Abstract
Gold mining is responsible for most Hg pollution in developing countries. The aims of this study were to assess the levels of total Hg (T-Hg) in human hair, fish, water, macrophyte, and sediment samples in the gold mining district of San Martin de Loba, Colombia, as well as to determine fish consumption-based risks for T-Hg ingestion. T-Hg levels were measured by electrothermal atomization and atomic absorption spectroscopy. The overall mean T-Hg level in hair for humans in the mining district of San Martin de Loba was 2.12 μg/g, whereas for the reference site, Chimichagua, Cesar, it was 0.58 μg/g. Mean T-Hg levels were not different when considered within localities belonging to the mining district but differed when the comparison included Chimichagua. T-Hg levels in examined locations were weakly but significantly associated with age and height, as well as with fish consumption, except in San Martin de Loba. High T-Hg concentrations in fish were detected in Pseudoplatystoma magdaleniatum, Caquetaia kraussii, Ageneiosus pardalis, Cyrtocharax magdalenae, and Triportheus magdalenae, whereas the lowest appeared in Prochilodus magdalenae and Hemiancistrus wilsoni. In terms of Hg exposure due to fish consumption, only these last two species offer some guarantee of low risk for Hg-related health problems. Water, floating macrophytes, and sediments from effluents near mining sites also had high Hg values. In mines of San Martin de Loba and Hatillo de Loba, for instance, the geoaccumulation index (Igeo) for sediments reached values greater than 6, indicating extreme pollution. In short, these data support the presence of a high Hg-polluted environment in this mining district, with direct risk for deleterious effects on the health of the mining communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are a term used to describe metals and metalloids that have been associated with contamination and potential toxicity (Duffus 2002). These elements have received increasing attention due to the better understanding of their toxicological importance in ecosystems and human health. Within this group of chemicals, mercury (Hg) is considered to be one of the environmental pollutants with the greatest impact on the biosphere (Miller et al. 2011). This metal has been utilized by humans since Roman mining operations 3,500 years ago. Today, its uses are equally numerous, ranging from electronics to gold mining. The process by which many developing countries use elemental Hg to extract gold is becoming one of the primary sources of Hg pollution (Tomiyasu et al. 2013). During gold mining, Hg is employed in its elemental form to produce gold-Hg amalgams. Once it reaches the ecosystems, Hg is distributed into the air, soil, water, and sediments, forming the largest metal deposits in the environment (Martinez-Finley and Aschner 2014). Elemental Hg is highly volatile and easily dispersed at the high temperatures that often occur at sites of amalgamation. Once in the air, Hg undergoes global long-range atmospheric transport and deposition that allows its accumulation in biota, with subsequent human health risks (Falandysz et al. 2014).
People living in close proximity to artisanal mining areas are vulnerable to Hg exposure. One of the major problems of Hg is its ability to cause neurotoxicity (Woods et al. 2013) and teratogenesis (Heinz et al. 2011), as well as lesions in organs such as the liver and kidneys (Sonne et al. 2013). Much of the neurotoxicity of Hg is associated with its ability to reach the brain by binding to cysteine, which uses the neutral amino acid transporter (Aschner and Aschner, 1990). Through the same process, the complex methylmercury (CH3Hg+)-cysteine also crosses the placenta and reaches the fetus where the developing nervous system is particularly sensitive to its deleterious effects (Kajiwara et al. 1996). Therefore, prenatal life is more susceptible to brain damage than adults (Cordier et al. 2002). Methylmercury also depletes cellular glutathione, resulting in the generation of oxidative stress (Lebel et al. 1998), and inhibits Se-dependent enzymes, key molecules in preventing neuronal oxidative damage. Therefore, Se-enriched diets are recommended to ameliorate organic Hg neurotoxicity symptoms (Ralston and Raymond 2010).
In Latin America, small-scale and artisanal gold extraction is one of the leading causes of Hg release to aquatic ecosystems (Veiga 2010). Although this activity contributes significantly to rural employment in Colombia, the apparent wealth resulting from gold mining contrasts with the loss of ecosystems, as well as both health and social impairment on communities. Despite the existing alternatives for avoiding Hg use in Colombian artisanal gold extraction, Hg pollution in mining areas has been almost exclusively associated with this activity (Olivero-Verbel et al. 2011; Marrugo-Negrete et al. 2008). The inappropriate use of Hg and the poor technical knowledge on handling and recovering of this metal have facilitated Hg contamination of water bodies and the atmosphere. Hg pollution possesses a daily threat to human health in towns surrounding these mines. The main objectives of this research were to determine the extent of Hg pollution derived from gold mining in the district of San Martin de Loba, one of the most prominent gold mining areas in Colombia, as well as assess sediment contamination and the health risk due to Hg intake via fish consumption.
Materials and methods
Study area
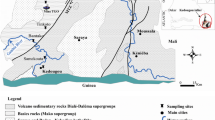
This study was conducted in the Mining District of San Martin de Loba, located in Southern Bolivar. This district registers all auriferous mining activities taking place in several municipalities, being San Martin de Loba, Barranco de Loba, and Hatillo de Loba, some of the most representative locations (Fig. 1). This area belongs to the Mompox Depression, a flood plain zone between the Magdalena and Cauca Rivers. Its main characteristic is the abundance of marshes and high temperatures throughout the year.
