Abstract
This is the first report on using Macleaya cordata for phytoextraction of uranium from the uranium contaminated soil in the greenhouse. Macleaya M. cordata was found to increase uranium concentration in the soil solution by increasing the dissolved organic carbon (DOC). The amendment experiments with citric acid (CA) and [S,S]-ethylenediamine disuccinic acid (EDDS) at the rates of 1.0, 2.5, 5.0, and 10.0 mmol kg−1 dry weight (DW) soil showed that EDDS was more efficient to increase uranium concentration in the shoot than CA when they were applied at the same rate. The applications of 5.0 mmol kg−1 EDDS and 10.0 mmol kg−1 CA were most appropriate for increasing uranium concentrations in the shoot of M. cordata. CA was more efficient to increase the solubility of uranium at the same application rates except for 2.5 mmol kg−1 application rate. There was a linear correlation between the uranium concentration in the shoot and the average uranium concentration of one planted pot during 14 days in soil solution after the application of different rates of EDDS and CA, respectively (r 2 = 0.972, P < 0.01; r 2 = 0.948, P < 0.01), indicating that uranium uptake was dependent on the soluble uranium concentration. The Fe-U-DOC and Mn-U-DOC complexes were probably formed after the application of CA. Soil solution pH and Fe, Mn, Ca, and DOC concentrations in soil solution were found to be changed by the chelates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium mining and processing, nuclear fuel fabrication, and weapon research can lead to soil contamination with uranium (Huang et al. 1998; Shahandeh and Hossner 2002). The uranium in soil can go into human bodies through food chain, and it can cause damage to people’s kidney, brain and heart, and even death (Jagetiya and Sharma 2013). Therefore, remediation of the uranium contaminated soil has been the research focus.
Phytoextraction has been widely accepted as a cost-effective, safe, and eco-friendly “green” remediation method (Stingu et al. 2012), and it is suitable for the remediation of diffusely contaminated areas where contaminants occur in the surface layer with a relatively low concentration (Rulkens et al. 1998). Successful phytoextraction requires plants with high metal uptake capacity, high biomass production, and high growth rate (Mitch 2002).
We have found that Macleaya cordata has high uptake capacity for uranium (Ding et al. 2010, 2011), and an adult M. cordata is usually 1–4 m high (Wu 1999). It is a perennial herb and can be harvested twice a year except for the seed germination in the first year. Therefore, it was selected for this study. But much has to be done on the root-induced changes in the rhizosphere of M. cordata in relation to the uranium uptake, the soil-to-plant transfer of uranium by M. cordata, and the enhancement of the availability of uranium in soil.
Previous studies show that the uptake of uranium by plant depends on the availability of heavy metals in soil for the plant (Evangelou et al. 2007; Duquène et al. 2009), uranium availability for plants can be enhanced by applying citric acid (CA; Huang et al. 1998; Shahandeh and Hossner 2002; Huhle et al. 2008; Duquène et al. 2009; Jagetiya and Sharma 2013), and [S,S]-ethylenediaminedisuccinic acid (EDDS), a structural isomer of ethylenediaminetetraacetic acid, is an effective chelate for enhancing the availability of metals in soil (Meers et al. 2005; Wu et al. 2007; Duquène et al. 2009; Lozano et al. 2011; Hseu et al. 2013).
The objectives of this study were (1) to investigate the effect of M. cordata on the concentration of uranium in the soil solution, (2) to evaluate the effect of EDDS and CA on the phytoextraction of uranium from the tailing soil by M. cordata, (3) to assess the effect of the chelates on the solubility of uranium in the tailing soil, and (4) to explore the variation of U, Fe, Mn, Ca, pH, and dissolved organic carbon (DOC) in the soil solution after the application of the chelates.
