Abstract
Biogas slurry, as a substitute for chemical fertilizers, is usually applied according to the needs of biogas slurry to be consumed at onetime. This poses a great risk of short-term nutrient overload in soils, resulting in high losses of nitrogen (N) and phosphorus (P) from farmland. Therefore, a field experiment was conducted to identify the optimal irrigation pattern for biogas slurry in paddy field by monitoring N and P losses via surface runoff, leaching, NH3 volatilization, and denitrification as well as rice yield and soil residue with five fertilizer application patterns: no fertilizer application, conventional chemical fertilizer, and three biogas slurry irrigation patterns including the current irrigation pattern of three times (BS1), irrigation pattern of five times (BS2), and 20% reduction of N input with five irrigations (BS3). BS2 treatment significantly increased the yield of rice by 13.91 and 23.37% compared to the CF and BS1 treatments, respectively. But the BS2 treatment promoted NH3 volatilization loss by 70% compared with the BS1 treatment. The BS3 treatment decreased the total N loss and P loss by 14.33 and 21.95%, respectively, compared to the BS1 treatment, along with a 10.26% increase in yield. Therefore, increasing the irrigation frequency of the biogas slurry to five times and reducing the fertilizer application rate by 20% was the optimal irrigation pattern in paddy field with the best agricultural and environmental benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Chinese livestock and poultry farming industry is developing at a high speed, along with the generation of larger quantities of manure, about 354 million tons in 2019 (Xu et al. 2022). Biogas engineering is an effective method for resource utilization of livestock and poultry manure and the more than 600 million m3 of biogas slurry (Lu et al. 2012), the residual liquid of livestock and poultry manure following anaerobic fermentation; these are produced from about 1.00 × 105 different biogas projects (NBSC 2010; Xiao 2020). Biogas slurry can be used to offset the use of chemical fertilizers because of its enormous nutrient content, including nitrogen (N), phosphorus (P), and potassium (K) content (Dahiya and Vasudevan 1986; Bonten et al. 2014; Kumar et al. 2023). The Chinese government has invested considerably in promoting the use of biogas slurry in crop cultivation for the reuse of agricultural waste and the reduction of mineral fertilizer input (Zhan et al. 2020).
Rice is a major food crop in China, with a large cultivation area of 29.9 million hm2 in 2021, accounting for 25.4% of the cultivated area for food crops (NBSC 2022). Generally, about 120–280 kg of N and 50.0–90.0 kg of P are applied per hm2 of rice field (MARAPRC, 2022). The demand for N and P nutrients in Chinese rice fields are up to 4.50 billion kg and 2.70 billion kg, respectively. Paddy fields with high fertilizer applications in China could be good places to use biogas slurry as a liquid fertilizer.
The large number of paddy fields that retain flooded water (Yoon 2009), provides abundant space to apply large amounts of biogas slurry during short-term irrigation. In addition, multiple fertilizer application operations, such as substrates, tillers, and spike fertilizers (Liu et al. 2016), are carried out during the flooding stage of rice (Feng 2008; Xu et al. 2013). Paddy fields have a purification effect on eutrophic wastewater, derived from the self-regulatory function of the soil-microbial-plant complex system (Matsuno et al. 2006; Yoon 2009; Shi 2010). Paddy fields are ideal for the natural purification of biogas slurry and for cycling and reusing nutrients in agricultural organic waste.
Biogas slurry application could increase crop yield (Huang et al. 2013; Rahaman et al. 2021; Kumar et al. 2023) and increase soil organic carbon stocks by simulating soil bacteria and fungi to participate in soil organic carbon cycling in wheat-rice rotation field (Tang et al. 2021). Minamikawa et al. (2021) also found biogas slurry application can mitigate CH4 and N2O emissions from a lowland rice paddy field without yield loss. Some studies concluded that the proper reuse of biogas slurry can increase nitrogen use efficiency (NUE) and did not cause environmental impacts (Abubaker 2012; Win et al. 2014; Yin et al. 2019). But biogas slurry typically has high concentrations of ammonium, accounting for 35–81% of total nitrogen (Möller and Müller 2012; Nkoa 2014) and high pH (Kumar et al. 2023). Once applied to the field, ammonium in biogas slurry can easily be volatilized (Bonten et al. 2014; Wang et al. 2014). Excessive irrigation of biogas slurry could lead to numerous NH3 volatilization, accounted for 13.0–16.55% of total N input in paddy field (Deng et al. 2011; Jiang et al. 2011). Biogas slurry application could also raise potential risk of surrounding water pollution via runoff and leaching losses in paddy field (Wang et al. 2016; Eltohamy et al. 2021).
Proper irrigation with biogas slurry in paddy field is crucial for its sustainable use as fertilizer. Oritate et al. (2016) applied biogas slurry according to the schedule of conventional rice cultivation with a total of three fertilization by pouring method. In China, farmers prefer applying biogas slurry to fields according to the needs of biogas slurry to be consumed at onetime (Wang et al. 2016), rather than the crop nutrient requirements at different growth stages. This often results in a short-term nutrient overload in the flooded water and a high risk of losses via NH3 volatilization, surface and leaching into water bodies (Phayom et al. 2012).
Many studies focused on the optimal application rate of biogas slurry. Yang et al. (2017) proposed that the reasonable biogas slurry sourced N input was 213.9 kg hm−2 in paddy field and on exceeding this the application rate, rice yield was not increased further. The study of Win et al. (2014) revealed that application of biogas slurry to rice fields at the 100 kg N hm−2 was considered to substitute chemical fertilizer utilization without additional environmental impacts in greenhouse gas emission and heavy metal uptake. Other studies investigated distribution methods of biogas slurry to fields. Pouring method with irrigation water was economical relative to split application by pipeline system (Oritate et al. 2016). But Phayom et al. (2012) found that periodic application of biogas slurry exhibited significantly higher levels of N uptake, agronomic efficiency and fertilizer N recovery efficiency than onetime application. Wang et al. (2016) also suggested that biogas slurry in paddy field should adopt the way of more irrigation times and fewer disposal amounts to decrease the risk of surface runoff losses resulting in agricultural pollution. Hence, it is practical to mitigate the loss risk of nutrients during biogas slurry irrigation by proper irrigation method, increasing the irrigation frequency and reducing the fertilizer rate of biogas slurry.