Geographic location of the study area in the Bolivar Department. HTL Hatillo de Loba, SML San Martin de Loba, BL Barranco de Loba, SCM Santa Cruz Mine, VKM Villa Kelly Mine, PM Palenquillo Mine, PMA Palenquillo Marsh, CM Catanga Mine, ChM Charco Marsh, CMA Chimi Marsh, MRPA Magdalena River, La Peña Area, RM Redonda Marsh
Hair collection
A total of 426 human hair samples were collected during October to December 2011 in the mining district: 200 from San Martin de Loba, 76 from Hatillo de Loba, and 167 from Barranco de Loba (Santa Cruz mine). In addition, 112 samples were obtained from Chimichagua, Cesar, a reference site located northeast of the mining area where fishing is a major economic activity, but no gold mining is present. A sample of approximately 100–200 mg of hair from the occipital scalp of each voluntary was collected using ethanol-cleaned scissors. After removal, samples were immediately deposited in white envelops, labeled, and transported to the laboratory. Hair strands were cut with stainless steel scissors into 1–2-mm pieces and stored in a desiccator before analysis. The participants were interviewed by trained health professionals who carefully explained the objectives of the study. Gathered information included sociodemographic characteristics and several possible factors that may be associated with hair Hg concentration. A written informed consent was signed by each voluntary after receiving detailed explanations of the study and its potential consequences prior to enrollment.
Collection of fish, sediment, and macrophyte samples
A total of 195 fish were caught from October 2011 to January 2012 with the help of local fishermen: 90, 6, and 99 fish specimens were obtained from San Martin de Loba, Barranco de Loba, and Hatillo de Loba, respectively. Moreover, some specimens of Pseudoplatystoma magdaleniatum (Bagre tigre) were purchased at San Martin de Loba due to their large size and food habits (carnivorous). After collection, specimens were stored in ice and transported to the laboratory. The total length and weight of each specimen were recorded. A dorsal muscle subsample was removed using plastic knives and kept at −20 °C until chemical analyses.
Forty-seven sediment samples were obtained by lowering an Eckman grab from a boat. At each station, at least three to four subsamples were collected to make a composite sample of approximately 500 g. Each sample was placed in plastic bags, labeled and packed on ice, transported to the lab, and stored at −20 °C for their subsequent freeze-drying (Labconco Freezone 2.5) at −50 °C for 20 h and then homogenized. During sediment sampling, 43 macrophyte (Eichhornia crassipes) samples were manually collected from different sites. The plants were rinsed with marsh water and transported on ice to the lab. Upon arrival, plants were washed twice with Milli-Q water, stored at −20 ° C, freeze-dried as previously described, and homogenized (Jampeetong et al. 2012; Mechora et al. 2014). Additionally, 50 water samples (250 mL per station) were collected in several sites from the mining district, and then poured into clean, acid-washed polyethylene containers, acidified with HNO3 to pH <2, and kept refrigerated until analysis within 1 week after collection (Marrugo-Negrete et al. 2008).
Mercury analysis in hair, fish, sediment, macrophytes, and water
The concentrations of total Hg (T-Hg) in human hair, fish muscle, sediments, and macrophytes were assessed employing 10, 140–160, 25, and 3–10 mg, respectively (Olivero-Verbel et al. 2009; Olivero-Verbel and Caballero-Gallardo 2013). Subsamples were pyrolyzed at 800 °C, and the vaporized Hg was detected by a RA-915+ Zeeman mercury spectrometer (Sholupov et al. 2004). T-Hg quantification was performed employing calibration curves constructed by measuring the absorbance for different weights of certified materials. Curves were considered optimal if the regression coefficient was ≥0.99. The accuracy of the method was assessed by analysis of blanks, and the use of certified reference materials such as IAEA-086 (human hair), IAEA-085 (human hair), IAEA-433 (marine sediment) from the International Atomic Energy Agency, NIES-13 (human hair) from National Institute for Environmental Studies, Japan, DORM-2 (dogfish muscle), and TORT-2 (lobster hepatopancreas) from National Research Council Canada. T-Hg in unfiltered water samples was measured using cold vapor atomic absorption spectroscopy (CVAAS) after digestion with diluted KMnO4-K2S2O8 solutions for 2 h at 95 °C (US-EPA 1994). Detection limits for analyzed matrices are reported elsewhere (Marrugo-Negrete et al. 2008).
Assessment of sediment contamination
The geochemical accumulation index (I geo) was employed as a quantitative tool to assess the level of Hg contamination in sediments. This index was calculated using Eq. 1, proposed by Muller (1969):
where Cn is the sediment metal concentration; A is the constant for modifying the fluctuation of the background value caused by lithological movement, usually 1.5 (Bhuiyan et al. 2010); and Bn is the geochemical background value of the metal. In this work, two reported background values were utilized: 0.06 (Lecce and Pavlowsky 2014) and 0.08 μg/g (Hortellani et al. 2013). Based on the results, Hg pollution in the sediment was classified into seven different categories: class 0 (unpolluted), I geo ≤ 0; class 1 (unpolluted to moderately polluted), 0 ≤ I geo ≤ 1; class 2 (moderately polluted), 1 ≤ I geo ≤ 2; class 3 (moderately to strongly polluted), 2 ≤ I geo ≤ 3; class 4 (strongly polluted), 3 ≤ I geo ≤ 4; class 5 (strongly to extremely polluted), 4 ≤ I geo ≤ 5; and class 6 (extremely contaminated), I geo > 5.
