Abstract
Mercury (Hg) used in gold amalgamation is a major source of contamination in developing countries. Water, sediments, plankton, fish, and human samples from Grande Marsh, a Hg-polluted marsh located in the south of Bolívar, municipality of Montecristo, Colombia, were collected during both the rainy and the dry seasons (2003–2006), at three different sampling sites, and analyzed for total Hg (T-Hg) content. Water, sediment, seston, phytoplankton, and zooplankton T-Hg concentrations were 0.33 ± 0.03 μg/L, 0.71 ± 0.03, 1.20 ± 0.06, 0.52 ± 0.03, and 0.94 ± 0.05 μg/g dry weight (wt), respectively. T-Hg levels in these compartments were highly pair-correlated (p < 0.05), and for all of them, except sediments, greater values were found during the dry season. Significant differences were observed for T-Hg concentrations in fish according to their trophic position. Average highest T-Hg values were found in carnivorous species such as Caquetaia kraussi (1.09 ± 0.17 μg/g fresh wt), Hoplias malabaricus (0.58 ± 0.05 μg/g fresh wt), and Plagioscion surinamensis (0.53 ± 0.07 μg/g fresh wt), whereas the lowest were detected in noncarnivorous species such as Prochilodus magdalenae (0.157 ± 0.01 μg/g fresh wt). In those fish species where seasonal comparisons were possible, specimens captured during the dry season had greater T-Hg levels in muscle. Although the T-Hg mean level for all fish samples (0.407 ± 0.360 μg/g fresh wt) did not exceed the recommended limit ingestion level, risk assessment based on the hazard index suggested that a fish intake of 0.12 kg per day (a small carnivorous specimen) could increase the potential health effects related to Hg exposure in the local human population, whose hair T-Hg median value was 4.7 μg/g, and presented a low but significant correlation with fish consumption (r = 0.250, p = 0.016). In short, Hg pollution from gold mining around Grande Marsh has permeated the food web, and currently levels in fish represent a serious concern for human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) is a priority pollutant due to its persistence, bioaccumulation and toxicity in the environment (Clarkson and Magos 2006). It may occur either naturally or as a result of anthropogenic activity. Globally, the major source of Hg to freshwater systems is atmospheric deposition from natural and anthropogenic sources (Swain et al. 1992; Morel et al. 1998), although discharges from industry, mining activities, and watershed runoff may increase regional loadings (Gill and Bruland 1990). In the environment, Hg deposited in sediments is converted into methylmercury (MeHg) by biomethylation of the inorganic form, and this species is bioaccumulated and biomagnified in food chains (WHO 1989; Morel et al. 1998; Ullrich et al. 2001), where, for example, fish might have more than 90% of their T-Hg content as MeHg (Lacerda et al. 1994; Morel et al. 1998). The presence of organic Hg in fish not only results in deleterious effects to themselves (Basu et al. 2007; Sandheinrich and Miller 2006), but also constitutes the major source of human exposure through fish consumption (Kehrig et al. 1998; WHO 1990).
Despite the fact that there is enough international awareness regarding Hg contamination, especially after the Minamata disaster, in regions such as Latin America, Southeast Asia, and sub-Saharan Africa, environmental pollution by this metal is a growing problem due to the expansion of artisanal gold mining during the past three decades (Appleton et al. 2001). This has been widely documented in the Amazon River basin (Lodenius and Malm 1998), but few works have been undertaken in other tropical zones where the use and discharges of the metal are massive and there exists less information on the ecology and natural history of species. A particular case is the north of Colombia, where the Magdalena and Cauca rivers form a vast complex of marshes rich in biodiversity but threatened by extensive artisanal gold-mining activities.
In Colombia, gold mining is the major contributor to Hg pollution (Olivero et al. 2004; Olivero and Johnson 2002; Olivero and Solano 1998). Artisanal gold mining occurs on a relatively large scale mainly in the south of Bolivar and northeast of Antioquia mining areas, where it has been estimated that ∼50,000 people are involved in these activities using Hg amalgamation (Olivero et al. 2002), and because of the lack of technologies to reduce Hg emissions and discharges, the metal is easily deposited in the sediments of nearby marshes, where Hg distribution in different compartments depends on factors such as sediment input (Kongchum et al. 2006), habitat type (Heim et al. 2007), and the presence of specific microenvironments that facilitate Hg methylation (Guimarães et al. 2000), among others.
Among the most important freshwater resources in Colombia is the Grande Marsh in the municipality of Montecristo, south of Bolívar. This ecosystem is strategic because it controls flooding events and regulates water currents from rivers, and it has diverse wildlife species, most of them pivotal for survival of nearby communities. Unfortunately, this body of water receives gold-mining residues from the Caribona River, where extensive gold mining is taking place. In its watershed, gold is recovered mostly from alluvial deposits and to a minor extent from underground mines. Dredge work is the method used to extract sedimentary deposits from the watercourse. These materials are subjected to particle size selection and sometimes to gravity concentration and then treated with elemental Hg for gold amalgamation. The gold-rich amalgam is then burned in open air to obtain the precious metal, without proper equipment to recover the Hg. This generally occurs along riverbanks, and Hg reaches the watercourse and the atmosphere during the amalgamation and the burning process, respectively. Similar gold-mining methods have also been described for the Brazilian Amazon (Kligerman et al. 2001).