Materials and methods
Soil preparation and analysis
Uranium tailing and campus soil were collected from a uranium mill tailings impoundment in South China and the university campus, respectively. The samples were air-dried, ground, and passed through a 2-mm nylon sieve. Two soils were prepared. The first one was tailing soil which consisted of a mixture of uranium tailing and campus soil in the ratio of 1:1 dry weight (DW), and the second one was campus soil. The campus soil was artificially contaminated with 18.0 mg uranium kg−1 (DW), and the uranium was in the form of uranyl nitrate [UO2(NO3)2 · 6 H2O]. The tailing soil and campus soil, each weighing 3.14 kg (DW), were transferred into plastic pots, respectively. The two kinds of soils were fertilized using the method by Shahandeh and Hossner (2002). Then, they were allowed to incubate for 30 days, and the moisture content was kept at 80 % of the saturation moisture content during this period by weighing the pots daily and adding the deionized water. The tailing soil and the campus soil were also homogenized and aerated by manual mixing in every other day to avoid anaerobic conditions. Properties of the soils analyzed by standard methods (Lu 2000; Zhang and Gong 2012) are shown in Online Resource 1. Exchangeable cations (Ca, Mg, K, and Na ) were analyzed by atomic absorption spectrometry (AAS, PinAAcle 900F, Singapore), Uranium concentration was measured with inductively coupled plasma mass spectrometry (ICP-MS, Agilent, 7700 X, USA). Soil sample (0.25 g) was added into Teflon vessel and digested with 6 mL 65 % HNO3 and 2 mL 40 % HF in microwave digestion system. Twelve milliliters 4 % H3BO3 solution was added directly into the Teflon vessel to remove fluoride ions in the digestion system (Chajduk et al. 2013).
Plant Culture
Seeds of M. cordata were disinfected with 1 % NaOCl for 10 min after the seed shells were artificially removed, and they were then washed 3 to 5 times with distilled water and soaked with distilled water for 12 h. Afterwards, the seeds were germinated in a light culture chamber at 28 °C on filter papers in Petri dishes containing 8 mL distilled water for 2 weeks. The germinated seeds were then planted in the tailing soil and the campus soil and kept growing for 2 weeks. Then, two seedlings were planted at a depth of 1.5 cm in the tailing soil and the campus soil pots, respectively, and thinned to one per pot after 4 weeks of growth. Planted pots were watered to 80 % of the saturation moisture content once a day by weighing the pots and adding the deionized water. A collection tray was placed beneath each pot in order that the leachate could be retained and re-applied to the same pot. Plants grew in a greenhouse under the following conditions: illumination time was 14 h; air temperature, 22–28 °C; relative humidity, 65–70 %; and illumination, 60 W m−2. In order to make all the plants grow under similar conditions, the location of the plant was changed in turns every week. To examine the effects of the plant and the chelates on the chemical properties of soil solution in the soils, a rhizon soil moisture sampler (type MOM, Eijkelkamp Agrisearch, Giesbeek, The Netherlands) was inserted vertically at the center of each pot.
Experimental setup
Soil solution samples (8 mL each) were collected via the rhizon soil moisture samplers with sterile syringes at 14 pm on the day before two seedlings were planted in the tailing soil and the campus soil, and at 4, 8, 12, 16, and 20 weeks after the first sampling. Sixteen weeks after thinning out seedlings, the plant shoots were cut in 1 cm above the soil surface and harvested. Unplanted pots as control were also kept under the same conditions as the planted pots. Each treatment was replicated three times, and this experiment had six planted pots and six unplanted pots.
One day before application of chelates, no water was added to tailing soil to ensure that its soil moisture content was less than 80 % of the saturation moisture content after the addition of chelates. When the plants started flowering (Wu et al. 2007; 14 weeks after thinning out seedlings), the pots were amended with EDDS and CA as solutions at doses of 1.0, 2.5, 5.0, and 10.0 mmol kg−1 dry soil, which were prepared with pure salts of Na3-S,S-EDDS and CA, respectively, and one set of pots was used as control. 300 mL of Na3-S,S-EDDS and CA treatment solutions for irrigating the pots were prepared by diluting 1 mol L−1 Na3-S,S-EDDS solution (pH 7.0) and 1 mol L−1 CA solution, respectively. Each treatment was replicated three times, and this experiment had 27 planted pots in total. Soil solution samples(8 ml each)were collected at 14 pm on the 2nd day before chelate application and at 1, 3, 5, 7, 10, and 14 days after chelate application. Soil moisture content was adjusted to 80 % of the saturation moisture content by adding the deionized water 24 h before sampling. Two weeks after chelate application, the plant shoots were cut in 1 cm above the soil surface and harvested (Shahandeh and Hossner 2002; Duquène et al. 2009).