Risk-based consumption limits
Risk factors were calculated according to the guidelines of the US Environmental Protection Agency (US-EPA 1989, 2000), previously reported by different authors (Marrugo-Negrete et al. 2008; Copat et al. 2013a, b). It was assumed that the ingestion dose was equal to the adsorbed T-Hg dose and that cooking had no effect on muscle T-Hg levels (Chien et al. 2002). Hg consumption limit calculations were based on the reference dose (RfDo) set by the US-EPA for methylmercury (MeHg). In summary, this risk was calculated using Eqs. 2 and 3 for the estimated daily intake per meal (EDIm) and for the target hazard quotient (THQ):
where EDIm is the estimated daily intake of Hg per meal size; MS is the standard portion size of 230 g for adults (Hosseini et al. 2013); C is the MeHg mean concentration in fish (MS = 0.90 × T-Hg) (Marrugo-Negrete et al. 2008); BW is the body weight of 70 kg for adults (Copat et al. 2013a); THQ is the target hazard quotient, the ratio between exposure and the reference dose, which indicates that systemic effects may occur when it results above 1. RfD for MeHg is 0.1 μg/kg/day (US-EPA 1989).
The allowable number of fish meals of a specific meal size that may be consumed over a given period of time was also evaluated. For noncarcinogenic effects, we obtained the maximum allowable fish consumption rate in meals/week (CRmw) (US-EPA 2000) that would not be expected to cause any chronic systemic effects.
Considering an average adult body weight of 70 kg (US-EPA 1994), the MeHg USEPA Acceptable Daily Intake (ADI) can be approximated as 7 μg/day/adult (49 μg Hg/week) (Hosseini et al. 2013).
Data analysis
Data are presented as mean ± standard error. ANOVA was used to evaluate mean differences for T-Hg concentrations between sampling locations, previously checking for normality and variance homogeneity, using Kolmogorov–Smirnov and Bartlett tests, respectively. When normality was not achieved, Kruskal–Wallis was used instead. Spearman correlation analysis was conducted to determine associations between T-Hg and other variables. Also, Student’s t test was done to compare between genders. The criterion of significance was set at P < 0.05.
Results
Sociodemographic and other characteristics of participants
The sociodemographic and other general characteristics of the communities evaluated in this study are summarized in Table 1. No significant differences were found between mining localities regarding age (P = 0.343). However, when considering the four groups, there were differences for fish intake habits, dental amalgam filling status, education, and gender (P < 0.05).
Hair mercury concentrations
The results of the analysis of T-Hg in human hair from the mining district of San Martin de Loba are presented in Fig. 2. The average concentration for all tested samples from the mining district was 2.12 ± 0.14 μg/g (0.12–34.39 μg/g). This value is in average four-fold greater than that registered in the reference site (0.58 ± 0.05 μg/g). Hair T-Hg values within localities decreased in the order San Martin de Loba (2.50 ± 0.26 μg/g) > Barranco de Loba 1.85 ± 0.21 > Hatillo de Loba (1.74 ± 0.15 μg/g). These values exceed the internationally accepted safety limits for T-Hg (1 μg/g) (Silbernagel et al. 2011).
The over-limit ratio values for T-Hg levels in hair from people living in evaluated localities are shown in Table 2. The risk threshold level for human health used in this study was 1.0 μg/g in hair, as suggested by USEPA (Silbernagel et al. 2011). Data showed that 73 % of volunteers from San Martin de Loba, 69.7 % from Hatillo de Loba, and 76.7 % from Barranco de Loba had T-Hg concentrations greater than this level, with no significant differences detected between sites (x 2 = 1.26, P = 0.532). However, when the analysis included the reference site, significant differences were recorded between the proportions (x 2 = 111.1, P < 0.001), indicating that people from the mining district indeed had greater T-Hg concentrations than a municipality where gold mining is not a major activity.
Hair T-Hg levels according to gender are shown in Fig. 3. In all cases, male has significant greater average T-Hg levels. Outliers in the community of San Martin de Loba included three males, two miners (23.3 and 27.8 μg/g), and a fisherman (31.4 μg/g). However, in Barranco de Loba, a female, a daughter of a miner, had T-Hg level of 34.4 μg/g.
According to Spearman correlation data (Table 3), age and height of volunteers correlated with T-Hg levels for all examined localities, including the reference site, being the maximum correlation found between T-Hg and age in Chimichagua. In the case of fish intake and T-Hg, with the exception of San Martin de Loba (ρ = 0.108, P = 0.136), all localities showed significant correlations.
Fish mercury concentrations
The average muscle T-Hg concentrations in fish are depicted in Fig. 4. The highest T-Hg concentrations were observed in fish collected in marshes from San Martin de Loba, Chimi Marsh (Caquetaia kraussii, 0.45 ± 0.16 μg/g; Cyrtocharax magdalenae, 0.42 ± 0.05 μg/g; and Triportheus magdalenae, 0.39 ± 0.09 μg/g) and Charco Marsh (C. magdalenae, 0.36 ± 0.09 μg/g). In Barranco de Loba and Hatillo de Loba, C. magdalenae presented lower T-Hg levels (0.29 ± 0.06 μg/g and 0.17 ± 0.02, respectively). Interestingly, Prochilodus magdalenae (0.02–0.03 μg/g) always showed low T-Hg levels, even when captured from water bodies impacted by gold mining.
T-Hg concentrations in muscle tissue of fish caught in the mining district of San Martin de Loba, Colombia. San Martin de Loba (a–c), Barranco de Loba (d), Hatillo de Loba (e, f). Data are presented as mean ± standard error for different sampling campaigns. Dotted lines correspond to the maximum international permissible levels in fish muscle (0.5 μg/g)
Large-size specimens of P. magdaleniatum obtained from San Martin de Loba area were tested for T-Hg in several edible parts along their bodies, and the results are presented in Fig. 5. T-Hg concentrations were similar within specimens, although significant differences were found when compared to each other. As expected, T-Hg levels in these specimens increased with fish length and weight.