The main objective of this paper is to describe Hg levels for different environmental matrices in this ecosystem and to evaluate changes in Hg distribution along different sampling sites and seasons, assessing environmental and human exposure to Hg.
Materials and Methods
Study Area
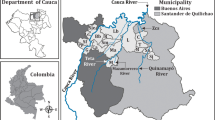
The Grande marsh is located in northwest Colombia (8°16’–8°22’N and 74°26’–74°32’W), covering an area of approximately 38 km2 (Fig. 1). It is located 30 m above sea level and within the largest gold production area in the country (southern Bolivar and northeastern Antioquia). The mean annual precipitation and temperature are 2350 mm and 27°C, respectively. The marsh is part of the Cauca River basin and receives waters from the Caribona River and the Dorada creek. The maximum water level occurs from July to November, and the lowest level in March, with changes that reach up to 3.3 m. The dry season goes from January to April, and the rainy season from May to December, with maximum and minimum water levels between July and November, and between December and March, respectively.
Sample Collection and Treatment
Environmental matrices and collection dates are presented in Table 1. Composite water samples (2 L per station) were collected with a polycarbonate Van Dorm bottle at three depths (bottom, middle, and 30 cm below the surface of the water) and then poured into clean, acid-washed polyethylene (HDPE) containers. Samples were acidified with HNO3 to pH < 2 and kept refrigerated until analysis within 1 week after collection.
Sediment samples were obtained by lowering a Van Veen grab from a boat. At each station four subsamples were collected at all cardinal points within a 3-m radius from the reference point. Samples were placed in plastic bags, labeled and packed in ice, transported to the lab, and dried at 40°C in a drying oven.
Phytoplankton and zooplankton samples were collected using 10-cm-diameter, 64- and 150-μm-mesh plankton nets, respectively (Watras et al. 1998). Samples were rinsed with marsh water and drained directly from the cod-end bucket into 1-L plastic bottles, double bagged, and transported on ice to the lab. Upon arrival, samples were centrifuged at 2000 g and the pellets dried at room temperature in a desiccator until a constant weight was achieved (Ullrich et al. 2007). Composite seston samples were taken as previously described for water. Samples were vacuum-filtered through 0.45-μm-mesh cellulose acetate membranes. All micro-organisms and particles retained on the membrane surface are designated here as seston (Morrison and Watras 1999; Caetano and Vale 2003), and they were dried at room temperature prior to analysis.
Local fishermen gathered fish during fishing campaigns. Selected species were those considered the most economically important, and all of them could be stratified into two different categories based on food habits: carnivorous and noncarnivorous. After length was measured, fish were eviscerated by removing all internal organs, placed individually in plastic bags, labeled, and transported on ice to the lab. Samples for analysis were obtained from dorsal muscle dissected with a plastic knife (Olivero et al. 1998).
Hair samples were collected from fishermen and their families living in La Raya, a small-scale fishing community located northwest of Grande Marsh. Hair strands taken closest to the scalp were removed from the occipital region and then placed in white envelopes with the root end stapled.
Great care was taken to avoid contamination during collection and preparation of the samples. The nets and bottles were carefully rinsed with filtered marsh water before and after each use and stored in clean plastic containers with a lid. Plastic gloves were used at all times throughout the collection and handling of samples. At the lab, all glasware used was cleaned with HCl and HNO3 and subsequently rinsed three times with double-distilled water.
Mercury Analysis
T-Hg in unfiltered water samples was measured using cold vapor atomic absorption spectroscopy (CVAAS) after digestion with diluted KMnO4-K2S2O8 solutions for 2 h at 95°C (USEPA 1994). Detection limit was 0.1 μg/L. Sediments and seston were digested with H2SO4-HNO3 (7:3, v/v) and KMnO4 (5%, w/v) at 100°C for 1 h (USEPA 1998), whereas fish and other biological samples were treated with H2SO4-HNO3 (2:1, v/v) at 100–110°C for 3 h (Sadiq et al. 1991). Detection limits for sediments and biological samples were 26.4 μg/kg dry weight (wt) and 13.1 μg/kg fresh wt, respectively. Quality control was performed with certified materials and spiked samples. The T-Hg concentration for the biological material (DORM-2, dogfish muscle; National Research Council Canada) was 4.46 ± 0.25 μg/g dry wt (certified value, 4.64 ± 0.26 μg/g dry wt), and that for sediments (CRM008-050; Resource Technology Corp.) was 0.74 ± 0.02 μg/g dry wt (certified value, 0.72 ± 0.03 μg/g dry wt). Hg recovery percentages from the spiked samples were 98.0 ± 4.2% and 95.2 ± 4.3% (n = 6) for sediments and biological material, respectively. In both methods the RSD was <10%. T-Hg concentrations in sediments, seston, and plankton are reported on a dry weight basis, whereas those in fish are expressed on a fresh or wet weight basis. Analyses of T-Hg in hair were performed by direct pyrolysis coupled with atomic absorption spectrometry (Sholupov et al. 2004), and calibration curves were prepared using certified materials (IAEA-085 and IAEA-086). Detection limit for hair T-Hg oscillated between 0.01 and 0.05 μg/g. Organic matter (OM) content in sediments was determined as reported by Coquery and Welbourn (1995). pH was measured in the sediment slurry using a pH-meter (Hanna Instruments, Italy) calibrated with its respective solutions before every determination.