Plant sample and soil solution analysis
Plant sample was prepared and analyzed using the method by Duquène et al. (2009). Soil solution pH was measured by the glass electrode method. DOC was determined with a TOC analyzer (Shimadzu, TOC-Vcpn, Japan). Concentrations of Ca, Fe, and Mn in soil solution were determined by AAS. Uranium concentration was measured by ICP-MS.
Statistical analyses
Statistical analysis of data was performed using SPSS Version 18.0. Mean values based on three replicates were calculated. Data were statistically evaluated by analysis of variance and significant difference was assessed by the Tukey’s multiple range test with P < 0.05. Linear correlations were assessed by Pearson’s linear correlation statistic.
Results and discussion
Uranium uptake into shoot and uranium release by M. cordata
The content of uranium in the shoot of M. cordata grown in campus soil significantly increased with that in tailing soil (F = 129.718, P < 0.05; Table 1). It can be found that the average concentration of uranium of 33.4 μg L−1 in the campus soil solution was significantly higher than that of 6.6 μg L−1 in the tailing soil solution (F = 66.085, P < 0.05; Table 1). In this study, the total amount of uranium in the campus soil is the same as that in the tailing soil; however, the total uptake of uranium into the shoot of M. cordata in the campus soil was 184 μg kg−1, which was 2.4 times that in the tailing soil. This probably indicates that the uptake of uranium in plant is dependent on the uranium concentration in the soil solution rather than the uranium concentration in soil. Recent studies also illustrated the similar results (Kim et al. 2010a; Abreu et al. 2014). M. cordata culture resulted in an increase of uranium concentration in the rhizosphere soil solution in the tailing soil and the campus soil (Table 1). The observation was consistent with the results of the previous studies (Takeda et al. 2008; Duquène et al. 2009). These results are probably due to DOC concentration increase (Takeda et al. 2008; Kim et al. 2010a). Plant roots contribute to the increase of DOC by exudation (Kim et al. 2010b). The uranium concentration in the rhizosphere soil solution in tailing soil and campus soil was positively correlated with the DOC concentration during the cultivation period (r 2 = 0.850, P < 0.01; r 2 = 0.604, P < 0.01; Online Resource 2). This result implies that most of uranium was complexed with the DOC in soil solution in the tailing soil and campus soil. DOC in soils may facilitate the release of the adsorbed heavy metals from the solid phase to the soil solution as metal-DOC complexes (Kim et al. 2010a).
Plant growth and dry mass after application of chelates
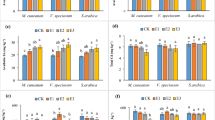
One to two days after application of 10.0 mmol kg−1 EDDS, M. cordata showed significant senescence, and only one plant of M. cordata in the three replicates survived, but the survived showed strong toxicity symptoms such as chlorosis and necrotic spots. After the treatment with 5.0 mmol kg−1 EDDS, only one plant of M. cordata in the three replicates exhibited a small number of necrotic spots (3 days after treatment). The other plants of M. cordata had no symptoms of toxicity.
The dry mass of the shoot of M. acordata did not exhibit significant decrease as compared with the control after the application of EDDS and CA except for 10.0 mmol kg−1 EDDS application (Fig. 1). The reduction in shoot dry biomass of the dead plants was 41 and 55 %, and that of the live plant was 13 %. Reduction in the shoot dry mass after EDDS treatment is probably due to the heavy metal concentration and the addition of the chelate that exceeds the capacity of plant for activating a defense system (Luo et al. 2006). Many reports have already shown negative effects of EDDS on the growth of plants (Evangelou et al. 2007; Wu et al. 2007; Duquène et al. 2009). Wu et al. (2007) reported that the EDDS itself or EDDS-metal complexes had detrimental effects on the growth of plant and the total chlorophyll and chlorophyll A contents decreased after the EDDS treatment.