T-Hg in muscle of P. magdaleniatum. Numbers inside represent the sample tissue used for results shown in B (a). Average T-Hg levels in different muscle sections of specimens (S1-S3) depicted on a (b). W weight (g) and L length (cm) measurements are shown for comparison. *Statistically different when compared to muscle samples from S3
Risk-based consumption limits
Estimated EDIm, THQ, and CRmw values are presented in Table 4. Overall data indicate that for several species, the EDIm is greater than that suggested by the Joint FAO/WHO Expert Committee on Food Additive (JECFA) online database for total Hg (JECFA 2010) (0.004 mg/kg weekly, thus 0.571 μg/kg daily). Of special concern are Arenca, Mojarra amarilla, and Chango collected from San Martin de Loba, Chango from Barranco de Loba, and Blanquillo from Hatillo de Loba. These results are in agreement with calculated THQ values. In fact, in addition to species mentioned in the previous paragraph, other fish species that represent a possible risk for chronic systemic effects derived from their Hg content are Doncella and Barbul, with THQ values greater than 2.0. Although unfortunately all the species could not be obtained in all sampling stations, it is of special interest to point out that from the data gathered for C. magdalenae (Chango), with THQ values higher than 5 throughout the gold mining district, it is possible to suggest that for other fish species with similar T-Hg content, consumption should be avoided. This information, together with CRmw results, indicates that the species that can be eaten with low risk for human health are Bocachico, Coroncoro, and Comelon, being the first one of the most commercially available in the region.
Water mercury concentrations
T-Hg concentrations in water samples collected from different sites along the mining district are presented in Table 5. Although all collected samples were detected with measurable levels of T-Hg, greatest concentrations were registered in water samples collected from mills where ores are crushed at Catanga Mine, in San Martin de Loba (39.18 ± 7.50 μg/L), whereas the lowest was observed at Villa Kelly Mine, Loma de Gallo Wetland (0.17 ± 0.01 μg/L).
Sediment mercury concentrations and geoaccumulation analysis
Levels of T-Hg in sediment samples revealed high variability (Table 6). Sediments from Catanga Mine presented the highest metal concentrations (63.46 ± 25.88 μg/g), followed by Palenquillo Marsh (50.32 ± 35.58 μg/g), and Villa Kelly Mine (31.75 ± 11.85 μg/g) in Hatillo de Loba. These samples were collected in nearby streams that conduct effluents from the mines to marshes or rivers. The lowest levels were observed in Charco Marsh (0.40 ± 0.09 μg/g) at San Martin de Loba.
The I geo data for the sampling sites are presented in Table 6. Based on these results, I geo class 6, this is highly polluted sediments, which were found in San Martin de Loba (Caño and Catanga Mines) and Hatillo de Loba (Juana Sanchez, Villa Kelly Mine and Palenquillo Marsh). I geo class 4, representing moderately to highly polluted sediments, was found only in Barranco de Loba. Moderately polluted sediments with I geo classes 2 and 3 were found in two sites from San Martin de Loba.
Macrophyte mercury concentrations
Mercury content in root samples of the floating macrophyte E. crassipes collected in the mining district is shown in Fig. 6. Macrophyte roots from San Martin de Loba (Caño Mine) and Hatillo de Loba (Loma de Gallo Wetland) had the highest T-Hg concentrations (Fig. 6a; 0.48 ± 0.14 and 0.22 ± 0.06 μg/g, respectively). Mercury levels in different parts of E. crassipes (Fig. 6b) collected at the most polluted site (Caño Mine) revealed that T-Hg levels decrease in the order roots > leaves > flowers > stem.
Discussion
Mercury is a persistent environmental pollutant considered a prominent toxicant among heavy metals (McAloon and Mason 2003) due to its high toxicity and association with historic events of contamination (Kasper et al. 2007). One of the most prominent uses of Hg in Latin America is artisanal gold mining. In this work, T-Hg levels in several environmental matrices were evaluated at the Mining District of San Martin de Loba, Northern Colombia, aiming to establish human health risks based on Hg exposure. Mean T-Hg concentrations in human hair reported here were greater than those registered back in 2006 (Olivero-Verbel et al. 2011), as follows: San Martin de Loba (2.50 ± 0.26 vs 0.92 ± 0.14 μg/g), Barranco de Loba (1.85 ± 0.21 vs 1.77 ± 0.19 μg/g), and Hatillo de Loba (1.74 ± 0.15 vs 1.09 ± 0.11 μg/g). This information indicates that the increase in Hg concentration was sustained in San Martin de Loba, probably as a result of a more extensive mining process taking place in that area. However, all mining towns of this district had greater T-Hg values than those found in Chimichagua, a location where mining is not practiced and fishing is one of the major economic activities. Overall, human Hg exposure usually occurs through inhalation of elemental Hg vapor via occupational or dental amalgam exposure, or by ingestion of contaminated fish (organic mercury) (WHO 1991; Richardson 1996). As the district is surrounded by river and marshes, Hg release from gold mining may be reaching humans both as a result of direct breathing of Hg vapor or through consumption of Hg-containing fish.
