Abstract
The availability of rapid and effective methodologies for assessing lotic systems with microphytobenthos is still quite scarce. Hence, the primary goal of this study was to optimize the growth conditions of the sensitive and ubiquous benthic diatom Navicula libonensis for laboratorial and field assessments. The effect of different conditions of temperature, photoperiod, initial cell density, test duration and cell encapsulation into calcium alginate beads was evaluated in a first set of experiments. There was a slight increase in the growth of free and immobilized cells at 23 °C, at lower initial cell densities and at the shortest experimental period (6 days). Through all the conditions, the growth profiles of free versus immobilized were fairly variable. A second experimental trial involved the validation of selected conditions, applied to the ecotoxicological testing of N. libonensis to two reference chemicals—3,5-dichlorophenol and potassium dichromate. A similar response of free and immobilized cells was observed between exposures to spiked stream water and synthetic medium, and through the conditions tested. This outcome suggests that N. libonensis may potentially provide reliable responses under direct in situ exposures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In lotic freshwater ecosystems, the benthic microalgae communities play an important role as they are in the basis of the trophic chain, are biostabilizers of sediments and regulate the benthic-pelagic nutrient cycling (Poulíčková et al. 2008). Among benthic microalgae, diatoms are the most used organisms as indicators of stream quality due to their ubiquity and sensitivity, as well as because their variability spans over most ecological conditions of the aquatic environment (Feio et al. 2009). Thereby, worldwide water quality monitoring programmes included diatoms as standard bioindicators (Brabec and Szoszkiewicz 2006) and its survey is mandatory in regulatory legislation such as the Water Framework Directive (Directive 2000/60/EC 2000). Although international guidelines such as OECD (2011), ISO8692 (1989) and USEPA (2002) mentioned the use of diatoms for ecotoxicological proposes, their use in ecotoxicological risk assessment as well as the development of methodologies for its use have been neglected and the focus is given to macrophytes and planktonic freshwater microalgae. Notwithstanding, much less interest has been paid to the species that compose the microphytobenthos and the development of methodologies for its use in ecotoxicological testing (Araújo et al. 2010; Moreno-Garrido et al. 2003; SETAC 1993). In fact, planktonic microalgae show high sensitivity to toxicants, often being more sensitive than other planktonic organisms (e.g. Marques et al. 2011; Pereira et al. 2009). However, in lotic systems, the prevalence of planktonic microalgae is negligible, strengthening the need to include sensitive and ecologically relevant benthic microalgae in test batteries for the ecotoxicological assessment of water column and sediment, either in the laboratory or under field conditions as highlighted by Moreira-Santos et al. (2005).
Under this rationale, the benthic diatom, Navicula libonensis, was herein selected as a potential model species for the development of toxicity testing methodologies, considering several meaningful criteria. This is a widely distributed benthic diatom that can be found in Europe (Rimet et al. 2007; Souffreau et al. 2010), including in the Iberian Peninsula and specifically in Portugal (de Oliveira 2007; Novais 2011), in North America (Sokal et al. 2008; Wilson et al. 1994) and in South America (Hassan et al. 2006; Seeligmann et al. 2008). From an environmental risk assessment perspective, such wide distribution naturally increases the ecological significance of the results yield in standard laboratorial tests and the suitability of in situ assays. Furthermore, N. libonensis was classified as sensitive to non-point source organic pollution by the Specific Pollution Sensitivity Index (SPI) (Cemagref 1982), and former studies indicate that the species is significantly more sensitive to the reference chemicals potassium dichromate and dichlorophenol than other microalgae (in some cases, the ECx values are one order of magnitude lower; Vidal et al. 2014). N. libonensis also provides a good handling compromise in the laboratory due to its relative larger size as compared to other diatoms.
Laboratorial test procedures for the culturing and toxicity testing with N. libonensis were already optimized in a former study (Vidal et al. 2014), but only free cells were considered so far. The immobilization of the diatoms in encapsulation matrices offers many advantages over the use of free cells, especially for in situ toxicity assessment as stated by Araújo et al. (2010). The encapsulation of microalgae has been successfully used in toxicity tests developed for application in freshwater (Moreira-Santos et al. 2002; Moreira-Santos et al. 2004b), estuarine and marine ecosystems (Moreno-Garrido et al. 2003, 2005, 2007).
The main aim of this work was the optimization of the conditions for conducting sensitive and cost-effective ecotoxicological assessment of water column and sediments using algal growth bioassays suitable for laboratory and in situ assessments, using the freshwater benthic diatom N. libonensis. As such, the effect of different conditions on N. libonensis growth was assessed by testing: (i) different incubation temperatures (23 and 15 °C) and (ii) photoperiods (24L and 12L:12D), which were both variables set considering standard laboratorial conditions and field scenarios; (iii) different initial cell density (104 and 105 cells mL−1), which was already proven to affect the test outcome in other species (Moreira-Santos et al. 2002; Moreno-Garrido 2008); (iv) cell immobilization in matrices with two distinct alginate percentages (1.3 and 1.5 %), thus addressing the effect of alginate concentration in exposure and encapsulation efficiency, versus free cells; (v) different test periods (T6, T9, T11), hence evaluating whether longer periods—generally required in tests run in the field due to variation in photoperiod and temperature—suit the species physiology and/or allow better detection of effects. The ecotoxicological response of N. libonensis under these conditions was still validated with the testing of reference chemicals (potassium dichromate and 3,5-dichlorophenol) in artificial medium (Chu 10) and in natural stream water (sample taken from a reference stream in Luso, Portugal; Silva 2008).