However, there are few experiments to investigate the effect of irrigation frequency of biogas slurry on yield in rice field and fewer that testing a new biogas irrigation pattern on less amount of biogas slurry with multiple irrigation on nutrient losses.
But more frequent irrigation with biogas slurry would increase the labor cost and the cost of transportation of biogas slurry from biogas digesters to fields (Phayom et al. 2012; Oritate et al. 2016). With the continuous increase in labor costs and rapid development of precision agriculture, automatic fertilization has become an important part of modern agriculture in China (Shi et al. 2021; Lu et al. 2022). The intelligent irrigation system effectively saved water with the water-saving rates of ∼2.9–6.5% and 8.8–19.3% in the dry and wet seasons respectively, and the yield and other agronomic traits remained unaffected (Zeng et al. 2023). Different irrigation scenarios could be tested based on an Internet-based smart irrigation system to find the optimal biogas slurry irrigation scenario for limiting phosphorus release from paddy soil (Eltohamy et al. 2021).
In this study, a precise irrigation system was developed to implement biogas slurry application in paddy fields. We aimed to optimize for the best irrigation strategy to minimize the risk of N and P loss and maintain yield. We hypothesized that the increase in irrigation frequency and reduction in the fertilizer rate of biogas slurry could mitigate N and P losses via runoff, leaching, and NH3 volatilization. A field experiment was conducted to test its reliability, and an irrigation mode with higher frequency and lower fertilizer rate was established in the system to elucidate the best irrigation strategy for minimizing N and P losses.
Material and methods
Site and soil description
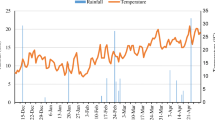
The field experiments were conducted in Tudian Town, Tongxiang City, Zhejiang Province, China (30°28′ N, 120°17′ E), 2021. The experimental site with the total area of 675 m2 is located on the Hangjiahu Plain, which has a typical subtropical monsoon climate with the mean annual temperature of 16.5 °C and mean annual precipitation of 1.43 × 103 mm (PGTxM, 2021). About 60% of the total annual precipitation occurs from June to November. The daily mean air temperature and rainfall at the experimental site during rice growing period, obtained from Tongxiang weather station (No. 58456) are shown in Fig. 1. The soil type was purple clayey paddy soil with the following basic soil physicochemical properties: pH 6.45–6.76, 1.17–1.82 g kg−1 total nitrogen (TN), 0.558–0.782 g kg−1 total phosphorus (TP), 13.4–18.5 g kg−1 total potassium content (TK), and 14.5–16.1 g·kg−1 organic matter.
Biogas slurry was obtained from the Shuangfeng Ecological Farming Cooperative, Tongxiang City, Zhejiang Province, using pig manure as the raw material. The basic physicochemical properties of the biogas slurry were: pH 7.94, 5.28 mS cm−1 conductivity, 1.04 × 103 mg L−1 TN, 387 mg L−1 NH4+–N, 15.0 mg L−1 NO3−–N, 89.2 mg L−1 TP, and 109 mg L−1 TK.
Experimental design
Experiments were conducted in a randomized complete block with five treatments, which were set based on input N loadings from biogas slurry or chemical fertilizer and irrigation pattern of biogas slurry using the automatic irrigation system (Table 1). The treatments were designed as follows: (1) a control (CK), no fertilizer was applied to rice; (2) a chemical fertilizer application (CF), applied with urea (N, 46.0%), calcium magnesium phosphate (CMP, P2O5, 16.0%), and KCl (K2O, 62.0%); (3) the conventional irrigation mode of biogas slurry (BS1), applied thrice as basal (40.0% NPK), tillering (40.0% NPK), and panicle fertilizers (20.0% NPK); (4) irrigation mode of biogas slurry as a total of five applications (BS2), with two applications each as tillering and panicle fertilizers (40.0–20.0–20.0–20.0–20.0% NPK); (5) irrigation mode with biogas slurry application five times similar to that in BS4 but at 20.0% reduced fertilizer rate (BS3). Each treatment was replicated thrice for 15 plots.
For CF, BS1, and BS2 treatments, the conventional fertilizer application rate was set at 240 kg N hm−2, 75.0 kg P2O5·hm−2, and 150 kg K2O·hm−2 while for BS3 treatment, the fertilizer rate was reduced to 192 kg N hm−2, 60.0 kg P2O5·hm−2, and 120 kg K2O·hm−2. To maintain equal amounts of P and K in the chemical fertilizer and biogas slurry treatments, we compensated for the CMP and KCl (Table 1).
For the CF and BS1 treatments, fertilization was performed on June 18th at the basal stage (D1) on June 27th (D10) at the tillering stage, August 1st at the panicle stage (D61). For BS2 and BS3 treatments, in addition to the above fertilization, the second tillering fertilizer was applied on July 5th (D18) and the second panicle fertilizer was applied on August 24th (D68). Rice seedlings were transplanted into the experimental field on June 19th (D2), and rice was harvested on November 15th, 2021 (D151).