In addition to be a great fishery resource, fish are considered excellent indicators for the assessment of Hg pollution (Kasper et al. 2007). In tropical ecosystems, several studies have reported that fish species with carnivorous habits accumulate more Hg than those not carnivorous (Marrugo-Negrete et al. 2008; Olivero-Verbel et al. 2009; Zhu et al. 2012). The results presented here for different fish species suggest that based on their Hg content, only few species are suitable for human consumption, in particular P. magdalenae and Hemiancistrus wilsoni, which usually report T-Hg concentrations below 0.1 μg/g fresh weight, even when caught at wetlands with consistent heavy Hg discharges from artisanal gold mining, such as the Redonda Marsh, near Santa Cruz mine (Barranco de Loba). On the other hand, species such as A. pardalis, P. magdaleniatum, C. kraussii, C. magdalenae, and T. magdalenae possess T-Hg concentrations that effectively constitute a risk for human health. Several of these species collected in other freshwater ecosystems of Colombia have similar or greater Hg levels (Marrugo-Negrete et al. 2008). The species C. magdalenae presented high concentrations of T-Hg in all sampling stations along the Mining District of San Martin de Loba. The high Hg bioaccumulation capacity of this species had not been reported in the literature.
The identification of fish species with high Hg bioaccumulation potential is of great importance for populations whose diet is based on fish, as they are the most vulnerable and the least aware of their danger. Regardless of whether these species are obtained from marshes impacted by gold mining or not, these should be avoided for human consumption, especially by children and pregnant women, since they are the most susceptible to the deleterious effects of the heavy metal (Sadagoparamanujam et al. 2011). Prenatal exposure to Hg is of particular concern because the fetal central nervous system is highly vulnerable to its effects, affecting neurodevelopment, primarily measured through decrements in IQ (Dickenson et al. 2013), although other cognitive and behavioral deficits have also been observed, including attention-deficit/hyperactivity disorder-related behavior in children (Llop et al. 2012; Jain 2013).
P. magdaleniatum is one of the most desirable species in the national market for the quality of its meat (CCI-INCODER 2007). These species usually reach large sizes, with piscivorous habits, and are highly distributed in the Magdalena basin (Carvalho-Costa et al. 2011), where the mining district is located. P. magdaleniatum samples from the Magdalena River have been found with T-Hg levels of 0.17 ± 0.06 μg/g (Alvarez et al. 2012) for specimens larger than those captured in this study. At the Dique Channel, approximately 200 km downstream of the mining district, this species has reported T-Hg levels of 0.07 ± 0.01 μg/g, suggesting a clear reduction in Hg exposure with distance from the source (Olivero-Verbel and Caballero-Gallardo 2013).
Data on consumption limits (Table 4) indicate that although the T-Hg concentrations detected in examined fish are under recommended limits (less than 0.5 μg/g), the intake per meal size in many cases exceeds the doses suggested by the JECFA (JECFA 2003, 2010). These data are the first to estimate the risk of eating fish from this mining area and confirm the potential public health risks based on Hg increases with trophic levels, the length of the food web, and body size (Gentès et al. 2013).
This research reports that most water samples have average T-Hg levels above the method detection limit (0.1 μg/L), which is a clear evidence of the extent of Hg pollution in the mining district of San Martin de Loba. The highest values of T-Hg in water were recorded in channels that collect effluents from Catanga Mine, San Martin de Loba (up to 73.2 μg/L). This value exceeded several times the T-Hg levels found in an abandoned gold mine in China (up to 9.3 μg/L) (Qiu et al. 2009). T-Hg concentrations found at Redonda Marsh and The Pacha Marsh were low (0.33 ± 0.06 μg/L), consistent with levels found in sediments and macrophytes. Interestingly, similar values were reported by Marrugo-Negrete et al. (2008) in water samples from Grande Marsh in South Bolivar.
T-Hg levels in sediments collected in the mines of the Mining District of San Martin de Loba (Table 6) reflect the high Hg contamination in liquid waste generated from gold mines. T-Hg values as high as 232.8 and 117.0 μg/g in sediments from mines at San Martin de Loba and Hatillo de Loba, respectively, demonstrated the magnitude of the problem. According to the I geo indexes, Hg pollution in these sediments classifies them as highly to strongly polluted (class 6), comparable to sediments found in an abandoned gold mining area in southern Minas Gerais State, Brazil (Cesar et al. 2011) and the Middle Odra River (Germany/Poland) (Boszke et al. 2004). These concentrations are far from 0.81 μg/g, which is the allowable limit suggested by the International Atomic Energy Agency (IAEA) (Kwaansa-Ansah et al. 2011). Due to its high density, the bulk Hg amounts deposited in nearby streams of the mining operations may move slowly to nearby marshes, polluting their sediments (Marrugo-Negrete et al. 2008).
One of the most predominant macrophyte species in the Magdalena River basin is E. crassipes (water hyacinth). This species has received considerable attention due to its ability to grow in polluted water bodies and its accumulation capacity of toxic metal ions and other contaminants (Lu et al. 2004; Mishra et al. 2007; Vesk et al. 1999; Zhu et al. 1999). Even under stress, this plant is usually able to grow without showing any symptoms of toxicity (Lage-Pinto et al. 2008). This plant is an effective Hg bioaccumulator, particularly if it is in direct contact with the sediment in shallow waters, as in water channels leading to nearby marshes (Caño mine, San Martin de Loba). In general, Hg concentrations in macrophytes from the marshes surrounding gold mines were usually fairly low, probably as a result of the short residence time experienced in these water bodies. Results presented here are similar to those reported by Marrugo-Negrete et al. (2010) in the Ayapel marsh from the Mojana Region, an ecosystem indirectly impacted by gold mining. Interestingly, T-Hg concentrations in the different organs of this macrophyte also have a differential distribution as observed in the Caño Mine, San Martin de Loba (Fig. 6), where maximum Hg values were recorded in roots, followed by leaves and flowers, and lower levels in the stems.