Material and methods
Test organism
N. libonensis (size: length range of 27–35 μm, width range of 5.9–7.0 μm and description; Spaulding et al. 2010) was purchased from the UTEX Culture Collection of Algae (University of Texas at Austin, USA; UTEX LB FD183). The cultures were maintained in 100-mL Erlenmeyer vessels containing 40 mL of Chu 10 medium (Chu 1942), at 20 ± 2 °C and continuous light supply (60–120 μE m−2 s−1, using cool white lamps). Under these conditions the exponential growth phase of cells starts at the 4th day and the decline phase at the 9th day according to Vidal et al. (2014), and inoculation of fresh medium was done during the exponential phase of previous cultures.
First trial—optimization of growth conditions
Cell immobilization
The effect of cell immobilization in the growth of N. libonensis was assessed by comparing between growth in cultures of free and immobilized cells. Algal cells were immobilized by encapsulation in beads of calcium alginate 1.3 and 1.5 % (w/v) concentrated, following the protocol suggested by Moreira-Santos et al. (2002) and Bozeman et al. (1989). Solutions of 1.3 and 1.5 % (w/v) sodium alginate (CAS no. 9005-38-3) were prepared with sterilized distilled water. Since the initial cell density is critical for the viability of immobilized cells because of nutrient availability, carbon dioxide diffusion and light penetration (Moreira-Santos et al. 2002), we tested two initial cell densities: 104 and 105 cells mL−1. They were established considering previous studies (e.g. Moreira-Santos et al. 2002; Moreno-Garrido et al. 2003) and the requirements of the OECD guideline for toxicity testing with microalgae (OECD 2011). Following previous optimization of procedures, an aliquot of an exponentially growing culture, concentrated by gravity rather than by centrifugation, was added to each alginate solution (1.3 and 1.5 %) to obtain an alginate-cell suspension with ca. 104 cells mL−1 and ca. 105 cells mL−1. Beads were then formed by dropwise adding (using a sterilized needle coupled to a 20-mL syringe) of each alginate-cell suspension into a 2 % (w/v) calcium chloride solution. Beads were gently stirred within the CaCl2 solution for approximately 45 min for gel hardening, then washed with distilled water and stored in dark at 4 °C (in 20× diluted Chu 10 medium). The beads presented a mean ± SD diameter of 3.14 ± 0.07 mm. Cell counting at the beginning and end of each test was carried out after disaggregating beads (in a total of three replicates of four beads, each replicate) in 1 mL of trisodium citrate solution [3 % (w/v); CAS no. 6132-04-3] upon smooth shaking. The bead and free cell countings were made using a tubular plankton chamber (Hydro-Bios, Germany) following previously optimized procedures (Hasle 1978). In brief, 1 mL sample was harvested from the homogenized (by short sonication and vortexing for 1–1.5 min) inoculum, which was added two drops of lugol to preserve the sample and facilitate diatoms settling for further counting (Olympus CKX 41; ×200 magnification). The beads were prepared 24 h (maximum period) before their use in the bioassays.
Experimental design and testing conditions
Eight separate experiments were conducted comprising all combinations of initial cell density, temperature and photoperiod to statistically test the effect of cell immobilization and test duration on diatom population growth. Table 1 provides an overview of the experimental design, clarifying the treatments set within each experiment. Test duration and immobilization factor levels were always tested within each of the other factor levels. In the first trial experiments, T11 was tested only at 15 °C because such temperature can slow down the species growth, (please see Vidal et al. 2014). The selected incubation conditions intended to represent the standard conditions described in the guidelines for testing with standard freshwater microalga (OECD 2011) versus field conditions in European temperate regions, including Portugal. The temperature chosen to represent field conditions was based on the average annual water temperatures for Portuguese streams (ca. 15 °C) (INAG 2008).
All experiments were conducted in sterile 50-mL glass test tubes containing 10 mL of Chu 10. Three replicates were considered per test condition. From the initial bulk culture, a small sample was collected after being homogenized and cell density was determined as previously described. After that, an estimated volume was transferred from the concentrate bulk culture to the 50-mL glass tubes which were then filled with the necessary volume of Chu 10 medium as to achieve an initial cell density of 104 or 105 cells mL−1 in a total test volume of 10 mL. Regarding encapsulated cells, four beads were used per replicate (average effective initial cell densities for the respective 104 and 105 factor levels are presented in Table S5). Their volume was neglected and 10 mL of Chu 10 medium or toxic solution was added. The tubes were covered with perforated Parafilm®. At the end of the test, the whole suspension was sonicated and vortexed in order to collect a homogenized sample of 1 mL, which was preserved with 100 μL of a Lugol’s solution 3.4 % (w/v) of iodine until further cell counting. The growth rates of free and immobilized cells of N. libonensis were determined on the basis of the cell density estimated as previously described (cf. “Cell immobilization” section) using the formula: μ i − j = (ln X j − ln X i ) / t j − t i (day−1), where μ i − j (days−1) is the average specific growth rate from time t i to t j (days), and X j and X i are, respectively, the cell density (cells mL−1) at time t j and t i . All the test preparation was performed near the flame in a flow chamber, guarantying the sterile conditions.