Each block was 30.0 m2 (5.00 m × 6.00 m), with a 0.300 m wide and 0.200 m high ridge around the plots to prevent infiltration. Each plot was equipped with a separate drainage outlet. A flowmeter was installed at the outlet to record the amount of runoff generated. The maximum depth of the flooded water in the paddy field was around 0.100 m, beyond which surface runoff would occur. In addition, a negative pressure soil pore water sampling tube was installed in each plot at a depth of 0.600 m for the in situ extraction of soil leachate.
The proposed automatic irrigation system
The irrigation system was developed based on five units: mobile device, server, industrial personal computer (IPC), programmable logic controller (PLC), fertilizer control, and delivery system that can apply biogas slurry to the field in a timely and precise manner according to the user setting (as shown in Fig. 2). First, the user enters the N content of the soil and biogas slurry into the developed application via a mobile device. This information is transmitted in a timely manner to a web platform, and the platform server calculates irrigation volumes according to the current growth stage (Table 1). Thereafter, the user can view the irrigation volume and send control commands. The platform server sends this command to the IPC via a 4G wireless network communication module and runs a Windows service program to control the pump and related valves. It also reads the system status information and monitors device feedback from the PLC. The PLC is used to control the unit and read its status. The fertilizer control and delivery system controls the fertilizer delivery and provides monitoring information and feedback on the operating status of the system.
Sampling analysis
Before rice transplantation and during each growth stage, fresh soil samples were collected from the 0–20.0 cm surface layer, air-dried, and sieved to determine the basic physical and chemical properties of the soil. Soil pH was determined using the acidity meter (soil: water = 1: 2.5); soil organic matter, using the potassium dichromate volumetric method; TN, using the Elementar Vario EL Cube high-precision elemental analyzer; TP, using the perchloric acid-sulfuric acid-molybdenum antimony anti-colorimetric method; and TK, using the flame photometric method.
Biogas slurry samples were collected before being poured into the storage tank. Soil leachate was collected every 7–10 days via a soil pore water sampling tube at a depth of 60 cm, and flooding water was sampled after the generation of surface runoff as warned by the remote-reading flowmeter. The pH of the biogas slurry was determined using an acidity meter and the conductivity of the biogas slurry was determined using a conductivity meter. The concentrations of TN, NH4+–N, and NO3−–N in the biogas slurry, soil leachate, and flooding water were determined using an automated discrete analyzer (SmartChem200, AMS Alliance, Italy). The concentration of dissolved P (DP), particulate P (PP) fractions was determined following the colorimetric method described by Pagel et al. (2008). The TK of the biogas slurry was measured using flame atomic absorption spectrophotometry.
At harvest, the grain, straw, and roots of the rice were collected from a 1 m × 1 m portion in the central area of each plot, and the yield was measured. They were oven-dried to a constant weight to measure dry matter. The TN content in the grain, straw, and root was determined using an element analyzer (Vario EL Cube, Elementar, Germany), and TP was measured with the Mo–Sb colorimetry method using an automated discrete analyzer (SmartChem200, AMS Alliance, Italy).
NH3 volatilization in each experimental plot was measured using the venting method (Wang et al. 2002). The venting method was performed using polyvinyl chloride (PVC) cylinders with two sponges placed at the top and middle as NH3 absorbers. The NH3 trapped in the middle absorbers was extracted, and the NH4+–N content of the extraction solution was determined colorimetrically using automated discrete analyzers (SmartChem200, AMS Alliance, Italy). Each plot contained three cylinders, and the samples were collected daily after fertilizer application until no differences were observed in the volatilization rates compared to that in the CK treatment.
To measure the denitrification rate, fresh soil samples collected from the surface layer (0–20 cm) of the three plots in the same treatment were mixed, and each test soil was weighed to a fresh soil equivalent of 10 g of dry soil. The weighed soil samples were placed into sealable 250 mL conical flasks with silicone stoppers and pre-incubated for one week in a 25 °C incubator protected from light. The soil-to-water ratio was 1:1. Ultrapure water was added at a water-soil ratio of 1:1, the mouth of the flask was sealed with a silicone stopper, and the flask was filled with high purity N2 after vacuuming using a vacuum pump; this was repeated thrice. C2H2 was immediately added to the conical flask with a 30 mL syringe to remove a certain amount of gas, and then the same amount of high-purity C2H2 was added to the conical flask at a final C2H2 concentration of 10%. The mouth was immediately sealed with a silicone plug to ensure that the environment inside the bottle was anaerobic. Finally, the conical flask was incubated on a shaker (25 °C, 180 rpm) for 6.5 h. Gas samples were collected at 0, 0.5, 1.5, 3.5, and 6.5 h. The gas collection was performed with a 20 mL syringe to collect 20 mL of gas from the upper space of the glass flask, and the N2O concentration was determined using a gas chromatograph (Agilent 7890A). The linear increase in N2O within 6.5 h after the addition of C2H2 was recorded to calculate the denitrification rate of the paddy soil.
Data analysis
The losses of N and P via surface runoff, leaching, NH3 volatilization, and denitrification were considered. The total surface runoff losses of N and P were calculated as follows:
where Li is the volume of i-th runoff; k is the total sampling time; and cr,s,i is the concentration of species in i-th runoff, including TN, NH4+–N, NO3−–N, TP, PP, and DP.
It was assumed that the daily content of species in the leachate during the two sampling days was the same. Therefore, the total leaching losses of species were calculated as follows:
, where j is the sampling time, m is the total sampling time, DWj is the amount of drainage water between two sampling times (cm) obtained from a satisfactory simulation using Hydrus-1D, and \({c}_{l,s,j}\) is the concentration of species at j-th leachate sampling time (kg m−3), including TN, NH4+–N, NO3−, TP, PP, and DP. The details of the water movement simulation using the Hydrus-1D model are shown in Supplementary Information (SI).