A Hazard Index (HI) approach based on the EPA’s Risk Assessment Guidance for Superfund (USEPA 1989) was calculated to assess the risk of deleterious effects of MeHg exposure through fish consumption. HI is defined as the ratio of a single substance exposure level (E) to a reference dose (RfD), the latter calculated as the concentration of a particular chemical that is assumed to be safe to ingest on a daily basis during a lifetime. According to the USEPA (1989), the RfD for MeHg is 0.1 μg/kg/day. Exposure due to MeHg consumption was obtained using the formula, E = (C × I)/W, where C is the MeHg mean concentration in fish (C = 0.90 × T-Hg), taking into account that 90% of the T-Hg in fish species has been found to be MeHg (Morel et al. 1998), I is the fish daily consumption (g/day), and W is the mean weight for a normal adult person (70 kg). Accordingly, when HI exceeds unity, there may be concern for potential health effects, in particular, for pregnant women and children, who are prone to develop neurological damage due to MeHg ingestion.
Statistical Analysis
Data for Hg analysis are presented as mean ± standard error or mean ± confidence interval (CI). Data normality and equality of variance were assessed using the Kolmogorov-Smirnov (K-S) and Barlett’s test, respectively. ANOVA was used to compare means between stations, followed by Tukey’s post-test. Kruskal-Wallis was applied when data did not follow a normal distribution and previous transformation did not normalize them. Correlation or multiple linear regression analysis was used to establish relationships between two variables. For all purposes, significance was set at p < 0.05.
Results
Mercury in Water and Sediments
Water and sediment T-Hg concentrations in different sampling sites are presented in Fig. 2. T-Hg concentration in water was 0.33 ± 0.03 μg/L (range, 0.16–0.46 μg/L), and on average, the T-Hg value at sampling site 1 (S1) was 41% greater than those observed at sites 2 and 3 (S2 and S3). Pooled data for all stations showed significant differences (p = 0.027) between rainy (0.25 ± 0.02 μg/L) and dry (0.32 ± 0.03 μg/L) seasons. In sediments, T-Hg concentration was 0.71 ± 0.03 μg/g dry wt (range, 0.49–1.08 μg/g dry wt); and similarly to the results observed for water, S1 had significant greater T-Hg concentrations than the other sampling sites. In contrast to T-Hg in water, sediment data considering all sampling sites revealed nonseasonal changes for T-Hg (p = 0.838). Organic matter and pH levels in sediments are reported in Table 2. No statistical differences were observed for these parameters according to sampling site.
Mercury in Seston, Phytoplankton, and Zooplankton
T-Hg levels found for seston, phytoplankton, and zooplankton at different sampling sites are presented in Fig. 3. Average values for pooled data were 1.20 μg/g dry wt (95% CI, 1.077 to 1.319 μg/g dry wt), 0.52 μg/g dry wt (95% CI, 0.459 to 0.573 μg/g dry wt), and 0.94 μg/g (95% CI, 0.823 to 0.966 μg/g dry wt), respectively. Seasonal changes in T-Hg content for phytoplankton and zooplankton collected at Grande Marsh are shown in Fig. 4. In these organisms, as well as in seston (rainy season, 1.06 ± 0.04 μg/g dry wt; dry season, 1.47 ± 0.04 μg/g dry wt, p < 0.001), T-Hg concentrations were greater during the dry season. Correlations between T-Hg levels in water, sediment, seston, phytoplankton, and zooplankton are presented in Table 3. All variables were significantly correlated (p < 0.05).
Mercury in Fish
T-Hg levels found in fish from Grande Marsh are presented in Fig. 5. Average T-Hg concentration in fish muscle was 0.407 ± 0.360 μg/g fresh wt. Carnivorous and noncarnivorous fish species had statistical differences (p < 0.05) for average T-Hg concentrations in muscle, with values of 0.534 ± 0.292 and 0.157 ± 0.067 μg/g fresh wt, respectively. Consequently, a ratio of 3.4:1 was found between these two trophic levels. Highest mean values within carnivorous species were found in Caquetaia kraussi (1.09 ± 0.17 μg/g fresh wt), Hoplias malabaricus (0.58 ± 0.05 μg/g fresh wt), and Plagioscion surinamensis (0.53 ± 0.07 μg/g fresh wt), whereas the lowest were measured in the noncarnivorous species (iliophagus) Prochilodus magdalenae (Bocachico) (0.157 ± 0.01 μg/g fresh wt). For all collected samples, the greatest concentration was recorded in a specimen of Plagioscion surinamensis (pacora; 2.04 μg/g fresh wt). No statistical differences in T-Hg levels were found between carnivorous species (p > 0.05). For some species (Fig. 5), statistical differences in T-Hg levels were found between the rainy and the dry season (p < 0.05), with highest values detected during the dry season. Linear regressions assessed to quantify the relationships between fish length and T-Hg in muscle are presented in Fig. 6. Regression slopes for carnivorous species were greater than those observed for noncarnivorous species.