The initial cell density, percent of alginate within the immobilization factor and test duration delivering higher growth rates within each multifactor experiments combination of temperature and photoperiod were selected to the second trial of the study.
Second trial—suitability of cell immobilization to assess the toxicity of chemical substances
The sensitivity of free and immobilized cells of N. libonensis to an organic (3,5-dichlorophenol, DCP) and a metallic (potassium dichromate, PD) reference compound was tested under different exposure conditions. The reference compounds were tested at their 6 days-EC50 values, according to the data obtained for this species in previous studies (Vidal et al. 2014). In order to evaluate the efficiency of the test apparatus and species response in the field, the tests were run under the same combinations of experimental conditions as set in the first test trial but, additionally, the chemical spiking was done in a natural stream water sample besides Chu 10. The water sample was collected in a pristine-like mountain stream (Luso—Northern Portugal). The water samples were characterized by measuring conductivity, pH, total suspended solids, dissolved oxygen level, biological oxygen demand (BOD5) (APHA 1995) and phaeophytin-corrected chlorophyll-a (chl-a) (Lorenzen 1967). The vacuum filtered sample (1.5 μm mesh pore size) was used for the colorimetric quantification of nitrites (NO2 −), nitrates (NO3 −), ammonia (NH4) and orthophosphates (PO4 3−) (APHA 1995). Ions and metals Mg2+, Ca2+, Si2+, K+ and Fe2+ were analysed through inductively coupled plasma mass spectrometry (ICP-MS) to verify whether their content was discrepant or not from the levels present in the artificial medium Chu 10 (APHA 1995). The original water sample was filtered through 0.45 μm mesh pore size filters (USEPA 2002) before use as test medium. An extra treatment of stream water enriched with nutrients (at the same concentrations as standing in Chu 10) was considered to prevent nutrient deficiency effects (USEPA 2002). In summary, nine treatments were considered in each bioassay: (i) blank Chu 10 medium (Chu10); (ii) DCP EC50 in Chu 10 (Chu10 + DCP); (iii) PD EC50 in Chu 10 (Chu10 + PD); (iv) blank stream water (SW); (v) DCP EC50 in stream water (SW + DCP); (vi) PD EC50 in stream water (SW + PD); (vii) nutrient-spiked blank stream water (SW + N); (viii) DCP EC50 in nutrient-spiked stream water (SW + N + DCP); (ix) PD EC50 in nutrient-spiked stream water (SW + N + PD). Cell immobilization was the second factor considered within each bioassay with two levels set: free cells and immobilized cells in 1.3 % alginate beads. Following previous optimization (see “First trial—optimization of growth conditions” section), the second trial bioassays were carried out for 6 days, starting from an initial cell density of 104 cells mL−1 (cf. “Results and discussion” section).
Data analysis
Regarding the first set of experiments, the variability of initial cell densities of the free cell and bead batches prepared immediately before beginning each experiment was analysed by computing dispersion measures from cell density data: average, standard deviation, coefficient of variation and minimum and maximum values. The influence of cell immobilization and test duration on the growth rates of N. libonensis was statistically analysed through a two-way analysis of variance (two-way ANOVA) run over the dataset of each eight separate independent experiments of fixed factor and factor level tested (e.g. 104 cells mL−1, 23 °C, 24L, T6, free cells; 104 cells mL−1, 23 °C, 24L, T6, beads 1.3 %; 104 cells mL−1, 23 °C, 24L, T6, beads 1.5 %). The factors used in each two-way ANOVA performed are indicated in Table 2 as “Source of variation”, while the dependent variable is N. libonensis growth rate within the respective combination of cell density and test condition. When no significant interaction was found, the simple main effects of each factor were then scrutinized by one-way ANOVA followed by the Tukey multiple comparison test. Whenever a significant interaction was found, the MSresidual of the two-way ANOVA was used as the denominator for calculating the F statistics of the one-way ANOVA over each factor and the q statistics for the Tukey multiple comparison tests (Quinn and Keough 2002). In order to search for significant differences between the two factor levels of alginate percentage (i.e. 1.3 and 1.5 %) or initial cell density (i.e. 104 and 105 cell mL−1), the Student’s t test (Quinn and Keough 2002) was applied within each test condition regarding temperature, photoperiod and exposure duration. These statistical analyses provided a more reliable support to decide which of the levels of those two factors better suited for N. libonensis growth.
In the second set of bioassays, the growth rate obtained in each treatment was expressed as a ratio of the respective controls (either free or immobilized cells exposed to Chu 10 medium) within each combination of temperature and photoperiod. The significant effects of cell immobilization and chemical spiked in different media on the diatom growth were assessed using the same approach as employed for the first trials. A significance level (α) of 0.05 was used in all analyses.