The N loss via NH3 volatilization was calculated using the daily NH3 volatilization rate (RAV).
where M is the amount of NH3–N collected using the PVC collector (mg), A is the cross-sectional area of the capturer (m2), and T is the continuous capture time for NH3 volatilization (d). i is the capture day and n is the total capture time.
The loss of N via denitrification was estimated using the measured denitrification rates (Cao et al. 2021) as follows:
, where RDN is the measured denitrification rate (kg N hm−2 d−1) of paddy soil for each rice growth stage, T is the time (d) of each rice growth stage, and EFD is the denitrification emission factor, here set to 27% according to the study of Pan et al. (2022).
N agronomic efficiency (AEN), N recovery efficiency (REN), P agronomic efficiency (AEP), and P recovery efficiency (REP) were selected as indicators of N and P utilization (Huang et al. 2016). The calculation is as follows:
, where Y0 and Y are the rice yields (kg hm−2) for the CK and other fertilizer treatments (CF, BS1, BS2, and BS3), respectively; NF is the cumulative N fertilizer input (kg N hm−2), and PF is the cumulative P fertilizer input (kg P hm−2). UN0 and UN are the cumulative plant N uptake (kg N hm−2) in the control and fertilizer treatments (CF, BS1, BS2, and BS3), respectively. Up0 and Up are the cumulative P uptake (kg P hm−2) in the control and fertilizer treatments (CF, BS1, BS2, and BS3), respectively.
Statistical analyses
The differences in the yield and N and P utilization indices (AEN, REN, AEP, REP), mineral N and P residues in the soil, N and P uptake by plants, and N and P losses via different methods (runoff, leaching, NH3 volatilization, and denitrification) in the paddy field among different treatments were compared through a one-way analysis of variance (ANOVA) with the Turkey’s HSD test. Statistical significance was set at p < 0.05. All statistical analyses were performed using the SPSS software package (version 26.0; SPSS Inc., Chicago, IL, USA). OriginPro 9.5 (OriginLab Corporation, USA) was used for the graphical processing.
Results
Runoff losses
A total of three runoffs were generated during the rice growing season, after continuous rainfall (as shown in Fig. 1), especially on the 42nd day (D42) for typhoon weather, with largest runoff volume up to 5.93 mm under the precipitation over 427 mm during D39–D42. There was no significant difference between the runoff volumes of the treatments.
Runoff losses of N and P under different treatments are shown in Fig. 3. Both the CF and BS1 treatments had higher TN runoff losses, up to 10.0 kg N hm−2, compared to that in the BS2 treatment. The BS3 treatment had the lowest TN runoff losses among the four applied fertilizer treatments, at a rate of 6.75 kg hm−2.
TN runoff losses accounted for 11.4, 10.1, 8.52, and 6.75% of the N fertilizer applied in the CF, BS1, BS2, and BS3 treatments, respectively (Table 2). The NH4+–N losses in the BS3 treatment was significantly lower than CF, BS1 and BS2 treatments. Both BS2 and BS3 treatments had lower NO3−–N runoff loss than the CF and BS1 treatments.
TP was composed of DP and PP, with a larger fraction of PP than DP (Fig. 3c). The TP runoff losses under the BS3 treatment, accounting for 0.292% of the fertilizer applied (Table 2), were significantly lower than those under CF, BS1 and BS2 treatments. The CF and BS1 treatments had higher DP runoff losses compared to BS2 and BS3 treatments. The PP runoff losses under the BS3 treatment were significantly lower than those under CF, BS1 and BS2 treatments (p < 0.05).
Leaching losses
The total water volume of leaching simulated by Hydrus-1D under BS3 treatment was the largest, up to 538 t hm−2 (Fig. 4a), equivalent to the water loss during one single runoff (Fig. 3a). The BS1 and CF treatments showed similar water losses via leaching, which were lower than those of the BS2 and BS3 treatments. TN loss via leaching under CF treatment was significantly the largest, accounting for 0.233% of fertilizer applied (Table 2); followed by that in BS1 and BS2; the one in BS3 was the lowest among fertilizer-applied treatments. The ratios of NH4+–N and NO3−–N leaching losses to TN leaching losses were lower than 50.0%, indicating that inorganic N was not the main form of TN during leaching.
TP leaching loss under different treatments accounted for 0.0189–0.0260% of the applied fertilizer. TP leaching losses under the CF and BS1 treatments were significantly higher than those under the BS2 treatment, while BS3 treatments had lowest TP leaching losses, whereas there was no significant difference between PP and DP leaching losses among the treatments.
NH3 volatilization loss
All treatments produced abundant NH3 volatilization within 15 d of fertilization (Fig. 5). The NH3 volatilization rate increased sharply after five days of base fertilizer application with deep plowing, and the BS1 treatment had the highest peak of approximately 1.00 kg N hm−2 day−1 on D7. After 3 days of tiller fertilizer application, NH3 volatilization reach the peak under all the fertilizer treatments, with BS1 and BS2 treatments having a higher peak of about 1.50 kg N hm−2 day−1. D20 witnessed the highest NH3 volatilization rate during the rice growing period, at 3.32 kg N hm−2 day−1 under the BS2 treatment one day after the second tiller fertilizer.
The NH3 volatilization rate was much lower after the application of the first- and second-spike fertilizers. The BS2 treatment generated the largest NH3 volatilization loss of 14.4 kg N hm−2 and BS3 treatment the second largest at 9.77 kg N hm−2, significantly larger than those of BS1 and CF treatments (p < 0.05) (Table 2). The BS2 treatment promoted NH3 volatilization loss by 70% compared with the BS1 treatment. The NH3 volatilization loss accounted for 5.21% of the fertilizer applied in the BS2 treatment, which was twice as high as that of the CF treatment. In addition, 2.48, 2.78, and 4.10% of the N fertilizer applied were lost via NH3 volatilization under the CF, BS1, and BS3 treatments, respectively.