Mercury in Human Hair
Frequency distribution of Hg levels in fishermen and their families living around Grande Marsh are presented in Fig. 7. These data do not follow a normal distribution (K-S distance = 0.126, p < 0.001). Spearman correlations between T-Hg values in hair and some anthropometrical parameters as well as fish consumption frequency are presented in Table 4. Human age, stature, and weight did not correlate significantly with T-Hg in hair. In contrast, a low but significant correlation was observed between fish consumption and T-Hg levels.
HIs for the noncarcinogenic effects of T-Hg based on a fish consumption of 120 g/day by people from Grande Marsh are presented in Table 5. Lowest and highest HI values were detected for the noncarnivorous fish species Prochilodus magdalenae (1.17) and the carnivorous species Plagioscion surinamensis (31.47), respectively.
Discussion
Mean T-Hg concentration in water from Grande Marsh (330 ± 30 ng/L) is 1.8 orders of magnitude greater than that estimated to be present in pristine freshwater ecosystems (5 ng Hg/L) (ATSDR 1997), and it is typical of sites severely polluted with Hg, such as those close to thermometer factories (Karunasagar et al. 2006). Water T-Hg values found in this study are also moderately greater than those reported for other rivers contaminated with artisanal gold mining in Ghana (162–164 ng/L [Donkor et al. 2006]) and Indonesia (up to 250 ng/L [Limbong et al. 2005]) but considerably lower than those observed in rivers located around gold-mining operation areas in Brazil (up to 800 ng/L [Palheta and Taylor 1995]). Interestingly, in some specific Amazonian rivers, such as in the Negro River, with low pH values and no history of Hg pollution, T-Hg levels in water can be as high as 3400–11,900 ng/L (Barbosa et al. 2003).
T-Hg concentrations found in sediments from Grande Marsh were greater than those reported for Mina Santa Cruz Marsh (gold-mining site), south of Bolivar, Colombia (0.140–0.355 μg/g dry wt [Olivero and Solano 1998]) and, also, higher than those observed in marshes located 40 km north of our study area (village of Caimito, San Jorge River basin: 0.155 ± 0.016 μg/g dry wt [Olivero et al. 2004]). On the other hand, considering a Hg threshold of 0.2 μg/g as the level of concern for Hg in continental water sediments (Salomons and Förstner 1984), on average, this zone of the Cauca River basin is categorized as highly polluted.
Based on sediment T-Hg levels, sampling sites represent two zones with significant differences between them (p < 0.05). Sampling site 1 (S1), located in the southwest part of the marsh, has higher concentrations, and it receives a direct impact from gold mining along the Caribona River, where Hg used during gold amalgamation reaches the river together with washed sediments. Recently, experiments using indoor microcosms have shown that sediments from tropical forest can undergo methylation of elemental Hg droplets, linking the amalgamation process with Hg transfer into the food chain (Dominique et al. 2007). Eventually, Hg from atmospheric deposition can also be taking place in Grande Marsh due to its proximity to the extraction sites, where amalgam burning releases this metal into the air (Mirlean et al. 2005). The second zone (S2 and S3), with a lower T-Hg concentration, corresponds to the middle and northeast area of the marsh, where contact with the gold-mining waters is less extensive. As shown in Fig. 1, Grande Marsh is small enough to allow for rapid mixing of water and sediments, and this may explain the relatively same T-Hg distribution in S2 and S3. However, independent from the sampling station, in this body of water, Hg methylation in sediments by sulfate reducing bacteria could be favored due to the their high organic matter content (9.24 ± 0.12%), relatively low pH levels (6.63 ± 0.01), and high temperatures (Morel et al. 1998; Ullrich et al. 2001).
The fact that current levels are far greater than 0.05 μg/g dry wt, the background T-Hg concentration reported for a similar marsh in the gold-mining area of Colombia (Olivero and Solano 1998), as well as the differences observed in T-Hg concentrations at the sampling stations (greater values at the station closer to Caribona River), suggests that this ecosystem is been impacted by gold-mining activities undertaken upstream of Caribona River. In Colombia, metallic Hg is directly incorporated into aquatic ecosystems and atmosphere from gold amalgamation and amalgam burning processes, respectively, without any significant effort to recover the metal (Olivero and Johnson 2002). Although Hg input into freshwater ecosystems from gold-mining areas in northern Colombia is similar to that observed for other South American countries (Appleton et al. 2001, 2006; Lacerda et al. 1994), it should be pointed out that environmental conditions in those marshes are different, compared, for instance, with some Amazonian ecosystems, where forest flooding is a crucial factor for the methylation rate of Hg, a critical step in its biomagnification potential (Guimarães et al. 2000).