Results and discussion
As a way to meet some evaluation requirements set in the WFD, it is worth investing research efforts in developing new strategies (e.g. using the responses of sensitive organisms) that could provide a valuable assessment under laboratorial and field conditions, by reliably responding to different environmental factors and contaminants. Thereby, this work gathers relevant data concerning the optimization of the growth of the benthic diatom N. libonensis for field and laboratorial assessments. This species was never used as a test organism despite its sensitivity to certain contaminants (Vidal et al. 2014).
First trial—optimization of growth conditions
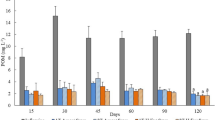
The results of the first set of experiments are shown in Fig. 1. The growth rates of N. libonensis were generally higher at 23 °C, although a similar outcome was obtained at 15 °C and 12L:12D photoperiod, particularly for experiments with initial cell density of 104 cells mL−1. A clear response pattern, however, could not be retrieved for the different photoperiods tested (Fig. 1)—for example, if at 23 °C, a full light cycle seems to produce better growth than a 12L:12D cycle, at 15 °C the opposite seems to occur. Indeed, Mayer et al. (1998) observed a more conspicuous effect of temperature variations on the growth of Selenastrum capricornutum than that triggered by light intensity, nitrogen source or pH. Similarly, Lewis et al. (2002) verified improved growth rates of the diatom Achnanthes longipes (free cells) as temperature increased from 10 °C up to 26 °C. Likewise, Faafeng et al. (1994) obtained higher growth rates of immobilized and particularly of free cells of S. capricornutum at 20 °C compared to 10 °C. Another study concluded that changes in the temperature and photoperiod regimes from field to standard laboratorial experiments significantly influenced the growth of the immobilized marine diatom Phaeodactylum tricornutum (Moreira-Santos et al. 2002). The raise of temperature and photoperiod tends to boost the metabolic rate of the microalgae within a certain optimal range, hence leading to the yield of higher biomass levels (Khoyi et al. 2009; Qian et al. 2010).
Growth rates of free and immobilized (at 1.3 and 1.5 % alginate concentration) cells of N. libonensis under different experimental conditions of temperature (15 and 23 °C), photoperiod (24L and 12L:12D) and initial cell density (104 and 105 cells mL−1). Error bars represent standard errors. Uppercase letters indicate significant differences (Tukey test; P < 0.05) between the responses of free and immobilized cells within each experimental period of 6 (T6; black bold letters), 9 (T9; grey bold letters) and 11 (T11; black letters). Lowercase letters indicate significant differences (Tukey test; P < 0.05) between the diatom response to different experimental periods as the growth of free cells (black bold letters), cells immobilized at 1.3 % alginate (grey bold letters) and cell immobilized at 1.5 % alginate (black letters) were assessed
For both initial cell densities analysed in this study, a 16-fold increase on the growth of free or immobilized cells—a validation criterion set in standard procedures—was never attained. Considering that we are dealing with a benthic diatom, there are some constraints yet to be surpassed in what concerns the handling and resuspension of the cells from the mucilaginous aggregates that they form, in order to allow a consistent control of the procedure and achievement of the initial densities intended. In fact, the yields of microalgae at the end of the test trials are greatly influenced by the initial cell density, especially if immobilized cells are considered (Moreno-Garrido 2008). As such, we analysed the variability of the effective initial cell densities in the different free and immobilized cell batches prepared for each experiment. From Table S1, a coefficient of variation <30 % for all free/immobilized cell batches can be noticed, hence indicating a good repeatability according to Environment Canada (2007) guidelines, and proving that the variation on initial cell densities did not seem to constrain the outcome of experiments. An initial density of 104 cells mL−1 led to improved growth rates of free and immobilized cells comparatively to 105 cells mL−1 (cf. Table S2 for the statistical differences of N. libonensis growth between the two cell densities). For this reason, the former was selected as the initial cell density for the second test trial with N. libonensis. Although it is extensively documented that higher initial cell densities of microalgae usually lead to higher growth rates at the end of an exposure period, e.g. Moreira-Santos et al. (2004a) or Moreno-Garrido (2008) found the opposite trend for Chlorella vulgaris immobilized in calcium alginate beads. Similarly, a previous study demonstrated that the cell division of immobilized P. tricornutum was accelerated when the initial number of cells per bead was lower (Moreira-Santos et al. 2002). The authors still found that the final growth rates only differed by ≤8 % among the upper and lower initial cell densities tested. It is commonly accepted that very high densities of immobilized microalgae may limit the diffusion of light, nutrients and carbon dioxide (Moreira et al. 2006). This is particularly critical if the test organism has a considerable cell size, such as N. libonensis (cf. “Cell immobilization”).
Within each combination of temperature, photoperiod and cell density a significant influence of cell encapsulation and test duration on the growth rate of N. libonensis was generally detected (Table 2, Fig. 1). Moreover, a significant interaction between these two factors was frequently determined (Table 2). The achievement of high cell densities when microalgae are subjected to temperature and light variations under field conditions usually demands longer test periods (Moreira-Santos et al. 2004a). Notwithstanding, the growth rates of N. libonensis were kept at similar or decreased levels as the assessment period enlarged (from 6 to 9 or 11 days), either for free or immobilized cells, irrespectively of the experimental conditions assessed (Fig. 1, Tables 2, S3, S4). Bearing on this outcome, a test duration of 6 days was set for the second stage trial, this being also in agreement with the growth curve previously determined for the species under standard conditions (i.e. 22 ± 2 °C and 24 L, Vidal et al. 2014).