Denitrification loss
Figure 6 show the denitrification loss under different treatments at different growing stages of rice, indicating that the spike poplar flowering period had the largest potential of denitrification loss, while the levels of denitrification loss at the transplanting, tiller pulling, and filling stages were similar to each other. The soil samples used to determine denitrification rates in the incubation experiment were mixed from the soil samples from three replicate plots of each treatment.
Yield and total losses of N and P
As shown in Table 3, the yield in the BS2 treatment was the highest (P < 0.05), whereas there was no significant difference between the BS1, BS3 and CF treatments. Both N and P uptake by plants under the BS1 treatment was significantly lower than those under the CF, BS2, and BS3 treatments (Table 2). The BS2 and BS3 treatments had significantly higher AEN, REN, REP and AEP values than those in the CF and BS1 treatments. The BS1 treatment had the lowest AEN, REN, REP and AEP.
As shown in Table 2, 11.5, 10.0, 10.7 and 9.27% of the N fertilizer applied were lost under the CF, BS1, BS2 and BS3 treatments, respectively. CF, BS1, BS2 and BS3 treatments lost 0.400, 0.321, 0.297 and 0.260% of the P fertilizer applied via runoff and leaching.
In the condition of fertilizer rate being equal, total N losses in the treatment of CF was significantly higher than that in BS1 treatment (P < 0.05), while there was no significant difference in total N losses between BS1 and BS2 treatment with different irrigation frequencies (P < 0.05). In the condition of fertilizer frequency being equal, BS3 treatment significantly reduced total N and P losses by 22.4% (P < 0.05), with comparison to BS2 treatment by decreasing fertilizer rate by 20%.
The BS3 treatment decreased the total N loss and P loss by 14.33 and 21.95%, respectively, compared to the BS1 treatment, along with a 10.26% increase in yield.
Discussion
Biogas slurry is a good source of crop nutrients, which contains some micronutrients, auximones, B vitamins, and humic acid to improve soil fertility, plant growth and enhance farm production (Dahiya and Vasudevan 1986; Ghoneim et al. 2008; Möller et al. 2008; Oritate et al. 2016; Chen et al. 2017; Rahaman et al. 2021). But in our study, the conventional irrigation mode of biogas slurry (BS1), which applied thrice as basal (40% NPK), tillering (40% NPK), and panicle fertilizers (20% NPK), had lowest nutrient use efficiency (AEN, REN, REP and AEP), owing to lower yield, plant N and P uptake. Dahiya and Vasudevan (1986) reported that replacement of N fertilizer with biogas slurry decreased the yields of major crops, due to the slower nutrient providing rate of biogas slurry but a higher dose of N is required by cereals and other major crops. Wang et al. (2018) also found that compared with the treatment of chemical fertilizer application alone, the rice growth indices decreased in the treatment with 100% chemical fertilizer N substitution by biogas slurry, with a risk of yield reduction.
The increased NH3 volatilization to replace chemical fertilizer with biogas slurry was considered as another risk on N supply deficiency for rice growth (Deng et al. 2011). Even though biogas slurry has nutrient profile contributed as a potential source of fertigation in agriculture fields, it also brings with various limitations such as bulkiness, low C/N ratios, N loss through NH3 volatilization and high pH (Möller and Müller 2012; Nkoa 2014). The pH of biogas slurry in our study was as high as 7.94 and NH4+–N concentration in biogas slurry accounted for 37.2% of TN. NH3 volatilization loss is considered to increase with higher soil pH because of the increased dissociation of NH4+ to NH3, thus increasing the potential of volatilization (Sharpe and Harper 1995). In our study, not surprisingly, the NH3 volatilization in the treatments with biogas slurry application were higher than CF treatment and increasing irrigation frequency accelerated NH3 volatilization in BS2 treatment.
But BS2 treatment had significantly higher nutrient use efficiency (AEN, REN, REP and AEP) than BS1, implying that increasing irrigation frequency could enhance N and P use efficiency. BS2 treatment also had higher yield, although not significantly based on Tukey’s HSD test. Kartika et al. (2018) also found that split fertilizer application from onetime to three times increased yield in rice cultivated by increasing weight of grains and number of filled spikelet. One reason was that increasing irrigation frequency reduced the N losses via runoff, leaching and denitrification (Table 1). Fertilizer application at early vegetative stage increases the risk of nutrient loss from the root zone, whereas fertilizer application during the period of rapid nutrient uptake at late vegetative and early reproductive stages can reduce the loss of applied fertilizer due to its rapid uptake by plants (Kartika et al. 2018). Similar results were concluded in previous studies that increasing the frequency of fertilizer application could reduce the total N emission load in farmland drainage (Liu 2016), nutrient leaching loss (Datta 1986; Sitthaphanit et al. 2010) and denitrification (Datta 1986). Increasing the frequency of fertilization kept the TN, NH4+–N, and NO3−–N contents in the flooded waters of the rice fields at low levels, whereas a large input of biogas slurry at onetime could quickly increase the N content in the accumulated water of rice fields, thereby increasing the risk of nutrient losses.
Another probable reason for higher nutrient use efficiency in BS2 treatment with comparison to BS1 treatment, was that increasing irrigation frequency could improve the availability of soil phosphorus and reduce the fixation of phosphorus fertilizer (Cahyono and Hartati 2013; Shen et al. 2023). But the total N losses in BS2 treatment was the highest among three treatments with biogas slurry irrigation, primarily contributed by high NH3 volatilization loss, especially after the second tiller fertilizer (Fig. 5), due to the high temperature over 30 °C (Fig. 1). NH3 volatilization loss could markedly increase with temperature (Terman 1980). And more exposure of high NH4+ concentration in soil surface during biogas slurry irrigation in BS2 treatment led to severe NH3 volatilization loss, consistent with previous studies (Terman 1980; Sharpe and Harper 1995). The advantage of increasing irrigation frequency was thereby weakened.