According to the results found in this study, environmental dynamics of Hg in the mining district of San Martin de Loba may occur in the following manner (Fig. 7). First, Hg used in gold mining is poured into the marshes and rivers where it is deposited in the sediments. Next, the Hg undergoes a process of methylation by bacteria present in it and is turned into methylmercury, which passes through planktonic organisms (phytoplankton and zooplankton) which are eaten by small fish, and these by larger fish like Bagre Tigre, Moncholo, Mojarra amarilla, and Pacora, or even bird species that are to be consumed by the population of the district. Similarly, the Hg reaches waterbodies by both wet and dry deposition, this last associated with dust particles on which Hg atoms in the air have been adsorbed in the atmosphere. Furthermore, metallic Hg deposited in sediments may pass the water column and enter air where it is dispersed. Hg from water could also be captured by aquatic plants and transported to nearby streams of the same ecosystems or, in some cases, remote sites. These plants may also die and settle to the bottom of the swamps where Hg is released, starting its biogeochemical cycle again. One aspect to highlight in the dynamics of Hg in the mining district of San Martin de Loba is that Hg not only affects people who are in direct contact with the metal in the mines. Humans living in urban areas are also affected when Hg is released into the atmosphere through burning processes of amalgamation, or directly above the ground where the miners carry contaminated mud on their shoes from the mines to the streets and houses of their municipalities.
Conclusions
Mercury pollution in the mining district of San Martin de Loba is extensive and covers environmental compartments and humans. Hg in fish from marshes located within the district possesses high risks for human health, as several species have Hg levels greater than those recommended by international agencies. This Hg is transported to marshes trough effluent channels where Hg is directly accumulated in sediments; from here, Hg biomagnification starts and moves forward through the food chain until reaching humans, who are exposed to Hg by fish consumption or by breathing elemental Hg during amalgam burning, and therefore susceptible to high risks of Hg-related health problems. While steps toward new technologies for gold extraction are been made, it is urgent to implement programs to prevent and mitigate environmental pollution by Hg.
References
Alvarez S, Kolok AS, Jimenez LF, Granados C, Palacio JA (2012) Mercury concentrations in muscle and liver tissue of fish from marshes along the Magdalena River, Colombia. Bull Environ Contam Toxicol 89(4):836–840. doi:10.1007/s00128-012-0782-9
Aschner M, Aschner JL (1990) Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav R 14(2):169–176. doi:10.1016/S0149-7634(05)80217-9
Bhuiyan MA, Parvez L, Islam M, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173(1):384–392. doi:10.1016/j.jhazmat.2009.08.085
Boszke L, Sobczynski T, Glosinska G, Kowalski A, Siepak J (2004) Distribution of mercury and other heavy metals in bottom sediments of the Middle Odra River (Germany/Poland). Pol J Environ Stud 13(5):495–502
Carvalho-Costa L, Piorski N, Willis S, Galetti JP, Ortí G (2011) Molecular systematics of the neotropical shovelnose catfish genus Pseudoplatystoma Bleeker 1862 based on nuclear and mtDNA markers. Mol Phylogenet Evol 59(1):177–194. doi:10.1016/j.ympev.2011.02.005
CCI-INCODER (Corporación Colombia Internacional CCI, Instituto Colombiano de Desarrollo Rural INCODER). (2007) Informe técnico regional cuencas del Magdalena, Sinú y Atrato. Convenio Bogotá (Colombia): CCIINCODER. Available at: http://www.agronet.gov.co/www/docs_agronet/2008924103627_Informetecnicoregional_magdalena_sinu_atrato.pdf
Cesar R, Egler S, Polivanov H, Castilhos Z, Rodrigues AP (2011) Mercury, copper and zinc contamination in soils and fluvial sediments from an abandoned gold mining area in southern Minas Gerais State, Brazil. Environ Earth Sci 64:211–222. doi:10.1007/s12665-010-0840-8
Chien LC, Hung TC, Choang KY, Yeh CY, Meng PJ, Shieh MJ, Han BC (2002) Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci Total Environ 285(1):177–185
Copat C, Arena G, Fiore M, Ledda C, Fallico R, Sciacca S, Ferrante M (2013a) Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: consumption advisories. Food Chem Toxicol 53:33–37. doi:10.1016/j.fct.2012.11.038
Copat C, Conti GO, Signorelli C, Marmiroli S, Sciacca S, Vinceti M, Ferrante M (2013b) Risk assessment for metals and PAHs by mediterranean seafood. Food Nut Sci 4(7A):10–13. doi:10.4236/fns.2013.47A002
Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, Josse D, White R, Amiel-Tison C (2002) Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res 89(1):1–11. doi:10.1006/enrs.2002.4349
Dickenson CA, Woodruff TJ, Stotland NE, Dobraca D, Das R (2013) Elevated mercury levels in pregnant woman linked to skin cream from Mexico. Am J Obstet Gynecol 209(2):e4–e5. doi:10.1016/j.ajog.2013.05.030