Mean T-Hg values found in sediments from Grande Marsh were lower than those detected in seston, whereas T-Hg levels in zooplankton were greater than in phytoplankton, with significantly higher values recorded for sampling site 1 (S1) in all these compartments as well as in water. Moreover, T-Hg values in sediments strongly correlated with T-Hg in water, seston, phytoplankton, and zooplankton. These observations are particularly relevant because they suggest that Hg is incorporated into the plankton not only directly from the bottom sediments, but also from re-suspended material and water. As zooplankton constitutes the first primary consumers, Hg present in these organisms is available to be transferred into the higher levels of the food web that feed on them, as changes in zooplankton density could determine Hg levels in both herbivorous and predatory fish (Chen and Folt 2005). Although some studies have pointed out the importance of specific zooplankton herbivore species in the biomagnification of Hg (Pickhardt et al. 2005), all those present in the seston could work as short-distance carriers for Hg and allow its distribution along the nearby ecosystems. Besides geographical differences, significant seasonal changes in T-Hg levels were also found for both phytoplankton and zooplankton, with greater values during the dry season (Fig. 4). This result is surprising considering that during the rainy season Hg deposited within the river sediments is resuspended and more Hg is going to be deposited mainly at sampling site 1, the closest point to the gold-mining area. On the other hand, the observed trend might not be related to sediment resuspension as seen in other watersheds (Gammons et al. 2006). Alternatively, it could involve changes in water physicochemical and biological variables, a more pronounced mining activity during the dry season that could account for more Hg release, and decreased water volumes (Hylander et al. 2000b), among others factors.
A comparison between the T-Hg levels found in sediments, seston, zooplankton, and phytoplankton from Grande Marsh with those measured in other locations around the world (Table 6) revealed that, at least in terms of sediments, they can be considered high with respect to other gold mining polluted sites and, of course, compared with places without a Hg history. However, actual values reported here are not as dramatic as those seen in contaminated places in Ecuador and Peru.
The highest T-Hg values found in fish during the dry season are probably due to more primary production, leading to increased Hg bioavailability in the food chain, augmented gold-mining operations, reduced water column, and changing habitats, among other factors (Zapata 1994; Hylander et al. 2000a). Although the overall mean T-Hg concentration was found to be <0.5 μg/g fresh wt, the maximum T-Hg concentration that should be present in fish for human consumption according to the World Health Organization (WHO 1990), 40 fish specimens (24 Hoplias malabaricus and 16 Plagioscion surinamensis), representing 30.0% of the total collected, presented values above this limit, most of them during the dry season (Figs. 5 and 6). To protect vulnerable people, in particular, pregnant women, those under 15 years of age, and frequent fish consumers, the WHO (1990) has recommended the lower T-Hg guideline of 0.2 μg/g fresh wt for those groups. T-Hg concentrations found in most carnivorous and some noncarnivorous species were higher than 0.2 μg/g, levels greater than those detected in similar species collected at Capote Marsh, a low-polluted body of water located approximately 250 km north of the study site (Olivero et al. 1998), and in other zones subjected to Hg pollution in Colombia (Olivero et al. 2004; Olivero and Solano 1998), but similar to those reported for gold-mining sites in Brazil (Barbosa et al. 2003; Malm et al. 1997; Porvari et al. 1995).
According to the relationships between fish length and T-Hg in muscle (Fig. 6), regression slopes for bivariate data on carnivorous species were greater than those observed for noncarnivorous species, indicating a more efficient Hg accumulation. In the case of the iliophagus Prochilodus magdalenae, exposure may change along the life span due to its migratory behavior. Correlation analysis performed on pooled data for all specimens showed that T-Hg in fish increases with size (r = 0.75, p < 0.001). Usually older individuals show higher Hg levels than younger ones as a consequence of a longer exposure time (Dixon and Jones 1994; Mauk and Brown 2001).
Median T-Hg concentration for T-Hg in hair from volunteers living around Grande Marsh was 4.7 μg/g, a value that is close to those published for polluted sites in Brazil (Telmer et al. 2006) but almost five times greater than the value recommended to protect pregnant woman and children from Hg poisoning (USEPA 2000). The low but significant correlation detected between T-Hg values in human hair and fish consumption (r = 0.250, p = 0.016), was also found for Caimito, another municipality close to Grande Marsh (Olivero et al. 2002).
The consumption of fish contaminated with Hg may represent a risk for human health. At a screening level, the EPA HI approach (USEPA 1989) assumes that there is a level of exposure for noncarcinogenic substances below which it is unlikely for even sensitive populations to experience adverse health effects. Results presented here (Table 5) revealed that there is a potential human health risk for people who consume fish from Grande Marsh, in particular, carnivorous fish.
Human T-Hg concentrations together with the HI data presented here should encourage more extensive and intensive ecological and environmental health studies in the region by local government. Although fish are considered the main source of Me-Hg exposure, they are the only source of protein from the daily food intake of many populations in this region. In addition, it should be pointed out that, besides fish eaters, miners are exposed to Hg but in its inorganic form, and this could also end up in severe health problems (Eisler 2004; Counter 2003) that require special attention from health authorities.
Until now, most research related to Hg pollution in Latin American countries has focused on Hg in rivers, and although much is generally known about Hg biomagnification in these freshwater ecosystems, little has been reported on Hg levels in all basic compartments of a food web in a polluted marsh. An integrated figure showing Hg levels in different compartments of the Grande Marsh is presented here (Fig. 8). On average, Hg levels at the top of the food web are more than four orders of magnitude greater than those found in water.
