Abstract
The concentrations of uranium, thorium, barium, nickel, strontium and lead in the samples of the tailings and plant species collected from a uranium mill tailings repository in South China were analyzed. Then, the removal capability of a plant for a target element was assessed. It was found that Phragmites australis had the greatest removal capabilities for uranium (820 μg), thorium (103 μg) and lead (1,870 μg). Miscanthus floridulus had the greatest removal capabilities for barium (3,730 μg) and nickel (667 μg), and Parthenocissus quinquefolia had the greatest removal capability for strontium (3,920 μg). In this study, a novel coefficient, termed as phytoremediation factor (PF), was proposed, for the first time, to assess the potential of a plant to be used in phytoremediation of a target element contaminated soil. Phragmites australis has the highest PFs for uranium (16.6), thorium (8.68), barium (10.0) and lead (10.5). Miscanthus floridulus has the highest PF for Ni (25.0). Broussonetia papyrifera and Parthenocissus quinquefolia have the relatively high PFs for strontium (28.1 and 25.4, respectively). On the basis of the definition for a hyperaccumulator, only Cyperus iria and Parthenocissus quinquefolia satisfied the criteria for hyperaccumulator of uranium (36.4 μg/g) and strontium (190 μg/g), and could be the candidates for phytoremediation of uranium and strontium contaminated soils. The results show that the PF has advantage over the hyperaccumulator in reflecting the removal capabilities of a plant for a target element, and is more adequate for assessing the potential of a plant to be used in phytoremediation than conventional method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Uranium mill tailings contains long-lived radioactive nuclides and heavy metals (Landa 2004). Although the uranium mill tailings is deposited in a specially designed and constructed repository, the radioactive nuclides and heavy metals will transport to the soil around the repository and pose a great threat to the ecosystem, agro-system and people’s health (Frostick et al. 2008). Therefore, it is necessary to study the remediation techniques for the uranium mill tailings contaminated soil and the tailings itself.
One of the promising strategies for treating the large-scale low-level contamination is the use of phytoremediation technique (AbdEl-Sabour 2007). In recent years, many researchers have studied the soil sampling and mapping of contamination, the relative uptake of radioactive nuclides by various plant species, and the improvement of the uptake by adding fertilizers, organic acids or chelating agents (Blanco Rodríguez et al. 2002; Shahandeh and Hossner 2002; Chen et al. 2005; Huang et al. 1998). It was found that the uptake of U and Th was mainly associated with the iron concentration in the plant and the phosphorus and alkaline earths in the substrate (Blanco Rodríguez et al. 2002), that sunflower and Indian mustard had the highest capability of accumulating U among thirty-four U-accumulating plant species collected from the U-contaminated soil (Shahandeh and Hossner 2002), that Lupine (Lupinus albus) and Ryegrass (Lolium perenne) had the highest uptake of 238U and 232Th among the crops on uranium mill tailings contaminated soil (Chen et al. 2005), and that the addition of citric acid could help to dissolve more U than the acidification or the other amendments tested (Huang et al. 1998).
However, the suitable plant species for phytoremediation of uranium tailings contaminated soil and the tailings itself have not been screened out so far, and the criteria for determining a candidate plant for the phytoremediation has not be established.
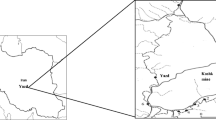
In South China, there is a uranium mill tailings repository. The repository has a subtropical continental climate with an annual average temperature of 17.9°C, an annual average rainfall of 1,452.0 mm, an annual average evaporation capacity of 1,324.5 mm, and the altitude from 210.5 m to 307.0 m above sea level. It covers an area of approximately 1.70 km2, and contains approximately 1.88 × 108 t of uranium mill tailings produced by a nearby uranium mill where the uranium ore was processed by the acid leaching from 1960s to 1990s. This repository is a natural laboratory for conducting research on phytoremediation of uranium tailings contaminated soil and the tailings itself.
The present study was to make a geo-botanical survey of the plant species at the uranium mill tailings repository in South China, to screen out the plant species capable of remediating the uranium mill tailings contaminated soil and the tailings itself, and to establish a criteria for screening a plant candidate for phytoremediation of the uranium mill tailings contaminated soil and the tailings itself.
Materials and Methods
In autumn, 2009, the tailings and plant samples were collected from the uranium mill tailings repository. The sampling site was defined by a 5 m × 5 m square, and the sample was taken at each corner and the intersection of its diagonals (Gramatica et al. 2006).