Our study found that a further cut in fertilizer rate by 20% in BS3 treatment not only guaranteed rice yield but also minimized the total N loss (Table 2). The N and P losses under the BS3 treatment was significantly lower than that under the BS2 treatment, indicating that reduced fertilization was an important factor in reducing nutrient losses from rice fields via runoff (Ding et al. 2020), leaching (Du et al. 2019), NH3 volatilization (Wang et al. 2022) and denitrification (Yin et al. 2019). It was reported that most N-loss pathway had exponential increases with additional N input (Ding et al. 2020). Hence, it was reasonable that the 20% reduction of biogas slurry application decreased 22.4% of total N loss in our study.
More than 90% of biogas fraction was water, for example, in BS1 treatment, 276.91 L of biogas slurry was added to the 30 m2 plot at the tiller stage (Table 1), equivalent to 9.23 mm of irrigation, thereby resulting in an increase in leachate (Fig. 4a). But the N leaching losses under treatments of biogas slurry application were significantly lower than that under the CF treatment (Table 1), because N from organic fertilizers was mainly concentrated in soil surface compared to that from chemical fertilizer, which was beneficial for plant uptake and thereby reduce leaching loss (Yu et al. 2011). Du et al. (2019) also observed similar results that biogas slurry decreased N leaching losses owing to the slower mineralization of complex organic N compounds in soil.
Our study also found that biogas slurry also reduced the risk of denitrification losses, consistent with previous observations that biogas slurry could inhibit NH3 oxidation and denitrification, regarded as positive to reduce N losses (Abubaker et al. 2015).
Given that no significant difference in rice yield between the CF and BS3 treatments, it is likely that 20% reduction of nutrient input using biogas slurry as substitutes for chemical fertilizer, would not risk the rice yield. This was probably because biogas slurry not only contains a large amount of fast-acting nutrients (Alburquerque et al. 2012), but also contains some micronutrients, auximones, B vitamins, and humic acid, which are beneficial to plant growth, improve soil fertility (Yu et al. 2011; Chen et al. 2017) and have positive effects on soil biological properties such as microbial biomass and enzyme activities (Yu et al. 2011), to enhance farm production. Moreover, 20% reduction of nutrient input with more frequent application of biogas slurry considerably improved both N and P use efficiency with less N and P losses, to maintain yield.
Conclusions
Replacing chemical fertilizer with biogas slurry at a general irrigation frequency of three times during the rice growing period reduced rice yield and plant uptake of N and P without a significant decrease in total N losses. Increasing the irrigation frequency of the biogas slurry from three to five times significantly increased the yield and reduced N loss via runoff, leaching, and denitrification, but induced a significant increase in NH3 volatilization, leading to greater total N losses. Therefore, increasing the irrigation frequency of biogas slurry from three to five times along with reducing the fertilizer application rate by 20% could efficiently minimize N loss via surface runoff, leaching, and denitrification with limited NH3 volatilization and maintain rice yield. This is considered the optimal irrigation pattern for the automatic irrigation control system, providing a strategy for biogas return to the field with the best agricultural and environmental benefits. However, NH3 volatilization losses under biogas slurry application are still non-negligible, and the above preliminary conclusion was derived from a short-term experiment using a newly developed irrigation system. Long-term field monitoring should be conducted in future studies and should focus on NH3 volatilization losses as well as the stability and reliability of the irrigation system.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Abubaker J, Risberg K, Jönsson E, Dahlin AS, Cederlund H, Pell M (2015) Short-term effects of biogas digestates and pig slurry application on soil microbial activity. Appl Environ Soil Sci 2015:1–15
Abubaker J, (2012) Effects of fertilisation with biogas residues on crop yield, soil microbiology and greenhouse gas emissions, Swedish Uni Agr Sci
Alburquerque JA, de la Fuente C, Campoy M, Carrasco L, Nájera I, Baixauli C, Caravaca F, Roldán A, Cegarra J, Bernal MP (2012) Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur J Agron 43:119–128
Bonten LTC, Zwart KB, Rietra RPJJ, Postma R, Nysingh SL (2014) Bio-slurry as fertilizer: is bio-slurry from household digesters a better fertilizer than manure?: a literature review. Alterra Rapport Wageningen Uni Res Centre. https://doi.org/10.13140/2.1.2811.1040
Cahyono O, Hartati S (2013) Improvement of phosphate fertilization method in wetland rice. AGRIVITA J Agric Sci. https://doi.org/10.17503/Agrivita-2013-35-1-p081-087
Cao H, Fan D, Yao M, Li X (2021) Methods for measuring soil denitrification rate. Chin J Appl Environ Biol 27:1102–1109 (in Chinese with English abstract)
Chen G, Zhao G, Zhang H, Shen Y, Fei H, Cheng W (2017) Biogas slurry use as N fertilizer for two-season Zizania aquatica Turcz. in China. Nutr Cycl Agroecosys 107:303–320
Dahiya AK, Vasudevan P (1986) Biogas plant slurry as an alternative to chemical fertilizers. Biomass 9:67–74