Duffus JH (2002) Heavy metals a meaningless term?(IUPAC Technical Report). Pure Appl Chem 74(5):793–807. doi:10.1351/pac200274050793
Falandysz J, Dryżałowska A, Saba M, Wang J, Zhang D (2014) Mercury in the fairy-ring of Gymnopus erythropus (Pers.) and Marasmius dryophilus (Bull.) P. Karst. mushrooms from the Gongga Mountain, Eastern Tibetan Plateau. Ecotoxicol Environ Saf 104:18–22. doi:10.1016/j.ecoenv.2014.02.012
Gentès S, Maury-Brachet R, Guyoneaud R, Monperrus M, André JM, Davail S, Legeay A (2013) Mercury bioaccumulation along food webs in temperate aquatic ecosystems colonized by aquatic macrophytes in south western France. Ecotoxicol Environ Saf 91:180–187. doi:10.1016/j.ecoenv.2013.02.001
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2011) Teratogenic effects of injected methylmercury on avian embryos. Environ Toxicol Chem 30(7):1593–1598. doi:10.1002/etc.530
Hortellani MA, Sarkis JE, Menezes LC, Bazante-Yamaguishi R, Pereira AS, Garcia PF, Maruyama L, Castro PM (2013) Assessment of metal concentration in the billings reservoir sediments, São Paulo State, Southeastern Brazil. J Braz Chem Soc 24(1):58–67. doi:10.1590/S0103-50532013000100009
Hosseini SM, Mirghaffari N, Sufiani NM, Hosseini SV, Ghasemi AF (2013) Risk assessment of the total mercury in Golden gray mullet (Liza aurata) from Caspian Sea. Intl J Aquat Biol 1(6):258–265
Jain RB (2013) Effect of pregnancy on the levels of urinary metals for females aged 17–39 years old: data from National Health and Nutrition Examination Survey 2003–2010. J Toxicol Environ Health Part A 76(2):86–97. doi:10.1080/15287394.2013.738171
Jampeetong A, Brix H, Kantawanichkul S (2012) Effects of inorganic nitrogen forms on growth, morphology, nitrogen uptake capacity and nutrient allocation of four tropical aquatic macrophytes (Salvinia cucullata, Ipomoea aquatica, Cyperus involucratus and Vetiveria zizanioides). Aq Bot 97(1):10–16. doi:10.1016/j.aquabot.2011.10.004
JECFA (2003) Joint FAO/WHO Expert Committee on Food Additives. Sixty-first meeting; 10–19 June; Rome. p. 9. Summary and Conclusion, JECFA/61/SC
JECFA (2010) Joint FAO/WHO Expert committee on food additives. Seventy-second meeting. Rome, 16–25 February 2010. Summary and conclusions. JECFA/72/SC. Food and Agriculture Organization of the United Nations World Health Organization. Issued 16th March 2010
Kajiwara Y, Yasutake A, Adachi T, Hirayama K (1996) Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol 70(5):310–314
Kasper D, Botaro D, Palermo EFA, Malm O (2007) Mercúrio em peixes-fontes e contaminação. Oecol Bras 11(2):228–239
Kwaansa-Ansah E, Agorku S, Nriagu J (2011) Levels of total mercury in different fish species and sediments from the Upper Volta Basin at Yeji in Ghana. Bull Environ Contam Toxicol 86(4):406–409. doi:10.1007/s00128-011-0214-2
Lage-Pinto F, Oliveira JG, Da Cunha M, Souza CM, Rezende CE, Azevedo RA, Vitória AP (2008) Chlorophyll fluorescence and ultrastructural changes in chloroplast of water hyacinth as indicators of environmental stress. Environ Exp Bot 64(3):307–313. doi:10.1016/j.envexpbot.2008.07.007
Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J (1998) Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res 79(1):20–32. doi:10.1006/enrs.1998.3846
Lecce SA, Pavlowsky RT (2014) Floodplain storage of sediment contaminated by mercury and copper from historic gold mining at Gold Hill, North Carolina, USA. Geomorphology 206:122–132
Llop S, Guxens M, Murcia M, Lertxundi A, Ramon R, Riaño I, Rebagliato M, Ibarluzea J, Tardon A, Sunyer J, Ballester F (2012) Prenatal exposure to mercury and infant neurodevelopment in a multicenter cohort in Spain: study of potential modifiers. Am J Epidemiol 175(5):451–465. doi:10.1093/aje/kwr328
Lu X, Kruatrachue M, Pokethitiyook P, Homyok K (2004) Removal of cadmium and Zinc by Water Hyacinth Eichhorrnia crassipes. Sci Asia 30:93–103
Marrugo-Negrete J, Benitez LN, Olivero-Verbel J (2008) Distribution of mercury in several environmental compartments in an aquatic ecosystem impacted by gold mining in northern Colombia. Arch Environ Contam Toxicol 55(2):305–316. doi:10.1007/s00244-007-9129-7
Marrugo-Negrete J, Benítez LN, Olivero-Verbel J, Lans E, Gutierrez FV (2010) Spatial and seasonal mercury distribution in the Ayapel Marsh, Mojana region, Colombia. Inter J Environ Health Res 20(6):451–459. doi:10.1080/09603123.2010.499451
Martinez-Finley EJ, Aschner M (2014) Recent advances in mercury research. Curr Environ Health Rep 1(2):163–171. doi:10.1007/s40572-014-0014-z
McAloon KM, Mason RP (2003) Investigations into the bioavailability and bioaccumulation of mercury and other trace metals to the sea cucumber, Sclerodactyla briareus, using in vitro solubilization. Mar Pollut Bull 46(12):1600–1608. doi:10.1016/S0025-326X(03)00326-6
Mechora Š, Germ M, Stibilj V (2014) Monitoring of selenium in macrophytes—the case of Slovenia. Chemosphere 111:464–470. doi:10.1016/j.chemosphere.2014.03.133