The dominant plant species were collected from the five plots at each sampling site. The whole plant was collected for the herbaceous plant, and the leaf and stalk were obtained for the ligneous plant. The plant samples were divided into the shoot (including seed, leaf and stalk) and the root, and they were gently washed with de-ionized distilled water for 3 min to remove the tailings particles adhered. Six specimens of each plant species were mixed to give a composite sample. The tailings sample was collected from the corresponding plant sampling site. In the laboratory, the plant and tailings samples were kept drying at room temperature for 2 weeks.
A subsample of each plant tissue and tailings was kept drying at 105°C for 24 h to reduce the bound water. All the data in this paper were on this dry weight basis (105°C). They were sealed in the clean polyethylene container and stored in a refrigerator for analysis. The weight of each plant tissue was precisely obtained by a scale before it was ground to powders using Wiley Mills.
The dried powders of plant and tailings samples were analyzed for the total concentrations of U, Th, Ba, Pb, Ni and Sr, respectively, using an ICP-MS (ELAN® DRC-e, Perkinelmer, American) at the State Key Lab of Ore Deposit Geochemistry, Guiyang Institute of Geochemistry, Chinese Academy of Sciences, China. The method was described by Liang et al. (2000).
The analytical reagent-grade HF and HNO3 were purified by sub-boiling distillation to give the low reagent blank and the corresponding low analytic limit of detection. The 18 MΩ/cm2 grade water was used in this experiment which was prepared by a Millipore purification system. Every PTFE bomb was cleaned with 8 mL of 3.6 M HNO3 and heated to 100°C and kept boiling for 1 h before use.
The characteristics of the uranium mill tailings sample were analyzed according to NY/T 1121 (2006). For each tailings sample, 0.050 g of the sample was taken and digested with HNO3 and HF. The method was described by Liang et al. (2000).
For each plant sample, 0.100 g of the sample was put in a PTFE bomb and 2 mL of 15.2 M HNO3 added in it. After that, the bomb was sealed and stood for overnight. Then the bomb was opened and kept on an electric oven at 150°C until the solution was boiled to dryness. After the bomb cooled, 2 mL of 15.2 M HNO3 and 0.2 mL of 21.8 M HF were added in it. Again, the bomb was sealed, heated to 190°C, and kept heating at that temperature for 20 h. After that, 1 mL of 15.2 M HNO3 was added in the bomb, and the solution was boiled to dryness. After the bomb cooled, 1 mL of 500 ng/mL Rh internal standard solution was added in it. The final residue in the bomb was re-dissolved by adding 1 mL of 7.8 M HNO3. Finally, the bomb was resealed, heated to 130°C, and kept heating at that temperature for 3 h. After it cooled, the digestion was diluted to 10 mL by adding distilled de-ionized water. The reagent blank was treated in the same way as the sample.
The stock solutions in the concentration of 1,000 mg/L of U, Th, Sr, Ba, Ni and Pb were prepared using the pure metals or pure metal oxide. The detection limit for each element was calculated by both the sum of background and three times of its standard deviation. The range of the detection limit was 0.001–0.005 μg/L for Th and U, 0.01–0.2 μg/L for Ni, Sr, Ba, and Pb, respectively. The accuracies of the ICP-MS analyses are estimated to be better than ±5% (relative) for the elements determined. The recoveries for the target elements in this study from fortified sample were 97.6%–110.5%.
The transfer factor (TF), which is defined as the ratio of target element concentration in the plant to that in the tailings, can be used as an index for the accumulation of a target element in the plant and its transfer from the tailings to the plant (Whicker et al. 1999).
Phytoremediation factor (PF) is defined as:
In this equation, the shoot refers to the ground tissue of the plant including the seed, leaf, and stalk. The phytoremediation factor can be used as an index for the capability of a plant to accumulate the target element from the tailings.
Results and Discussion
The characteristics of the uranium mill tailings are presented in Table 1. The pH value of the tailings ranged from 5.49 to 5.86, indicating that it was a media with weak acidity. The concentrations of U, Th, Sr, Ba, Ni and Pb in the tailings sample at each sampling site are presented in Table 2. It is obvious that there is a great difference between the minimum and maximum values of the concentrations of the determined elements in the tailings. Three explanations for this may be given. First, the tailings had the acid nature, this resulted in the dissolution of 6 target elements from the tailings and they could transport with the help of the rainfall from one site to another. Second, the tailings was deposited at the repository during different periods from 1963 to 1994, and this resulted in the different releasing rates for 6 target elements. Third, plant species growing on different sampling sites had different uptake activities for 6 target elements.