Uranium concentrations in shoot after application of chelates
Uranium concentrations in the shoot increased with the increasing rate of chelates (Fig. 2 and Online Resource 3), and the uranium concentrations in the shoot of the dead plants were 26.9 and 23.5 ug plant−1 or 2,983.9 and 2,425.1 ug kg−1. This result was in agreement with the results of the previous studies (Huang et al. 1998; Shahandeh and Hossner 2002; Jagetiya and Sharma 2013). The maximum uranium concentration in the shoot increased 17 times that for the control after the application of 10.0 mmol kg−1 EDDS. There was a linear correlation between the uranium concentrations in the shoot and the average uranium concentrations of one planted pot during 14 days in soil solution after the application of different rates of EDDS and CA, respectively (r 2 = 0.972, P < 0.01; r 2 = 0.948, P < 0.01; Fig. 3). This again indicates that the uptake of uranium by plant is dependent on the uranium concentration in soil solution. The slope of the regression equation after application of EDDS (2.744) was significantly higher than that after application of CA (0.818). This result indicates that EDDS was more efficient to increase the uranium concentration in the shoot than CA when they were applied at the same rate, and uranyl–EDDS complexes were more easily utilized by M. cordata than uranyl–citrate complexes. EDDS has been detected in roots, shoots and xylem sap of sunflower (Tandy et al. 2006a, b), and in xylem sap and leaves of Elsholtzia splendens (Wu et al. 2007), and this indicates that metal complexes or free chelates are taken up into the xylem and translocated to the shoots (Wenger et al. 2005). Available uncoordinated free EDDS may destroy the physiological barriers in roots by removing Fe2+, Ca2+ and other divalent cations from the plasma membrane (Quartacci et al. 2007), which is important for the selectivity properties of the root (Duquène et al. 2009). This probably explained why the dry mass and the growth status of M. cordata were affected by addition of 10.0 mmol kg−1 EDDS, and why EDDS was more efficient to increase the uranium concentration in shoot. When 10.0 mmol kg−1 EDDS was applied, only one plant of M. cordata in the three replicates survived and had toxicity symptoms. This indicates that although M. cordata is a perennial, it would not shoot out buds in the coming years after it was cut. Meanwhile, the uranium concentrations in the shoot were 8.8 and 6.1 μg plant−1 after the application of 5.0 mmol kg−1 EDDS and CA (Fig. 2), respectively. After the application of 10.0 mmol kg−1 CA, the concentration of uranium in the shoot was 8.5 μg plant−1, and it showed no significant decrease compared with that after the application of 5.0 mmol kg−1 EDDS (F = 0.195, P = 0.682). Therefore, at the same rate of chelates, application of 5.0 mmol kg−1 EDDS was the optimal scheme to enhance the uranium uptake from the tailing soil for phytoextraction, and application of 10.0 mmol kg−1 CA was also the optimal scheme because CA could more easily be degraded than the EDDS (Ebbs et al. 1998; Meers et al. 2005). In this study, the phytoremediation factor (Li et al. 2011) of M. cordata was 56 times that of the Indian mustard by Jagetiya and Sharma (2013) after the application of 2.5 mmol kg−1 CA (data not shown). The uranium concentrations in the shoot of Indian mustard (Duquène et al. 2009) were higher than that in the shoot of M. cordata after the application of 5.0 mmol kg−1 EDDS and CA. This may be due to the low solubility and bioavailability of uranium in uranium tailings since the uranium is present as insoluble forms of uranite (uranous oxide), tyuyamunite (hydrous calcium uranium vanadate), and autunite (hydrous calcium uranium phosphate; Shahandeh and Hossner 2002).