T-Hg concentrations in different environmental compartments of the Grande Marsh ecosystem. Average concentrations are shown for each component in the food web. For comparison purposes, values for T-Hg in fish are also reported as dry weight using a 75% moisture content for fish tissue, an average value obtained from those reported by several authors (78.5% [Schmitt and Brumbaugh 2007], 72.2% [Butala et al. 2007], 69–82% [Kojadinovic et al. 2006])
Taken together, these results show evidence that Grande Marsh is heavily impacted by gold mining and that Hg present in sediments and water has contaminated food webs to such extent that human fish consumption represents a potential health risk. Governmental intervention in several aspects, such as applying law regulations to develop environmentally friendly gold-mining practices, educational programs about Hg pollution, programs for forest protection in river basins, and reforestation, among others, would be pivotal action to protect this food source from Hg contamination.
References
Appleton J, Williams T., Orbea H, Carrasco M (2001) Fluvial contamination associated with artisanal gold mining in the Ponce Enríquez, Portovelo-Zaruma and Nambija areas, Ecuador. Water Air Soil Pollut 131:19–39
Appleton JD, Weeks JM, Calvez JP, Beinhoff C (2006) Impacts of mercury contaminated mining waste on soil quality, crops, bivalves, and fish in the Naboc River area, Mindanao, Philippines. Sci Total Environ 354:198–211
ATSDR (1997) Toxicological profile for mercury. Draft for public comment (update). Prepared by Research Triangle Institute under Contract No. 205-93-0606. Prepared for U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, August
Back R, Gorski P, Cleckner L, Hurley J (2003) Mercury content and speciation in the plankton and benthos of Lake Superior. Sci Total Environ 304:349–354
Barbosa A, de Souza J, Dórea J, Jardim W, Fadini P (2003) Mercury biomagnification in a tropical black water, Rio Negro, Brazil. Arch Environ Contam Toxicol 45:235–246
Bastos WR, Gomes JP, Oliveira RC, Almeida R, Nascimento EL, Bernardi JV, de Lacerda LD, da Silveira EG, Pfeiffer WC (2006) Mercury in the environment and riverside population in the Madeira River Basin, Amazon, Brazil. Sci Total Environ 368: 344–351
Basu N, Scheuhammer AM, Evans RD, O’Brien M, Chan HM (2007) Cholinesterase and monoamine oxidase activity in relation to mercury levels in the cerebral cortex of wild river otters. Hum Exp Toxicol 26:213–220
Butala SJ, Scanlan LP, Chaudhuri SN, Perry DD, Taylor RJ (2007) Interlaboratory bias in the determination of mercury concentrations in commercially available fish utilizing thermal decomposition/amalgamation atomic absorption spectrophotometry. J Food Prot 70:2422–2425
Caetano M, Vale C (2003) Trace-element Al composition of seston and plankton along the Portuguese coast. Acta Oecol 24:S341–S349
Chen CY, Folt CL (2005) High plankton densities reduce mercury biomagnification. Environ Sci Technol 39:115–121
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Coquery M, Welbourn P (1995) The relationship between metal concentration and organic matter in sediments and metal concentration in the aquatic macrophytes Eriocaulon septangulare. Water Res 29:2094–2102
Counter SA (2003) Neurophysiological anomalies in brainstem responses of mercury-exposed children of Andean gold miners. J Occup Environ Med 45:87–95
Dixon R, Jones B (1994) Mercury concentrations in stomach contents and muscle of five fish species from the northeast coast of England. Mar Pollut Bull 28:741–745
Dominique Y, Muresan B, Duran R, Richard S, Boudou A (2007) Simulation of the chemical fate and bioavailability of liquid elemental mercury drops from gold mining in Amazonian freshwater systems. Environ Sci Technol 41:7322–7329
Donkor AK, Bonzongo JC, Nartey VK, Adotey DK (2006) Mercury in different environmental compartments of the Pra River Basin, Ghana. Sci Total Environ 368:164–176
Eisler R (2004) Mercury hazards from gold mining to humans, plants, and animals. Rev Environ Contam Toxicol 181:139–198
Gammons CH, Slotton DG, Gerbrandt B, Weight W, Young CA, McNearny RL, Cámac E, Calderón R, Tapia H (2006) Mercury concentrations of fish, river water, and sediment in the Río Ramis-Lake Titicaca watershed, Peru. Sci Total Environ 368:637–648
Guimarães J, Fostier A, Forti M, Melfi J, Kehrig H, Mauro J, Malm O, Krug J (1999) Mercury in Human and Environmental Samples fromTwo Lakes in Amapá, Brazilian Amazon. Ambio 28:296–301
Guimarães JR, Roulet M, Lucotte M, Mergler D (2000) Mercury methylation along a lake-forest transect in the Tapajós river floodplain, Brazilian Amazon: seasonal and vertical variations. Sci Total Environ 261:91–98
Gill GA, Bruland KW (1990) Mercury speciation in surface freshwater systems in California and other areas. Environ Sci Technol 24:1392–1400