To screen the candidates for phytoremediation of uranium mill tailings containing 6 target elements, 15 dominant plant species belonging to 9 families were collected from the uranium mill tailings repository in South China (Table 2). Among the plant samples collected, Cyperus iria accumulated the highest concentration (36.4 μg/g) of U in the shoot. Juncellus serotinus accumulated the highest concentrations of Th (3.66 μg/g), Ba (179 μg/g) and Ni (25.2 μg/g) in the root. Juncellus serotinus accumulated the highest concentration of Pb (91.0 μg/g) in the shoot. Parthenocissus quinquefolia was one species which accumulated the largest amount of Sr (190 μg/g) in the shoot, but the lowest concentrations of other metals. Although the hyperaccumulators for U and Sr have not been defined so far, Baker and Brooks (1989) defined the hyperaccumulator for metals in two ways. First, the concentration of an element accumulated in an organism can be higher than that in the soil. Second, the amount of an element accumulated in an organism can be greater than that in other organisms investigated. Based on this view, Cyperus iria and Parthenocissus quinquefolia have satisfied the criteria for a hyperaccumulator for U and Sr, respectively. The highest concentration of Pb in the shoot of Juncellus serotinus was 91.0 μg/g, but this value did not satisfy the criteria for a hyperaccumulator for Pb (Baker and Brooks 1989). These results suggested that different plant species had different accumulation characteristics for a target element.
The potential of a plant to be used in phytoremediation does not merely depend on the concentration of a target element in the plant (Verma et al. 2007). It has been proposed that the plant with low dry biomass would share a low resultant capability of removing an element and not be suitable for phytoremediation though the concentration of the target element would be very high in this plant (Robinson et al. 1997). The dry biomass of the plant is considered as an important factor. In the present study, the removal capabilities of the plant samples collected for a target element was assessed by multiplying the concentration of the target element in the plant with the its dry biomass. The results are shown in Table 3. Phragmites australis had the greatest removal capabilities for U, Th and Pb. Miscanthus floridulus had the greatest removal capabilities for Ba and Ni, and Parthenocissus quinquefolia had the greatest removal capability for Sr.
The concentration of a target element in the tailings is another important factor that determines the time duration it takes to complete the phytoremediation. For preliminary search of a plant for phytoremediation, the transfer factor is one of the criteria for identification of the plant that can further be used to improve its removal capability for a target element by various breeding techniques (Whicker et al. 1999). As shown in Table 4, Cyperus iria had a higher TF for U (5.48), than the reported U accumulated plants (Shahandeh and Hossner 2002; Chen et al. 2005). But, the relatively small amount of biomass in Cyperus iria may be a limiting factor for phytoremediation in this study (Tables 2, 3). TFs for other plant species were smaller than one. The factors such as the concentration of a target element, speciation and pH of the tailings, the plant age and ecotype may modify the uptake and ratio of the content of the element in the plant shoot to that in the plant root (Florijn et al. 1993; Jiang and Singh 1994; Tu et al. 2002).
In sum, phytoremediation of the tailings depends on three parameters including the plant biomass, the target element concentration in the plant, and the target element concentration in the tailings. In order to further assess the potential of a plant for phytoremediation, a novel coefficient is proposed and termed as phytoremediation factor (PF). This factor is the ratio of the total amount of a target element accumulated in the plant shoot to the concentration in the tailings at the site where the plant grows. The PFs of the plant samples collected were calculated and presented in Table 4. As shown in Table 4, Phragmites australis has the highest PFs for U, Th, Ba and Pb, respectively. Miscanthus floridulus has the highest PF for Ni. Broussonetia papyrifera and Parthenocissus quinquefolia have the relatively high PF for Sr compared with other plants collected. PF extends the conventional definition of hyperaccumulator, and it can easily be obtained. Although the concentration of a target element in a plant does not satisfy the criteria for a hyperaccumulator, the plant may also be considered as the candidate for phytoremediation if it has relatively high biomass. Therefore, PF may provide a novel reference for identification of a plant capable of remediating the tailings and soil contaminated by the radioactive nuclides and heavy metals on a large scale. It is necessary that further studies should be performed to improve this factor, since the plant biomass at a unit area of land is not considered in this factor at present.