For all conditions tested and comparing the growth rates of free vs. immobilized cells, it can be concluded that the growth of N. libonensis as free cells was either not significantly different in some conditions or significantly higher or lower than that observed in beads, in another conditions (of 1.3 and/or 1.5 % alginate) (Fig. 1, Table S2). The growth profiles of free versus immobilized cells documented in other studies under different incubation conditions are quite variable as well. Previous works with chlorophyta, cyanobacteria and diatom microalgae, either from freshwater or estuarine/marine environments, demonstrated higher (Faafeng et al. 1994; Moreira-Santos et al. 2004a; Moreno-Garrido et al. 2007), similar or lower (e.g. Mallick 2002; Rai and Mallick 1992; Twist et al. 1997) growth rates for free cells relatively to the immobilized cells. Several factors were pointed out as possible explanations to this variation (Moreno-Garrido 2008). Specifically, Hoogenhout and Amesz (1965) concluded that the culturing conditions can largely modulate the responses of these organisms, possibly by acting through their physiological condition. Indeed, contrary to our expectations, under 15 °C a considerable growth of immobilized diatoms was generally noticed (104 cells mL−1 at 12L:12D, 105 cells mL−1 for both photoperiods), which frequently was statistically similar to the growth of free cells (Fig. 1, Table S2). Regarding the two percentages of alginate used, significant differences on diatom growth rate between 1.3 and 1.5 % alginate for some experiments were determined (Table S5). However, the overall trend corresponded to higher growth rates under 1.3 % of alginate, which was in tandem with the percentage of alginate used in previous studies with immobilized diatoms (e.g. Moreira-Santos et al. 2002). For that reason, this alginate concentration was selected for the second experimental trial (Fig. 1). In fact, tightened matrices resulting from a higher percentage of alginate (Gombotz and Wee 1998) may limit the uptake of resources by the diatom hence constraining its growth. In any case, the matrix of encapsulation neither was toxic for the diatom nor affected its morphological integrity, and even promoted the growth of N. libonensis, seemingly to act as a protective barrier under lower temperatures (e.g. 104 cell mL−1; 15 °C 12L:12D, T6). Different studies brought up to discussion the influence of temperature and light intensity on the production, quality, quantity and biological activity of carbohydrate-rich exopolymeric substances (EPS) by benthic diatoms (e.g. Lam et al. 2005). Wolfstein and Stal (2002) observed a reduced production of EPS by Cylindrotheca closterium under lower temperatures and irradiances, though the outcome was indirectly affected by the amount of algal cells that could produce EPS. Since sodium alginate is a polysaccharide, it is likely that the immobilized N. libonensis cells, under stress conditions (e.g. low temperature), may take advantage of the alginate matrix to cope with an inhibited ability to produce EPS for their adhesion. Moreover, Jiménez-Pérez et al. (2004) had also observed that P uptake efficiency was higher in immobilized planktonic green algae than in free cells, which may favour algae growth.
Second trial—validation of the optimized test procedure
The growth response of N. libonensis after exposure to the reference substances spiked into Chu 10 and into natural stream water (cf. Table 3 for its physical and chemical characteristics) is illustrated in Fig. 2. Although the relative growth rates and the sensitivity of the diatom to chemical substances under 15 °C were slightly below those observed under 23 °C, no consistent pattern could be clearly defined; the same conclusion can be drawn when different photoperiods are compared (Fig. 2). The individual or combined effect of temperature and photoperiod on the accumulation of phenol by S. capricornutum (Newsted 2004), and on the transcription of photosynthesis-related genes to Cd in C. vulgaris (Qian et al. 2010), has been stressed out. If such or similar negative effects occur in N. libonensis exposed to DCP or PD, the growth endpoint assessed does not capture the physiological impairment consistently.
Relative growth rates (simple ratio between the growth rates found in blank Chu10 and each test condition) of free and immobilized cells of N. libonensis exposed to different treatments of stream water (SW) and Chu10 non-spiked and spiked with 3,5-dichlorophenol (DCP; left-hand panel) or potassium dichromate (PD; right-hand panel). Error bars represent standard errors. Different letters above error bars indicate significant differences (P < 0.05) between responses yield under different treatments when tested within free cells (bold letters) and within immobilized cells (light black letters). The asterisks stand for significant differences between the responses of free and immobilized cells within each treatment
Regardless the test incubation conditions, the overall relative growth rates of free cells in SW and SW + N was, respectively, of more than 41 and 70 % of that obtained under Chu10, which was set as the reference rate for calculations, while for beads it was of more than 49 and 89 % of the rates obtained for immobilized cells under Chu10 (Fig. 2). The reduction of N. libonensis relative growth rates in plain stream water irrespective of the test conditions was consistent with previously reported data (e.g. Marques et al. 2011; Moreira-Santos et al. 2002, 2004a). Whenever natural samples are being assayed in the laboratory, it is important to discern a toxic effect from that caused by nutrient deprivation, the latter being avoided by adding nutrients to the test water (USEPA 2002). In this case study, the addition of nutrients to SW indeed promoted the diatom growth rates, although these were only significantly higher than in plain SW for immobilized cells at 23 °C, 24L and for free cells at 15 °C, 24L (Fig. 2, Tables 4, S6, S7).