Heim WA, Coale KH, Stephenson M, Choe KY, Gill GA, Foe C (2007) Spatial and habitat-based variations in total and methyl mercury concentrations in superficial sediments in the San Francisco Bay-Delta. Environ Sci Technol 41:3501–3507
Hylander L, Meili M, Oliveira L, Castro E, Guimaraes J, Araujo D, Neves R, Barros A, Silva G (2000a) Relationship of mercury with aluminum, iron and manganese oxy-hydroxides in sediments from the Alto Pantanal, Brazil. Sci Total Environ 260:97–107
Hylander L, Pinto F, Guimaraes J, Meili M, Oliveira L, Castro E (2000b) Fish mercury concentrations in the Alto Pantanal, Brazil: influence of season and water parameters. Sci Total Environ 261:9–20
Ikingura JR, Akagi H, Mujumba J, Messo C (2006) Environmental assessment of mercury dispersion, transformation and bioavailability in the Lake Victoria goldfields, Tanzania. J Environ Manage 81:167–173
Kainz M, Lucotte M (2002) Can flooded organic matter from sediments predict mercury concentrations in zooplankton of a perturbed lake? Sci Total Environ 293:151–161
Kamman NC, Driscoll CT, Estabrook R, Evers DC, Miller E (2003) Biogeochemistry of mercury in Vermont and New Hampshire lakes—an assessment of mercury in waters, sediments and biota of Vermont and New Hampshire lakes. Comprehensive Final Project Report to USEPA. Vermont Department of Environmental Conservation, Waterbury
Karunasagar D, Balarama Krishna MV, Anjaneyulu Y, Arunachalam J. (2006) Studies of mercury pollution in a lake due to a thermometer factory situated in a tourist resort: Kodaikkanal, India. Environ. Pollut 143(1):153–158
Kehrig H, Malm O, Akagi H, Guimaraes J, Torres J (1998) Methylmercury in fish and hair samples from the Balbina reservoir, Brazilian Amazon. Environ Res Sect A 77:84–90
Kligerman DC, La Rovere EL, Costa MA (2001) Management challenges on small-scale gold mining activities in Brazil. Environ Res 87:181–198
Kojadinovic J, Potier M, Le Corre M, Cosson RP, Bustamante P (2006) Mercury content in commercial pelagic fish and its risk assessment in the western Indian Ocean. Sci Total Environ 366:688–700
Kongchum M, Devai I, DeLaune RD, Jugsujinda A (2006) Total mercury and methylmercury in freshwater and salt marsh soils of the Mississippi river deltaic plain. Chemosphere 63:1300–1303
Lacerda L, Bidone E, Guimaraes A, Pfeiffer W (1994) Mercury concentrations in fish from the Itacaiúnas-Parauapebas river system, Carajás region, Amazon. An Acad Bras Cien 66:373–379
Limbong D, Kumampung J, Ayhuan D, Arai T, Miyazaki N (2005) Mercury pollution related to artisanal gold mining in north Sulawesi Island, Indonesia. Bull Environ Contam Toxicol 75:989–996
Lodenius M, Malm O (1998) Mercury in the Amazon. Rev Environ Contam Toxicol 157:25–52
Malm O, Pfeiffer WO, Souza CMM, Reuther R (1990) Mercury pollution due to gold mining in the Madeira river basin, Brazil. Ambio 19(1):11–15
Malm O, Guimaraes JR, Castro M, Bastos W, Viana J, Branches F, Silveira E, Pfeiffer W (1997) Follow up of mercury in fish, human air and urine in Madeira and Tapajos basins, Amazon, Brazil. Water Air Soil Pollut 97:45–51
Mauk RJ, Brown ML (2001) Selenium and mercury concentrations in brood-stock walleye collected from three sites on Lake Oahe. Arch Environ Contam Toxicol 40:257–263
Mirlean N, Larned ST, Nikora V, Kutter VT (2005) Mercury in lakes and lake fishes on a conservation-industry gradient in Brazil. Chemosphere 60:226–236
Morel F, Kraepiel A, Amyot M (1998) The chemical cycle and bioacumulation of mercury. Annu Rev Ecol Syst 29:543–566
Morrison KA, Watras CJ (1999) mercury and methylmercury in freshwater seston: direct determination at picogram per litre levels by dual filtration. Can J Fish Aquat Sci 56:760–766
Nguyen HL, Leermakers M, Kurunczi S, Bozo L, Baeyens W (2005) Mercury distribution and speciation in Lake Balaton, Hungary. Sci Total Environ 340:231–246
Olivero J, Jhonson B (2002) La contaminación con mercurio en el Sur de Bolívar. El Lado gris de la minería del oro. Editorial. Universidad de Cartagena
Olivero J, Solano B (1998) Mercury in environmental samples from a waterbody contaminated by gold mining in Colombia, South America. Sci Total Environ 217:83–89
Olivero J, Solano B, Acosta I (1998) Total mercury in muscle of fish from two marshes in Goldfields, Colombia. Bull Environ Contam Toxicol 61:182–187
Olivero J, Jhonson B, Arguello E (2002) Human exposure to mercury due to fish consumption in San Jorge river basin, Colombia (South America). Sci Total Environ 289:41– 47
Olivero J, Johnson B, Mendoza C, Paz R, Olivero R (2004) Mercury in the aquatic environment of the village of Caimito in the Mojana region, north of Colombia. Water Air Soil Pollut 159:409– 420
Palheta D, Taylor A (1995) Mercury in environmental and biological samples from a gold mining area in the Amazon region of Brazil. Sci Total Environ 168:63–69