U, Fe, and Mn concentrations in soil solution after application of chelates
The uranium concentration in soil solution increased with the increasing concentration of CA and EDDS (Table 2). The highest concentration of uranium in soil solution increased 278 times as compared with the control 1 day after the application of 10.0 mmol kg−1 CA. The average uranium concentrations in the soil solution increased 93.3, 37.6, 10.5, and 1.9 times as compared with the control after the application of 10.0, 5.0, 2.5, and 1.0 mmol kg−1 EDDS, respectively, and increased 102.7, 52.1, 6.0, and 3.5 times in relation to the control after the application of 10.0, 5.0, 2.5, and 1.0 mmol kg−1 CA, respectively. Therefore, CA was more efficient to increase the solubility of uranium in tailing soil than EDDS at the same application rates except for 2.5 mmol kg−1 application rate. This result was consistent with the findings by Duquène et al. (2008) and Lozano et al. (2011). The increase of solubility of uranium in the tailing soil may be due to the chelation of uranium with CA and EDDS (Huang et al. 1998; Shahandeh and Hossner 2002; Lozano et al. 2011), and the enhancement of the desorption of uranium from solid-phase uranium particles in the uranium tailing since CA can remove coatings of amorphous iron and aluminum sesquioxide (Huang et al. 1998). Liang et al. (2000) and Logue et al. (2004) found that amorphous iron was readily dissolved after the application of CA. We also observed that Fe and Mn concentrations significantly increased after the application of chelates (Online Resource 4). Similar results were observed in previous studies (Francis and Dodge 1998; Yip et al. 2009). The significant correlations between U and Fe concentrations and between U and Mn concentrations in soil solution were found on the first day after the treatment with different rates of CA and EDDS (r 2 = 0.887 − 0.960, P < 0.01, n = 12). This result suggests that most of the uranium in soil solution probably comes from MnO X -U and FeO X -U in the tailing soil. Meanwhile, the correlations between U and Fe concentrations and U and Mn concentrations in soil solution during the whole growth stage can be described linearly after the application of CA (r 2 = 0.798 − 0.889, P < 0.05, n = 7; r 2 = 0.753 − 0.977, P < 0.05, n = 7), but this is not the case for EDDS (r 2 = 0.203 − 0.584, P = 0.118 − 0.369, n = 7; r 2 = 0.012 − 0.647, P = 0.054 − 0.833, n = 7, respectively). These similar results were found by Huang et al. (1998) and Duquène et al. (2008). This result may be due to formation of Fe-U-DOC and Mn-U-DOC complexes after application of CA. Meanwhile, we observed that there was a linear correlation between U and DOC concentrations, and this was to be described in the following sections. Previous study also found the simulated fractional distribution of U(VI) species including Fe-U-citrate complexes (Kantar and Honeyman 2006).
The applications of 5.0 mmol and 10.0 mmol kg−1 EDDS were very effective in the release of uranium from the tailing soil (Table 2). Soluble uranium concentration in the soil solution increased with time, decreased on the seventh and the tenth day, and increased on the fourteenth day after the application of 10.0 mmol kg−1 EDDS. Similar change was observed after the application of 5.0 mmol kg−1 EDDS. These changes may be due to re-adsorption of free and complexed EDDS or degradation of uranyl–EDDS complexes, and renewed solubilization (Tsang et al. 2009; Yip et al. 2009; Lozano et al. 2011). Adsorbed EDDS may substantially enhance mineral dissolution through the destabilization of metal-oxygen bonds (Yip et al. 2009). Soluble uranium concentration in soil solution was 418.3 μg L−1 14 days after the application of 10.0 mmol kg−1 EDDS, and it was higher than 14 days after the application of 10.0 mmol kg−1 CA. It is likely that the biodegradation of uranyl–citrate acid complexes is faster than that of uranyl–EDDS complexes in the tailing soil (Ebbs et al. 1998; Meers et al. 2005).
Ca and DOC concentrations and pH in soil solution after application of chelates
The concentration of Ca (Fig. 4) in soil solution increased as compared with the control 1 day after the application of different rates of EDDS and CA. It is possible that the chelate and its complex with Ca decreased activity of Ca in soil solution, hence, promoting the dissolution of CaCO3 and the release of carbonates to the solution (Duquène et al. 2008). These results supported our speculation of formation of uranium bicarbonate or carbonate complexes in soil solution after the addition of chelates. Some investigators also reported an increased concentration of Ca in soil solution after the treatment with CA and EDDS (Francis and Dodge 1998; Gramss et al. 2004; Tandy et al. 2004; Hauser et al. 2005). Then, the concentrations of Ca in soil solution diminished with time afterwards. The possible explanation is the uptake of Ca by plant and the subsequent redistribution of ions onto soil exchange sites at lower ionic strength (Lorenz et al. 1997; Takeda et al. 2008), and the Ca-CA or EDDS complexes are biodegraded (Francis and Dodge 1998).