Datta SK (1986) 10. Improving nitrogen fertilizer efficiency in lowland rice in tropical Asia. Fertilizer Res 9:171–186
Deng O, Jiang L, Chen D, Sun S, Lv J (2011) Ammonia volatilization from the biogas slurry irrigation paddy field. J Soil Water Conserv 25:233–236 (in Chinese with English abstract)
Ding W, He P, Zhang J, Liu Y, Xu X, Ullah S, Cui Z, Zhou W (2020) Optimizing rates and sources of nutrient input to mitigate nitrogen, phosphorus, and carbon losses from rice paddies. J Clean Prod 256:120603
Du H, Gao W, Li J, Shen S, Wang F, Fu L, Zhang K (2019) Effects of digested biogas slurry applicationmixed with irrigation water on nitrate leaching during wheat-maize rotation in the North China Plain. Agr Water Manage 213:882–893
Eltohamy KM, Liu C, Khan S, Niyungeko C, Jin Y, Hosseini SH, Li F, Liang X (2021) An internet-based smart irrigation approach for limiting phosphorus release from organic fertilizer-amended paddy soil. J Clean Prod 293:126254
Feng G, (2008) Simulation on Soil water dynamics of rice field, Nanjing Uni infor, Nanjing
Ghoneim A, Ueno H, Ebid A, Asagi N, Darag IAE (2008) Analysis of nitrogen dynamics and fertilizer use efficiency in rice using the nitrogen15 isotope dilution method following the application of biogas slurry or chemical fertilizer. Int J Soil Sci 3:11–19
Huang H, Cao J, Chang Z, Cao Y (2013) Effects of digested pig slurry application on yields, nitrogen and phosphorous uptakes by rice and wheat. Soils 45:412–418
Huang L, Yu J, Yang J, Zhang R, Cai Y, Sun C, Zhuang H (2016) Relationships between yield, quality and nitrogen uptake and utilization of organically grown rice varieties. Pedosphere 026:85–97
Jiang L, Wang Q, Chen D, Li A, Qian S, Fu J, Wang J (2011) Effects of paddy field disposal of biogas slurry on rice production, soil quality and environmental safety. J Agro-Environ Sci 30:1328–1336 (in Chinese with English abstract)
Kartika K, Lakitan B, Sanjaya N, Wijaya A, Kadir S, Kurnianingsih A, Widuri LI, Siaga E, Meihana M (2018) Internal versus edge row comparison in jajar legowo 4:1 rice planting pattern at different frequency of fertilizer applications. AGRIVITA J Agric Sci. https://doi.org/10.17503/agrivita.v40i0.1715
Kumar A, Verma LM, Sharma S, Singh N (2023) Overview on agricultural potentials of biogas slurry (BGS): applications, challenges, and solutions. Biomass Conv Bioref 13:13729–13769
Liu F (2016) Effects of irrigation and fertilization in paddy field on discharge of agricultural non-point-source pollution in different-scale districts. Acta Agr Jiangxi 28:105–109 (in Chinese with English abstract)
Liu X, Wang H, Zhou J, Hu F, Zhu D, Chen Z, Liu Y (2016) Effect of N fertilization pattern on rice yield, N use efficiency and fertilizer–N fate in the Yangtze River Basin. China Plos One 11:e166002
Lu J, Jiang L, Chen D, Toyota K, Strong PJ, Wang H, Hirasawa T (2012) ontamination of anaerobically digested slurry in a paddy field ecosystem in Jiaxing region of China. Agr Eco Environ 146:13–22
Lu F, Bao-jie C, He-zhong D (2022) Cotton cultivation technology with Chinese characteristics has driven the 70-year development of cotton production in China. J Integr Agr 21:597–609
Matsuno Y, Nakamura K, Masumoto T, Matsui H, Kato T, Sato Y (2006) Prospects for multifunctionality of paddy rice cultivation in Japan and other countries in monsoon Asia. Paddy Water Environ 4:189–197
Minamikawa K, Huynh KC, Uno K, Tran NS, Nguyen CH (2021) Cattle biogas effluent application with multiple drainage mitigates methane and nitrous oxide emissions from a lowland rice paddy in the Mekong Delta. Vietnam Agr Eco Environ 319:107568
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARAPRC) (2022) Guideline on Scientific Fertilization of Rice in 2022. http://www.moa.gov.cn/xw/zxfb/202203/t20220329_6394524.htm
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci 12:242–257
Möller K, Stinner W, Deuker A, Leithold G (2008) Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr Cycl Agroecosys 82:209–232
NBSC (2010) The first national census bulletin of pollution sources. National Bureau of statistics of China
NBSC (2022) China Statistical Yearbook. National Bureau of statistics of China
Nkoa R (2014) Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron Sustain Dev 34:473–492
Oritate F, Nakamura M, Nguyen DP, Dang HVB, Nguyen KD, Yuyama Y, Yamaoka M, Kitagawa I, Sakoda A, Mochidzuki K (2016) Feasibility for use of digested slurry by the pouring method in paddy fields of Southern Vietnam. Paddy Water Environ 14:429–438
Pagel H, Ilg K, Siemens J, Kaupenjohann M (2008) Total phosphorus determination in colloid-containing soil solutions by enhanced persulfate digestion. Soil Sci Soc Am J 72:786–790
Pan B, Xia L, Lam SK, Wang E, Zhang Y, Mosier A, Chen D (2022) A global synthesis of soil denitrification: driving factors and mitigation strategies. Agr Eco Environ 327:107850
People's Government of Tongxiang Municipal (PGTxM) (2021) Regional Overview of Tongxiang City in 2019. http://www.tx.gov.cn/art/2021/3/4/art_1229398295_59024185.html
Phayom W, Iwashita K, Iwata M, Tanaka M (2012) Study of a slurry irrigation system by methane fermentation digestion for wet rice cultivation. Eng Agr Environ Food 5:57–64
Rahaman MA, Zhang Q, Shi Y, Zhan X, Li G (2021) Biogas slurry application could potentially reduce N2O emissions and increase crop yield. Sci Total Environ 778:146269