Pataranawat P, Parkpian P, Polprasert C, Delaune RD, Jugsujinda A (2007) Mercury emission and distribution: Potential environmental risks at a small-scale gold mining operation, Phichit Province, Thailand. J Environ Sci Health A Tox Hazard Subst Environ Eng 42:1081–1093
Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD (2005) Impacts of zooplankton composition and algal enrichment on the accumulation of mercury in an experimental freshwater food web. Sci Total Environ 339:89–101
Porvari P (1995) Mercury levels of fish in Tucurui hydroelectric reservoir and in river Mojú in Amazonia in the state of Pará, Brazil. Sci Total Environ 175:109–117
Prahalad AK, Seenayya G (1988) In situ partitioning and biomagnification of mercury in industrially polluted husainsagar lake, hyderabad, india. Water Air Soil Pollut 39:81–87
Roulet M, Lucotte M, Guimarães JR, Rheault I (2000) Methylmercury in water, seston, and epiphyton of an Amazonian river and its floodplain, Tapajós River, Brazil. Sci Total Environ 261:43–59
Sadiq M, Zaidi T, Al-Mohana M (1991) Sample weight and digestion temperature as critical factors in mercury determination in fish. Bull Environ Contam Toxicol 47:335–341
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, New York
Sandheinrich MB, Miller KM (2006) Effects of dietary methylmercury on reproductive behavior of fathead minnows (Pimephales promelas). Environ Toxicol Chem 25:3053–3057
Schafer J, Blanc J, Audry S, Cossa D, Bossy C (2006) Mercury in the Lot–Garonne River system (France): sources, fluxes and anthropogenic component. Appl Geochem 21:515–527
Schetagne R, Doyon JF, Fournier JJ (2000) Export of mercury downstream from reservoirs. Sci Total Environ 260:135–145
Schmitt CJ, Brumbaugh WG (2007) Evaluation of Potentially nonlethal sampling methods for monitoring mercury concentrations in smallmouth bass (Micropterus dolomieu). Arch Environ Contam Toxicol 53:84–95
Sholupov S, Pogarev S, Ryzhov V, Mashyanov N, Straganov A (2004) Zeeman atomic absorption spectrometer RA-915+ for direct determination of mercury in air and complex matrix samples. Fuel Process Technol 85:475–485
Swain EB, Engstrom DR, Brigham ME, Henning TA, Brezonik PL (1992) Increasing rates of atmospheric mercury deposition in midcontinental North America. Science 257:784–787
Telmer K, Costa M, Simões R, Araujo ES., Maurice Y (2006) The source and fate of sediment and mercury in the Tapajós River, Pará, Brazilian Amazon: ground- and space-based evidence. J Environ Manage 81:101–113
Tremblay I, Lucotte M, Rowan D (1995) Different factors related to mercury concentration in sediments and zooplankton of 73 Canadian lakes. Water Air Soil Poll 80:961–970
Ullrich S, Tanton T, Abdrashitova S (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
Ullrich SM, Ilyushchenko MA, Kamberov IM, Tanton TW (2007) Mercury contamination in the vicinity of a derelict chlor-alkali plant. I: Sediment and water contamination of Lake Balkyldak and the River Irtysh. Sci Total Environ 381:1–16
USEPA (1989) Risk assessment guidence for Superfund. Vol 1. U.S. Environmental Protection Agency, Washington, DC, p 54
USEPA (1994) Methods 2451 for determination of mercury in water. U.S. Environmental Protection Agency, Cincinnati, OH
USEPA (1998) Method 7471B for determination of mercury in solid or semisolid waste. U.S. Environmental Protection Agency, Cincinnati, OH
USEPA (2000) Reference dose for mercury. External review. National Center for Environmental Assessment NCEA-S-0930. U.S. Environmental Protection Agency, Cincinnati, OH
Watras CJ, Back RC, Halvorsena S, Hudson RJM, Morrisona KA, Wente SP (1998) Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ 219:183–208
WHO (1989) Mercury—environmental aspects. Environmental Health Criteria 86. World Health Organization, Geneva, Switzerland
WHO (1990) Methylmercury. Environmental Health Criteria 101. World Health Organization, Geneva, Switzerland
Zapata J (1994) Environmental impacts study of gold mining in the Madeira River Bolivian-Brazilian border. In: Environmental mercury pollution and its health effects in Amazon River basin. National Institute for Minamata Disease and Institute of Biophysics of the Universidad Federal do Rio de Janeiro, Rio de Janeiro, Brazil, pp 23–24
Acknowledgments
The authors thank the Universidad de Cordoba, Montería (Grant 011-2003), the Universidad de Cartagena, Cartagena, and Colciencias, Bogotá, Colombia (Grant 1107-04-16346), for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marrugo-Negrete, J., Benitez, L.N. & Olivero-Verbel, J. Distribution of Mercury in Several Environmental Compartments in an Aquatic Ecosystem Impacted by Gold Mining in Northern Colombia. Arch Environ Contam Toxicol 55, 305–316 (2008). https://doi.org/10.1007/s00244-007-9129-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9129-7