The exposure of free or immobilized cells of N. libonensis to SW, SW + N and Chu10 spiked with DCP or PD generally resulted in significant growth inhibition. Under some conditions, however, the inhibition of growth rates by DCP or PD was not statistically significant as the chemical treatments were compared to the corresponding natural water controls (i.e. SW and SW + N) (Fig. 2, Table S7). The growth inhibition detected under SW + DCP or SW + PD may actually be an effect of nutrient deficiency in the SW, hence increasing the probability of accepting false positives. It should be recognized in this context that under a few conditions, the addition of nutrients to SW + DCP (i.e. SW + N + DCP) or SW + PD (i.e. SW + N + PD) led to a significant reduction of the toxicity of both compounds (free cells: 15 °C-12L:12D for PD, beads: 23 °C-24L for PD, 15 °C-12L:12D for DCP and PD). This can relate to a lower bioavailability of the compounds due to complexation or adsorption onto other dissolved chemicals and organic matter present in SW + N (cf. also Table 3) (Moreira-Santos et al. 2002; Newsted 2004). Nevertheless, the growth rates detected under SW + DCP or SW + PD were generally not significantly different from those calculated under Chu10 + DCP or Chu10 + PD for free and immobilized cells, for all the combinations of temperatures and photoperiods. In fact, the lowest growth rates were normally observed under those four treatments. Hence, this benthic diatom provided similar ecotoxicological response levels to DCP and PD when approaching standard artificial conditions (represented by temperature, photoperiod and Chu 10 medium) to field scenarios (represented by temperature, photoperiod and stream water).
The encapsulation of cells, though offering advantages for in situ testing (Moreno-Garrido 2008; Twist et al. 1997), should allow the effective exposure of cells to the surrounding environment and guarantee that reliable responses to the contaminants in combination with environmental factors are being assessed. Free and immobilized N. libonensis cells exposed to natural stream water and artificial medium non-spiked and spiked with DCP or PD generally elicited similar sensitivity, although the statistics found significantly different responses in particular cases (Fig. 2, Table S6). Most of these were associated with SW and/or SW + N treatments, either spiked or non-spiked with chemicals. Except for free cells exposed to SW at 23 °C, 12L:12D, and SW + DCP and SW + N + DCP at 15 °C, 12L:12D, the remaining significant differences between the response of free and immobilized cells resulted from an enhanced growth of immobilized diatoms. Moreno-Garrido (2008) discussed the protection that the immobilization matrix may give against toxicity. The reason for that could be the partial removing of toxicants and their adsorption to the alginate polymer (Awasthi and Rai 2005), and the lower diffusion of toxicants through the matrix (Jang 1994). Nevertheless, the alginate matrix did not seem to limit the exposure of the diatom, since in Chu10 + DCP or Chu10 + PD the growth of free and immobilized cells was usually not significantly different. This outcome even suggests that under controlled conditions of temperature and photoperiod, free and immobilized cells respond similarly to the reference chemicals.
Conclusions
N. libonensis as benthic microalgae may be a potential alternative to planktonic ones for toxicity testing in lotic environments. In the first optimization trial, a slight increase of the diatom growth at higher temperatures, lower initial cell densities and shorter exposures under the artificial medium Chu 10 were the conditions that most favour them. However, at temperatures close to the average found in Portuguese natural streams (15 °C), there was apparently a protective effect of the alginate beads over N. libonensis that promoted its growth. Free cells either evidenced higher, lower or similar rates comparatively to the immobilized cells. In the second set of experiments, the combined scenarios of temperatures and photoperiods did not apparently influence the sensitivity of this diatom (free and immobilized cells) to the reference stream water and chemicals tested. The growth of the diatom under a plain field water sample was usually slightly lower than that under nutrient-enriched samples. In any case, the sensitivity of N. libonensis to the standard chemicals spiked into plain stream water was similar to that obtained in Chu 10 when used as dilution medium, both for free and immobilized cells. Thus, immobilized N. libonensis might retrieve a reliable ecotoxicological assessment under field scenarios. However, this should be further tested under more realistic scenarios involving the co-occurrence of different confounding factors, during direct exposures in in situ trials.