Conclusions
-
1.
The concentrations of the U, Th, Ba, Ni, Sr and Pb in the plant species vary greatly and depend on the accumulation characteristics of the plants and the concentrations of the elements in the tailings from sampling sites where the plants grow.
-
2.
The phytoremediation factor (PF) is proposed for the first time in this paper. It takes into consideration the concentration of a target element in a plant, the plant shoot biomass, and the concentration of the target element in the tailings or soil surrounding the root of the plant. The parameter can be used to indicate the removal capability of the plant for the target element from the tailings or soil as well as the adaptability of the plant to the environment.
-
3.
Based on the PF, Phragmites australis would be the candidate for phytoremediation of U, Th, Ba and Pb contaminated soil, Miscanthus floridulus, the candidate for phytoremediation of Ni contaminated soil, and Broussonetia papyrifera and Parthenocissus quinquefolia, the candidates for phytoremediation of Sr contaminated soil in the future.
References
AbdEl-Sabour MF (2007) Remediation and bioremediation of uranium contaminated soils. EJEAFChem 6:2009–2023
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic-a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Blanco Rodríguez PB, Vera Tome F, Lozano JC (2002) About the assumption of linearity in soil-to-plant transfer factors for uranium and thorium isotopes and 226Ra. Sci Total Environ 284:167–175
Chen SB, Zhu YG, Hu QH (2005) Soil to plant transfer of 238U, 226Ra and 232Th on a uranium mining-impacted soil from southeastern China. J Environ Radioact 82:223–236
Florijn PJ DE, Knecht JA, Van Beusichem ML (1993) Phytochelatin concentrations and binding state of Cd in roots of maize genotypes in shoot/root Cd partitioning. J Plant Physiol 142:537–542
Frostick A, BollhÖfer A, Parry D, Munksgaard N, Evans K (2008) Radioactive and radiogenic isotopes in sediments from Cooper Creek, Western Arnhem Land. J Environ Radioact 99:468–482
Gramatica P, Battaini F, Giani E, Papa E, Jones RJA, Preatoni D, Cenci RM (2006) Analysis of mosses and soils for quantifying heavy metal concentrations in Sicily: a multivariate and spatial analytical approach. Environ Sci Pollut Res 13:28–36
Huang JW, Blaylock MJ, Kapulnik Y, Ensley BD (1998) Phytoremediation of uranium-contaminated soils: role of organic acids in triggering uranium hyperaccumulation in plants. Environ Sci Technol 32:2004–2008
Jiang QQ, Singh BR (1994) Effect of different forms and sources of arsenic on crop yield and arsenic concentration. Water Air Soil Pollut 74:321–343
Landa ER (2004) Uranium mill tailings: nuclear waste and natural laboratory for geochemical and radioecological investigations. J Environ Radioact 77:1–27
Liang Q, Jing H, Conrad Gregoire D (2000) Determination of trace elements in granites by inductively coupled plasma mass spectrometry. Talanta 51:507–513
NY/T 1121 (2006) The standard for soil testing, published by ministry of agriculture of the people’s republic of China (in Chinese)
Robinson BH, Brooks RR, Howes AW, Kirkman JH, Gregg PEH (1997) The potential of the high-biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J Geochem Explor 60:115–126
Shahandeh H, Hossner LR (2002) Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut 141:165–180
Tu C, Ma LQ, Bondada B (2002) Arsenic accumulation in the hyperaccumulator Chinese brake and its utilization potential for phytoremediation. J Environ Q 31:1671–1675
Verma VK, Singh YP, Rai JPN (2007) Biogas production from plant biomass used for phytoremediation of industrial wastes. Bioresour Technol 98:1664–1669
Whicker FW, Hinton TG, Orlandini KA, Clark SB (1999) Uptake of natural and anthropogenic actinides in vegetable crops grown on a contaminated lake bed. J Environ Radioact 45:1–12
Acknowledgments
This research is supported by Scientific Research Foundation of Education Department (grant No. 10A103 and grant No. 10C1134) and Science and Technology Department (grant No. 2010GK2025) of Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Gy., Hu, N., Ding, Dx. et al. Screening of Plant Species for Phytoremediation of Uranium, Thorium, Barium, Nickel, Strontium and Lead Contaminated Soils from a Uranium Mill Tailings Repository in South China. Bull Environ Contam Toxicol 86, 646–652 (2011). https://doi.org/10.1007/s00128-011-0291-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0291-2