Sharpe RR, Harper LA (1996) Soil, plant and atmospheric conditions as they relate to ammonia volatilization. In: Ahmad N (ed) Nitrogen economy in tropical soils. Springer Netherlands, Dordrecht, pp 149–158. https://doi.org/10.1007/978-94-009-1706-4_15
Shen Y, Li R, Xu M, Zhou H, Liu P, Sun N (2023) Responses of soil available phosphorus and phosphorus forms to phosphorus fertilizer application times. J Agr Sci Tech Iran 25:173–181
Shi Y (2010) The potential capacity for paddy field ecosystem to decontaminate biogas slurry and its risks assessment. Zhejiang University, Hangzhou, p 142 (in Chinese with English abstract)
Shi S, Paudel K, Chen F (2021) Mechanization and efficiency in rice production in China. J Integr Agr 20:1996–2008
Sitthaphanit S, Limpinuntana V, Toomsan B, Panchaban S, Bell R (2010) Growth and yield responses in maize to split and delayed fertilizer applications on sandy soils under high rainfall regimes. Kasetsart J Nat Sci 44:991–1003
Tang Y, Luo L, Carswell A, Misselbrook T, Shen J, Han J (2021) Changes in soil organic carbon status and microbial community structure following biogas slurry application in a wheat-rice rotation. Sci Total Environ 757:143786
Terman GL (1980) Volatilization losses of nitrogen as ammonia from surface-applied fertilizers, organic amendments, and crop residues. Adv Agron 31:189–223
Wang Z, Liu X, Ju X, Zhang F (2002) Field in situ determination of ammonia volatilization from soil: Venting method. Plant Nutr Fertilizer Sci 8:205–209 (in Chinese with English abstract)
Wang Z, Cai K, Wang L, Li W, Wang G (2014) Influence of surface application of biogas slurry on ammonia volatilization and dynamic distribution of soil nitrogen. T Chin Soc Agr Mach 45:136–143 (in Chinese with English abstract)
Wang Z, Liang Y, Sheng J, Guan Y, Wu H, Chen L, Zheng J (2016) Analysis of water environment risk on biogas slurry disposal in paddy field. T Chin Soc Agr Eng 32:213–220 (in Chinese with English abstract)
Wang G, Kou X, Zhang J, Wang S, Xu R, Han G, Tang H, Zhu L, Bi J, Wu L (2018) Effect of chemical fertilizer nitrogen substitution by biogas slurry on the growth and quality of rice. Chin J Ecol 37:2672–2679 (in Chinese with English abstract)
Wang J, Wu G, Yuan M, Jing Y, Wang W, Zhang X, Sun Y (2022) Effects of different nitrogen application rates and methods on ammonia volatilization and nitrogen use efficiency in paddy fields. Soil Fertilizer Sci Chin 54:10–17 (in Chinese with English abstract)
Win AT, Toyota K, Win KT, Motobayashi T, Ookawa T, Hirasawa T, Chen D, Lu J (2014) Effect of biogas slurry application on CH4 and N2O emissions, Cu and Zn uptakes by whole crop rice in a paddy field in Japan. Soil Sci Plant Nutr (tokyo) 60:411–422
Xiao J (2020) Application status and development measures of biogas fertilizer in agricultural production. Asian Agr Res 12:54–61
Xu J, Peng S, Hou H, Yang S, Luo Y, Wang W (2013) Gaseous losses of nitrogen by ammonia volatilization and nitrous oxide emissions from rice paddies with different irrigation management. Irrigation Sci 31:983–994
Xu S, Chen W, Xie L, Chai S, Jia K, Wei Y (2022) Organic waste resources and nutrient utilization potential in China. J Plant Nutr Fertil 8:1341–1352 (in Chinese with English abstract)
Yang R, Sun Q, Zhao H, Zou G, Liu B, Li L (2017) Precision application of biogas slurry and its environmental effects in paddy fields. J Agro-Environ Sci 36:1566–1572 (in Chinese with English abstract)
Yin G, Wang X, Du H, Shen S, Liu C, Zhang K, Li W (2019) N2O and CO2 emissions, nitrogen use efficiency under biogas slurry irrigation: a field study of two consecutive wheat-maize rotation cycles in the North China Plain. Agr Water Manage 212:232–240
Yoon CG (2009) Wise use of paddy rice fields to partially compensate for the loss of natural wetlands. Paddy Water Environ 7:357–366
Yu Y, Xue L, Yang L (2011) Effects of nitrogen management of nitrogen leaching of paddy soil in Taihu Lake region. Acta Petrol Sin 48:988–995 (in Chinese with English abstract)
Zeng Y, Chen C, Lin G (2023) Practical application of an intelligent irrigation system to rice paddies in Taiwan. Agr Water Manage 280:108216
Zhan X, Zhang A, Zhang Q (2020) Controlling agricultural non-point source pollution: Thinking and practice in the era of agricultural green high-quality development. T Chine Soc Agr Eng 20:1–7 (in Chinese with English abstract)
Acknowledgements
This work was supported by the Zhejiang Province Three Rural and Nine Party Science and Technology Cooperation Plan [grant number 2022SNJF005] and National Natural Science Foundation of China [grant number 42277045]
Funding
This work was supported by the Zhejiang Province Three Rural and Nine Party Science and Technology Cooperation Plan [Grant Number 2022SNJF005] and National Natural Science Foundation of China [Grant Number 42277045].
Author information
Authors and Affiliations
Contributions
Yinxiu Liu, Shanshan Ying, Linping Luo and Peikun Jiang designed and directed the project; Ming Li and Yinxiu Liu performed the experiments; Ming Li and Shanshan Ying performed the model simulation and analyzed the data; Shanshan Ying took the lead in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Liu, Y., Luo, L. et al. Effects of different biogas slurry application patterns on nitrogen and phosphorus losses in a paddy field. Paddy Water Environ (2024). https://doi.org/10.1007/s10333-024-00982-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10333-024-00982-w