References
2000/60/EC (2000): Water Framework Directive of the European Parliament and the Council, of 23 October 2000, establishing a framework for community action in the field of water policy. Official Journal of the European Communities L 327, pp. 1–72
APHA (American Public Health Asssociation) (1995) Standard methods for the examination of water and wastewater, 19th edn. APHA, Washington DC
Araújo CV, Blasco J, Moreno-Garrido I (2010) Microphytobenthos in ecotoxicology: a review of the use of marine benthic diatoms in bioassays. Environ Int 36:637–646
Awasthi M, Rai LC (2005) Toxicity of nickel, zinc, and cadmium to nitrate uptake in free and immobilized cells of Scenedesmus quadricauda. Ecotoxicol Environ Saf 61:268–272
Bozeman J, Koopman B, Bitton G (1989) Toxicity testing using immobilized algae. Aquat Toxicol 14:345–352
Brabec K, Szoszkiewicz K (2006) Macrophytes and diatoms—major results and conclusions from the STAR project. Hydrobiologia 566:175–178
Cemagref (1982): Étude des mèthodes biologiques quantitatives d’appréciation de la qualit des eaux .Rapport Division Qualité des Eaux Lyon - Agence financière de Bassin Rhône - Méditerranée - Corse, Pierre-Bénite, 218 pp
Chu S (1942) The influence of the mineral composition of the medium on the growth of planktonic algae: part I. Methods and culture media. J Ecol 30:284–325
de Oliveira NS 2007: Caracterização físico-química e ecológica (diatomáceas) das linhas de água de Aveiro. M.Sc. thesis, University of Aveiro. 345pp
Environment Canada. 2007. Guidance document on statistical methods for environmental toxicity tests. Report EPS 1/RM/46, 241 p
Faafeng BA, van Donk E, Källqvist ST (1994) In situ measurement of algal growth potential in aquatic ecosystems by immobilized algae. J Appl Phycol 6:301–308
Feio MJ, Almeida SFP, Craveiro SC, Calado AJ (2009) A comparison between biotic indices and predictive models in stream water quality assessment based on benthic diatom communities. Ecol Indic 9:497–507
Gombotz WR, Wee S (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31:267–285
Hasle G (1978) The inverted-microscope method. In: Sournia A (ed) Phytoplankton manual. Monographs on oceanographic methodology. United Nations Educational, Scientific and Cultural Organzation (Unesco), Paris, pp 88–96
Hassan GS, Espinosa MA, Isla FI (2006) Modern diatom assemblages in surface sediments from estuarine systems in the Southeastern Buenos Aires Province, Argentina. J Paleolimnol 35:39–53
Hoogenhout H, Amesz J (1965) Growth rates of photosynthetic microorganisms in laboratory cultures. Arch Microbiol 50:10–25
INAG I. P. (2008): Tipologia de Rios em Portugal Continental no âmbito da implementação da Directiva Quadro da Água. I - Caracterização abiótica. Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional. Instituto da Água, I.P.
ISO8692 (1989) Water quality—fresh water algal growth inhibition test with Scenedesmus subspicatus and Selenastrum capricornutum. International Organization for Standardization, Geneva
Jang LK (1994) Diffusivity of Cu2+ in calcium alginate gel beads. Biotechnol Bioeng 43:183–185
Jiménez-Pérez V, Sánchez-Castillo P, Romera O, Fernández-Moreno D, Pérez-Martínez C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzym Microb Technol 34:392–398
Khoyi ZA, Seyfabadi J, Ramezanpour Z (2009) Effects of light intensity and photoperiod on the growth rate, chlorophyll a and beta-carotene of freshwater green micro alga Chlorella vulgaris. Comp Biochem Physiol A Mol Integr Physiol 153A:S215–S215
Lam C, Harder T, Qian P-Y (2005) Growth conditions of benthic diatoms affect quality and quantity of extracellular polymeric larval settlement cues. Mar Ecol Prog Ser 294:109–116
Lewis RJ, Johnson LM, Hoagland KD (2002) Effects of cell density, temperature, and light intensity on growth and stalk production in the biofouling diatom Achnanthes longipes (Bacillariophyceae)1. J Phycol 38:1125–1131
Lorenzen C (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. BioMetals 15:377–390
Marques CR, Pereira R, Gonçalves F (2011) Toxicity evaluation of natural samples from the vicinity of rice fields using two trophic levels. Environ Monit Assess 180:521–536
Mayer P, Frickmann J, Christensen ER, Nyholm N (1998) Influence of growth conditions on the results obtained in algal toxicity tests. Environ Toxicol Chem 17:1091–1098
Moreira SM, Moreira-Santos M, Guilhermino L, Ribeiro R (2006) Immobilization of the marine microalga Phaeodactylum tricornutum in alginate for in situ experiments: bead stability and suitability. Enzym Microb Technol 38:135–141
Moreira-Santos M, Moreno-Garrido I, Gonçalves F, Soares A, Ribeiro R (2002) An in situ bioassay for estuarine environments using the microalga Phaeodactylum tricornutum. Environ Toxicol Chem 21:567–574
Moreira-Santos M, Soares A, Ribeiro R (2004a) A phytoplankton growth assay for routine in situ environmental assessments. Environ Toxicol Chem 23:1549–1560
Moreira-Santos M, Soares A, Ribeiro R (2004b) An in situ bioassay for freshwater environments with the microalga Pseudokirchneriella subcapitata. Ecotoxicol Environ Saf 59:164–173
Moreira-Santos M, da Silva E, Soares A, Ribeiro R (2005) In situ and laboratory microalgal assays in the tropics: a microcosm simulation of edge-of-field pesticide runoff. Bull Environ Contam Toxicol 74:48–55