Miller MB, Gustin MS, Eckley CS (2011) Measurement and scaling of air–surface mercury exchange from substrates in the vicinity of two Nevada gold mines. Sci Total Environ 409(19):3879–3886. doi:10.1016/j.scitotenv.2011.05.040
Mishra KK, Raí UN, Prakash O (2007) Bioconcentration and phytotoxicity of Cd in Eichhornia crassipes. Environ Monit Assess 130:237–243. doi:10.1007/s10661-006-9392-5
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2(3):108–118
Olivero-Verbel J, Caballero-Gallardo K (2013) Nematode and mercury content in freshwater fish belonging to different trophic levels. Parasitol Res 112(6):2187–2195. doi:10.1007/s00436-013-3378-3
Olivero-Verbel J, Caballero-Gallardo K, Negrete-Marrugo J (2011) Relationship between localization of gold mining areas and hair mercury levels in people from Bolivar, North of Colombia. Biol Trace Elem Res 144(1–3):118–132. doi:10.1007/s12011-011-9046-5
Olivero-Verbel J, Caballero-Gallardo K, Torres-Fuentes N (2009) Assessment of mercury in muscle of fish from Cartagena Bay, a tropical estuary at the north of Colombia. Inter J Environ Health Res 19(5):343–355. doi:10.1080/09603120902749090
Qiu G, Feng X, Wang S, Fu X, Shang L (2009) Mercury distribution and speciation in water and fish from abandoned Hg mines in Wanshan, Guizhou province, China. Sci Total Environ 407(18):5162–5168. doi:10.1016/j.scitotenv.2009.06.007
Ralston NV, Raymond LJ (2010) Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 278(1):112–23. doi:10.1016/j.tox.2010.06.004
Richardson M (1996) The safety of dental amalgam, ISBN 0-662-24873-2. Minister of Health, Canada
Sadagoparamanujam V, Wilson DT, Ramanujam CL, Lederman RP, Grady JJ, Alcock NW (2011) Mercury exposure through diet in pregnant women and women of childbearing age. Toxicol Environ Chem 93(10):2098–2110. doi:10.1080/02772248.2011.625621
Sholupov S, Pogarev S, Ryzhov V, Mashyanov N, Stroganov A (2004) Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Process Technol 85(6):473–485. doi:10.1016/j.fuproc.2003.11.003
Silbernagel SM, Carpenter DO, Gilbert SG, Gochfeld M, Groth E, Hightower JM, Schiavone FM (2011) Recognizing and preventing overexposure to methylmercury from fish and seafood consumption: information for physicians. J Toxicol 2011:1–7. doi:10.1155/2011/983072
Sonne C, Leifsson PS, Dietz R (2013) Liver and renal lesions in mercury-contaminated narwhals (Monodon monoceros) from North West Greenland. Toxicol Environ Chem 95(3):1–14. doi:10.1080/02772248.2013.783666
Tomiyasu T, Kono Y, Kodamatani H, Hidayati N, Rahajoe JS (2013) The distribution of mercury around the small-scale gold mining area along the Cikaniki river, Bogor, Indonesia. Environ Res 125:12–19. doi:10.1016/j.envres.2013.03.015
US-EPA (1989) Risk Assessment Guidance for Superfund, vol. I. Human Health Evaluation Manual (Part A), Interim Final. EPA 540/1–89/002. United States Environmental Protection Agency, Washington, DC
US-EPA (1994) Methods 245.1 for determination of mercury in water. U.S. Environmental protection Agency. Cincinnati. Ohio
US-EPA (2000) Guidance for Assessing Chemical Contamination Data for Use in Fish Advisories, vol. II. Risk Assessment and Fish Consumption Limits EPA/823-B94-004. United States Environmental Protection Agency, Washington, DC
Veiga M (2010) Antioquia, Colombia: the world’s most polluted place by mercury: impressions from two field trips. United Nations Industrial Development Organization, Vienna, pp 1–24
Vesk PA, Nockolds CE, Allaway WG (1999) Metal localization in water hyacinth root from an urban rainyland. Plant Cell Environ 22:149–158
Woods JS, Heyer NJ, Russo JE, Martin MD, Pillai PB, Farin FM (2013) Modification of neurobehavioral effects of mercury by genetic polymorphisms of metallothionein in children. Neurotoxicol Teratol 39:36–44. doi:10.1016/j.ntt.2013.06.004
World Health Organization (1991) Inorganic mercury: environmental health criteria 118, in International Programme on Chemical Safety. World Health Organization, Geneva
Zhu L, Yan B, Wang L, Pan X (2012) Mercury concentration in the muscle of seven fish species from Chagan Lake, Northeast China. Environ Monit Assess 184(3):1299–1310. doi:10.1007/s10661-011-2041-7
Zhu YL, Zayed AM, Qian JH, De Souza M, Terry N (1999) Phytoaccumulation of trace elements by wetland plants: II. Water Hyacinth. J Env Qual 28(1):339–344. doi:10.2134/jeq1999.00472425002800010042x
Acknowledgments
The authors thank the Program to Support Research Groups, sponsored by the Vice-Rectory for Research of the University of Cartagena (2013–2014); the Ph. D Program in Environmental Toxicology at the same institution; the National Program for Doctoral Formation (COLCIENCIAS, 567–2012); the Government of Bolivar State, Colombia; and Leonor Cervantes.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Olivero-Verbel, J., Caballero-Gallardo, K. & Turizo-Tapia, A. Mercury in the gold mining district of San Martin de Loba, South of Bolivar (Colombia). Environ Sci Pollut Res 22, 5895–5907 (2015). https://doi.org/10.1007/s11356-014-3724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3724-8