es981203e.
Ping, L. F., Luo, Y. M., Zhang, H. B., Li, Q. B., & Wu, L. H. (2007). Distribution of polycyclic aromatic hydrocarbons in thirty typical soil profiles in the Yangtze River Delta region, east China. Environmental Pollution, 147(2), 358–365. doi:10.1016/j.envpol.2006.05.027.
Placha, D., Raclavska, H., Matysek, D., & Rummeli, M. H. (2009). The polycyclic aromatic hydrocarbon concentrations in soils in the Region of Valasske Mezirici, the Czech Republic. Geochemical Transactions, 10, 12. doi:10.1186/1467-4866-10-12.
Rey-Salgueiro, L., Martinez-Carballo, E., Garcia-Falcon, M. S., & Simal-Gandara, J. (2008). Effects of a chemical company fire on the occurrence of polycyclic aromatic hydrocarbons in plant foods. Food Chemistry, 108(1), 347–353. doi:10.1016/j.foodchem.2007.10.042.
Shen, C. F., Chen, Y. X., Huang, S. B., Wang, Z. J., Yu, C. N., Qiao, M., et al. (2009). Dioxin-like compounds in agricultural soils near e-waste recycling sites from Taizhou area, China: chemical and bioanalytical characterization. Environment International, 35(1), 50–55. doi:10.1016/j.envint.2008.07.005.
Song, Y. F., Jing, X., Fleischmann, S., & Wilke, B. M. (2002). Comparative study of extraction methods for the determination of PAHs from contaminated soils and sediments. Chemosphere, 48(9), 993–1001.
Song, Y. F., Wilke, B. M., Song, X. Y., Gong, P., Zhou, Q. X., & Yang, G. F. (2006). Polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and heavy metals (HMs) as well as their genotoxicity in soil after long-term wastewater irrigation. Chemosphere, 65(10), 1859–1868. doi:10.1016/j.chemosphere.2006.03.076.
Tao, S., Cui, Y. H., Xu, F., Li, B. G., Cao, J., Liu, W., et al. (2004). Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Science of the Total Environment, 320(1), 11–24. doi:10.1016/s0048-9697(03)00453-4.
USEPA (1993). Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons. US Environmental Protection Agency, EPA/600/R-693/089.
Wang, Y., Pan, Y. C., Yan, B. J., Li, A. Y., & Wang, J. H. (2008). Secure production of farm produce-oriented management and spatial decision-making system for producing area. Computer and Computing Technologies in Agriculture, 2(259), 909–916.
Wang, W. T., Simonich, S. L. M., Xue, M. A., Zhao, J. Y., Zhang, N., Wang, R., et al. (2010). Concentrations, sources and spatial distribution of polycyclic aromatic hydrocarbons in soils from Beijing, Tianjin and surrounding areas, North China. Environmental Pollution, 158(5), 1245–1251. doi:10.1016/j.envpol.2010.01.021.
Wilcke, W. (2000). Polycyclic aromatic hydrocarbons (PAHs) in soil—a review. Journal of Plant Nutrition and Soil Science-Zeitschrift Fur Pflanzenernahrung Und Bodenkunde, 163(3), 229–248.
Wilcke, W., Krauss, M., Safronov, G., Fokin, A. D., & Kaupenjohann, M. (2005). Polycyclic aromatic hydrocarbons (PAHs) in soils of the Moscow region—concentrations, temporal trends, and small-scale distribution. Journal of Environmental Quality, 34(5), 1581–1590. doi:10.2134/jeq2005.0005.
Yin, C. Q., Jiang, X., Yang, X. L., Bian, Y. R., & Wang, F. (2008). Polycyclic aromatic hydrocarbons in soils in the vicinity of Nanjing, China. Chemosphere, 73(3), 389–394. doi:10.1016/j.chemosphere.2008.05.041.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515.
Zhang, H. B., Luo, Y. M., Wong, M. H., Zhao, Q. G., & Zhang, G. L. (2006). Distributions and concentrations of PAHs in Hong Kong soils. Environmental Pollution, 141(1), 107–114. doi:10.1016/j.envpol.2005.08.031.
Acknowledgments
We gratefully acknowledge the financial support received from the National Natural Science Foundation of China (grants nos. 20977094, 41171155 and 40930739).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, X.F., Liu, M., Song, Y.F. et al. Composition, sources, and potential toxicology of polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in Liaoning, People’s Republic of China. Environ Monit Assess 185, 2231–2241 (2013). https://doi.org/10.1007/s10661-012-2704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2704-z