The concentration of DOC in soil solution increased with the increasing concentration of chelates (Fig. 5). The concentration of DOC increased 118 times and 85 times as compared with the control 1 day after the application of 10.0 mmol kg−1 EDDS and CA, respectively. The highest concentrations of DOC were obtained 1 day after addition of chelates. Then, they quickly decreased during the remaining observation period. The increase of DOC concentration is mainly attributed to the application of chelates (Römkens et al. 2002; Duquène et al. 2008). The soil solution became yellow after the application of high rates of EDDS (5.0 and 10.0 mmol kg−1) and CA (2.5, 5.0, and 10.0 mmol kg−1). It is probably due to the protonation and simultaneous dissolution of humic substances (Hauser et al. 2005; Huhle et al. 2008). There was a significant linear correlation between uranium and DOC concentrations in soil solution during the whole growth stage after the application of different rates of CA (r 2 = 0.814 − 0.997, P < 0.05, n = 7). This result implies that most uranium complexed with DOC in soil solution after the addition of different rates of CA. This correlation was not significant after the application of different rates of EDDS (r 2 = 0.001 − 0.568, P = 0.084 − 0.948, n = 7). This may result from the formation of uranium bicarbonate or carbonate complexes and from the adsorption of uranium by microorganisms. Meanwhile, the soil solution pH was between 7.26 and 7.69 at the end of the experiment (Fig. 6a), and this would readily produce stable uranium carbonate complexes (Duff and Amrhein 1996), especially after the application of 10.0 and 5.0 mmol kg−1 EDDS.
At the end of the experiment, soil solution pH increased by 0.36–1.11 unit as compared with the control after application of chelates (Fig. 6). This result is probably attributed to the presence of carbonates in the tailing soil (Ebbs et al. 1998; Duquène et al. 2008). Another possible explanation is that the acidity provided by CA was neutralized by conversion of carbonates to bicarbonates, which helped to rise the pH (Duquène et al. 2008). A third possible explanation is due to the microbial degradation of carboxylic acids in which H+ are consumed and OH− are liberated (Perez and Jeffries 1992; Barekzai and Mengel 1993). The soil solution pH significantly reduced from 6.60 to 4.32 and to 5.26 on the first day after application of 10.0 mmol and 5.0 mmol kg−1 CA, respectively (F = 42.859, P < 0.01; F = 8.807, P < 0.05). Ebbs et al. (1998) also found that the addition of 10 mmol and 2 mmol kg−1 CA decreased the soil pH from 6.8 to 2.6 and to 3.6 on the first day, respectively. However, this result was contrary to previous studies (Huhle et al. 2008; Duquène et al. 2008; Lozano et al. 2011). In the present study, the decrease of the soil solution pH was due to the acidification of CA (Ebbs et al. 1998).
Conclusions
It was found that M. cordata roots contributed to the increase of DOC and uranium concentrations in soil solution, and the soluble uranium concentration was related to the DOC concentration in soil solution during the cultivation period. CA was more efficient to increase the solubility of uranium than EDDS at the same application rates except for 2.5 mmol kg−1 application rate, and EDDS was more efficient to increase uranium concentration in the shoot than CA when they were applied at the same rate. However, the application of high rate of EDDS for the uptake of uranium by M. cordata was not feasible because of its toxicity. It was also found that the uranium concentration in shoot was dependent on the soluble uranium concentration, and the Fe-U-DOC and Mn-U-DOC complexes were probably formed after the application of CA.