Moreno-Garrido I (2008) Microalgae immobilization: current techniques and uses. Bioresour Technol 99:3949–3964
Moreno-Garrido I, Hampel M, Lubián L, Blasco J (2003) Sediment toxicity tests using benthic marine microalgae Cylindrotheca closterium (Ehremberg) Lewin and Reimann (Bacillariophyceae). Ecotoxicol Environ Saf 54:290–295
Moreno-Garrido I, Campana O, Lubián L, Blasco J (2005) Calcium alginate immobilized marine microalgae: experiments on growth and short-term heavy metal accumulation. Mar Pollut Bull 51:823–829
Moreno-Garrido I, Lubián L, Blasco J (2007) Sediment toxicity tests involving immobilized microalgae (Phaeodactylum tricornutum Bohlin). Environ Int 33:481–485
Newsted JL (2004) Effect of light, temperature, and pH on the accumulation of phenol by Selenastrum capricornutum, a green alga. Ecotoxicol Environ Saf 59:237–243
Novais MHCG 2011: Benthic diatoms in Portuguese watercourses. Ph.D. thesis, University of Évora, 224 pp
OECD (2011) Guidelines for testing chemicals in freshwater and cyanobacteria. Organization for Co-operation and Development, Paris
Pereira JL, Antunes SC, Castro BB, Marques CR, Gonçalves AMM, Gonçalves F, Pereira R (2009) Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology 18:455–463
Poulíčková A, Hašler P, Lysáková M, Spears B (2008) The ecology of freshwater epipelic algae: an update. Phycologia 47:437–450
Qian H, Li J, Pan X, Jiang H, Sun L, Fu Z (2010) Photoperiod and temperature influence cadmium’s effects on photosynthesis-related gene transcription in Chlorella vulgaris. Ecotoxicol Environ Saf 73:1202–1206
Quinn G, Keough M (2002) Experimental design and data analysis for biologists. University Press, Cambridge
Rai L, Mallick N (1992) Removal and assessment of toxicity of Cu and Fe to Anabaena doliolum and Chlorella vulgaris using free and immobilized cells. World J Microbiol Biotechnol 8:110–114
Rimet F, Gomà J, Cambra J, Bertuzzi E, Cantonati M, Cappelletti C, Ciutti F, Cordonier A, Coste M, Delmas F (2007) Benthic diatoms in western European streams with altitudes above 800 M: characterisation of the main assemblages and correspondence with ecoregions. Diatom Res 22:147–188
Seeligmann C, Maidana NI, Morales M (2008) Diatomeas (Bacillariophyceae) de humedales de altura de la Provincia de Jujuy-Argentina. Bol Soc Argent Bot 43:1–17
SETAC (1993): Guidance document on sediment toxicity tests and bioassays for freshwater and marine environments. From the “workshop on Sediment Toxicity Assessment”, November, Renesse, The Netherlands
Silva M (2008) Evaluation and integrated monitoring of the water quality from the Cértima River. M.Sc. thesis, University of Aveiro, 119 pp
Sokal MA, Hall RI, Wolfe BB (2008) Relationships between hydrological and limnological conditions in lakes of the Slave River Delta (NWT, Canada) and quantification of their roles on sedimentary diatom assemblages. J Paleolimnol 39:533–550
Souffreau C, Vanormelingen P, Verleyen E, Sabbe K, Vyverman W (2010) Tolerance of benthic diatoms from temperate aquatic and terrestrial habitats to experimental desiccation and temperature stress. Phycologia 49:309–324
Spaulding S, Lubinski D, Potapova M (2010) Diatoms of the United States. http://westerndiatoms.colorado.edu Accessed on 14 May, 2013.
Twist H, Edwards AC, Codd GA (1997) A novel in-situ biomonitor using alginate immobilised algae (Scenedesmus subspicatus) for the assessment of eutrophication in flowing surface waters. Water Res 31:2066–2072
USEPA (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. U.S. Environmental Protection Agency EPA-821-R-02-013, Washington DC
Vidal T, Pereira J, Abrantes N, Almeida SP, Soares AVM, Gonçalves F (2014) Toxicity testing with the benthic diatom Navicula libonensis (Schoeman 1970): procedure optimisation and assessment of the species sensitivity to reference chemicals. Bull Environ Contam Toxicol 1–7
Wilson SE, Cumming BF, Smol JP (1994) Diatom-salinity relationships in 111 lakes from the Interior Plateau of British Columbia, Canada: the development of diatom-based models for paleosalinity reconstructions. J Paleolimnol 12:197–221
Wolfstein K, Stal LJ (2002) Production of extracellular polymeric substances (EPS) by benthic diatoms: effect of irradiance and temperature. Mar Ecol Prog Ser 236:13–22
Acknowledgments
Tânia Vidal, Catarina Marques, Joana Luísa Pereira and Nelson Abrantes received individual research grants from the Portuguese Foundation for Science and Technology (FCT) (SFRH/BD/48046/2008, SFRH/BPD/47292/2008, SFRH/BPD/44733/2008 and SFRH/BPD/84833/2012, respectively). This study was funded by national funds through FCT, and by the European Regional Development Fund (ERDF) through the Competitiveness Factors Operational Programme (COMPETE), under the scope of the projects PTDC/AAC-AMB/112438/2009 and PEst-C/MAR/LA0017/2013.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Vidal, T., Marques, C., Abrantes, N. et al. Optimization of growth conditions for laboratory and field assessments using immobilized benthic diatoms. Environ Sci Pollut Res 22, 5919–5930 (2015). https://doi.org/10.1007/s11356-014-3713-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3713-y