References
Abreu MM, Neves O, Marcelino M (2014) Yield and uranium concentration in two lettuce ( Lactuca sativa L.) varieties influenced by soil and irrigation water composition, and season growth. J Geochem Explor 142:43–48
Barekzai A, Mengel K (1993) Effect of microbial decomposition of mature leaves on soil pH. J Plant Nutr Soil Sci 156:93–94
Chajduk E, Bartosiewicz I, Pyszynska M, Chwastowska J, Polkowska-Motrenko H (2013) Determination of uranium and selected elements in Polish dictyonema shales and sandstones by ICP-MS. J Radioanal Nucl Chem 295:1913–1919
Ding D, Li G, Hu N, Yulong L, Wang Y, Yang L, Ma S, Chen X (2010) Study on phyto-mining of uranium from uranium mill sand. Min Metall Eng 30:58–60 (in Chinese)
Ding D, Li G, Hu N, Liu Yulong, Wu Y, Xie H, Wang Y, Yan J, Hu J, Liu Yang, Ma S (2011) Method for restoring uranium milltailings by utilizing plants ZL200910044229.4 (in Chinese)
Duff MC, Amrhein C (1996) Uranium (VI) adsorption on goethite and soil in carbonate solutions. Soil Sci Soc Am J 60:1393–1400
Duquène L, Tack F, Meers E, Baeten J, Wannijn J, Vandenhove H (2008) Effect of biodegradable amendments on uranium solubility in contaminated soils. Sci Total Environ 391:26–33
Duquène L, Vandenhove H, Tack F, Meers E, Baeten J, Wannijn J (2009) Enhanced phytoextraction of uranium and selected heavy metals by Indian mustard and ryegrass using biodegradable soil amendments. Sci Total Environ 407:1496–1505
Ebbs SD, Norvell WA, Kochian LV (1998) The effect of acidification and chelating agents on the solubilization of uranium from contaminated soil. J Environ Qual 27:1486–1494
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003
Francis AJ, Dodge CJ (1998) Remediation of soils and wastes contaminated with uranium and toxic metals. Environ Sci Technol 32:3993–3998
Gramss G, Voigt K, Bergmann H (2004) Plant availability and leaching of (heavy) metals from ammonium-, calcium-, carbohydrate-, and citric acid-treated uranium-mine-dump soil. J Plant Nutr Soil Sci 167:417–427
Hauser L, Tandy S, Schulin R, Nowack B (2005) Column extraction of heavy metals from soils using the biodegradable chelating agent EDDS. Environ Sci Technol 39:6819–6824
Hseu Z-Y, Jien S-H, Wang S-H, Deng H-W (2013) Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. J Environ Eng 117:58–64
Huang JW, Blaylock MJ, Kapulnik Y, Ensley BD (1998) Phytoremediation of Uranium-Contaminated Soils: Role of Organic Acids in Triggering Uranium Hyperaccumulation in Plants. Environ Sci Technol 32:2004–2008
Huhle B, Heilmeier H, Merkel B (2008) Potential of Brassica juncea and Helianthus annuus in phytoremediation for uranium. In: Merkel B, Hasche-Berger A (eds) Uranium, Mining and Hydrogeology. Springer Berlin, Heidelberg, pp 307–318
Jagetiya B, Sharma A (2013) Optimization of chelators to enhance uranium uptake from tailings for phytoremediation. Chemosphere 91:692–696
Kantar C, Honeyman BD (2006) Citric acid enhanced remediation of soils contaminated with uranium by soil flushing and soil washing. J Environ Eng 132:247–255
Kim K-R, Owens G, Naidu R (2010a) Effect of root-induced chemical changes on dynamics and plant uptake of heavy metals in rhizosphere soils. Pedosphere 20:494–504
Kim K-R, Owens G, Naidu R, Kwon S (2010b) Influence of plant roots on rhizosphere soil solution composition of long-term contaminated soils. Geoderma 155:86–92
Li G, Hu N, Ding D, Zheng J, Liu Y, Wang Y, Nie X (2011) Screening of plant species for phytoremediation of uranium, thorium, barium, nickel, strontium and lead contaminated soils from a uranium mill tailings repository in South China. Bull Environ Contam Tox 86:646–652
Liang L, Hofmann A, Gu B (2000) Ligand-induced dissolution and release of ferrihydrite colloids. Geochim Cosmochim Acta 64:2027–2037
Logue BA, Smith RW, Westall JC (2004) Role of surface alteration in determining the mobility of U (VI) in the presence of citrate: implications for extraction of U (VI) from soils. Environ Sci Technol 38:3752–3759
Lorenz SE, Hamon RE, Holm PE, Domingues HC, Sequeira EM, Christensen TH, McGrath SP (1997) Cadmium and zinc in plants and soil solutions from contaminated soils. Plant Soil 189:21–31
Lozano JC, Blanco Rodríguez P, Tomé FV, Calvo CP (2011) Enhancing uranium solubilization in soils by citrate, EDTA, and EDDS chelating amendments. J Hazard Mater 198:224–231
Lu R (2000) Analytical Methods for Soil and Agrochemistry. Agricultural Science and Technology Press, Beijing, China
Luo C, Shen Z, Lou L, Li X (2006) EDDS and EDTA-enhanced phytoextraction of metals from artificially contaminated soil and residual effects of chelant compounds. Environ Pollut 144:862–871
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack FMG (2005) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58:1011–1022
Mitch ML (2002) Phytoextraction of toxic metals: a review of biological mechanism. J Environ Qual 31:109–120
Perez J, Jeffries TW (1992) Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol 58:2402–2409
Quartacci MF, Irtelli B, Baker AJM, Navari-Izzo F (2007) The use of NTA and EDDS for enhanced phytoextraction of metals from a multiply contaminated soil by Brassica carinata. Chemosphere 68:1920–1928
Römkens P, Bouwman L, Japenga J, Draaisma C (2002) Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ Pollut 116:109–121
Rulkens WH, Tichy R, Grotenhuis JTC (1998) Remediation of polluted soil and sediment: perspectives and failures. Water Sci Technol 37:27–35
Shahandeh H, Hossner LR (2002) Enhancement of Uranium Phytoaccumulation From Contaminated Soils. Soil Sci 167:269–280
Stingu A, Volf I, Popa VI, Gostin I (2012) New approaches concerning the utilization of natural amendments in cadmium phytoremediation. Ind Crop Prod 35:53–60
Takeda A, Tsukada H, Takaku Y, Akata N, Hisamatsu S (2008) Plant induced changes in concentrations of caesium, strontium and uranium in soil solution with reference to major ions and dissolved organic matter. J Environ Radioact 99:900–911
Tandy S, Bossart K, Mueller R, Ritschel J, Hauser L, Schulin R, Nowack B (2004) Extraction of heavy metals from soils using biodegradable chelating agents. Environ Sci Technol 38:937–944
Tandy S, Schulin R, Nowack B (2006a) The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere 62:1454–1463
Tandy S, Schulin R, Nowack B (2006b) Uptake of metals during chelant-assisted phytoextraction with EDDS related to the solubilized metal concentration. Environ Sci Technol 40:2753–2758
Tsang DCW, Yip TCM, Lo IMC (2009) Kinetic interactions of EDDS with soils. 2. Metal − EDDS complexes in uncontaminated and metal-contaminated soils. Environ Sci Technol 43:837–842
Wenger K, Tandy S, Nowack B (2005) Effects of chelating agents on trace metal speciation and bioavailability. In: Nowack B, VanBriesen J (eds) Biogeochemistry of chelating agents, vol 910, ACS Symposium SeriesWashington, D C: American Chemical Society., pp 204–224
Wu Z (1999) Flora of China. http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200009159. Accessed 15 Nov 2012
Wu LH, Sun XF, Luo YM, Xing XR, Christie P (2007) Influence of [S, S]-EDDS on Phytoextraction of Copper and Zinc by Elsholtzia Splendens From Metal-Contaminated Soil. Int J Phytoremed 9:227–241
Yip TCM, Tsang DCW, Ng KTW, Lo IMC (2009) Kinetic Interactions of EDDS with Soils. 1. Metal Resorption and Competition under EDDS Deficiency. Environ Sci Technol 43:831–836
Zhang G, Gong Z (2012) Soil Survey Laboratory Methods. Science Press, Beijing, China
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11305087, 51274124) and the Development Program for Science and Technology for National Defense (B3720132001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Chang-wu Li and Nan Hu are contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 262 kb)
Rights and permissions
About this article
Cite this article
Li, Cw., Hu, N., Ding, Dx. et al. Phytoextraction of uranium from contaminated soil by Macleaya cordata before and after application of EDDS and CA. Environ Sci Pollut Res 22, 6155–6163 (2015). https://doi.org/10.1007/s11356-014-3803-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3803-x