Abstract
As a simple and effective biorefinery method, deep eutectic solvent (DESs) treatment was proposed to isolate lignin from lignocellulose. In this study, choline chloride/lactic acid (ChCl/Lac) was applied to extract high-purity and antioxidative lignin. Under the optimized conditions (120 °C for 12 h), the lignin profiling indicated that the recovered lignin had a high purity (94.18%), low molecular weight (Mw 1967 g/mol), and excellent thermal stability as compared with milled wood lignin. The lignin samples were characterized by using Fourier transform infrared spectrometry and nuclear magnetic resonance (NMR) spectra. In addition, the NMR spectra indicated the functional groups of the lignin extracted via DESs were less damaged and the backbone structure was not significantly modified. To further determine the potential application of DES-Lignin, the antioxidant activity was evaluated by radical scavenging ability. The IC50 values of a treatment time of about 12 h was 0.174. As expected, DES-Lignin showed obvious advantage in oxidation resistance. In short, the proposed process was considered as a promising biorefinery strategy for lignin first production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
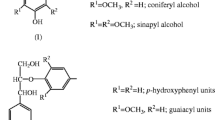
With the depletion of fossil fuels and the aggravation of environmental pollution, lignocellulosic biomass as a renewable and green resource has attracted more attention in the field of transportation fuels and commercial chemicals production (Dwivedi et al. 2009). Lignocellulose has a complex structure, which consists of lignin, hemicellulose, and cellulose. Compared with cellulose, lignin is considered to be a kind of underutilized component that could be converted into industrial products in biorefinery (Inkrod et al. 2018). Lignin, an aromatic polymer compound, consists of three alcohol precursor monomers of guaiacyl (G), sinapyl (S), and p-hydroxyphenyl (H), which could be developed as a kind of potential natural antioxidant because it contains abundant active functional groups (Del Rio et al. 2015; Salanti et al. 2010). Therefore, it is necessary to find a low-cost and environmentally friendly solvent to break the linking bonds among the lignocellulose components for extracting high-purity lignin.
The study found that ionic liquids exhibit the great advantage of destroying the crystal structure of cellulose and removing lignin/hemicellulose from the lignocellulose (Yang et al. 2019). However, high price and toxicity restricted the ionic liquids application to industrial production. Deep eutectic solvent (DESs) is a kind of eutectic liquid composed of hydrogen bond acceptors and hydrogen bond donors, providing similar performance to ionic liquids. In a recent study, DESs was synthesized with choline chloride (ChCl) and lactic acid (Lac) that exhibited the highest selectivity for extracting lignin from lignocellulose under relatively mild conditions (Wang et al. 2019). For this solvent, Lac as a conventional biomass-derived chemical could be produced by the fermentation of biomass, and ChCl as a kind of low-cost, environment friendly chemical that can also be extracted from biomass resources. Hence, DESs has a great potential value owing to its low cost, higher selectivity and nontoxicity (Abbott et al. 2004). Compared with other organic solvents, the novel DES system has the advantage of separating lignin of high purity, low molecular weight and intact structure, which is good for value-added utilization of lignin in the biorefinery (Lynam et al. 2017). To further understand DES-Lignin, it is necessary to study its internal basic structure and compare it with MWL which can well represent the original lignin structure (Rencoret et al. 2015). Due to the rich active groups in lignin, especially phenolic hydroxyl groups, it has significant advantages in scavenging of free radicals. Therefore, more attention should be paid to value-added utilization of DES-Lignin, especially in terms of antioxidant activity, that can be used as a natural preservative for food and cosmetics (Liu et al. 2020).
To the authors` knowledge, there are few studies on the morphological structure and value-added utilization of lignin from Cunninghamia lanceolata by using DES treatment. The previous researchers were mostly concentrated on hardwood or herbaceous biomass, and they ignored the lignocellulose of softwood. Therefore, the purpose of this work is to explore the morphological structure and the antioxidant activity performance of DES-Lignin from Cunninghamia lanceolata. The DES system was synthesized by mixing ChCl and Lac to extract high-purity lignin. Furthermore, the antioxidant capacity of DES-Lignin is based on the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging test. In this work, the structural and morphological transformations of the DES-Lignin and MWL were fully characterized and analyzed using FTIR, gel permeation chromatography (GPC), nuclear magnetic resonance (NMR), and thermal gravimetric analyzer (TGA) techniques. It is expected that the above research could provide some valuable information for researchers in terms of the structural and antioxidant activity of DES-Lignin in biorefinery.
Materials and methods
Materials
The Cunninghamia lanceolata chips were obtained from Shandong furniture company (Linyi, Shandong, China). Wood chips were washed three times by distilled water then air-dried for 7 days. The chips were ground into powder of 40–60 mesh and extracted with ethanol/toluene (1:2, v/v), followed by vacuum drying for 24 h at 80 °C. The composition analysis of Cunninghamia lanceolata is shown in Table 1. All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd.
Isolation of MWL
About 100 g of oven-dried powder was evenly placed in ZrO2 containers and the sample was milled by using a planetary ball mill. Each sample was ground for 24 h with 1 h breaks, then 1 h working, followed by extraction with dioxane/water mixture (85/15, v/v) for about 72 h based on previous literature method (Huang et al. 2011; You et al. 2013).
DES treatment
The DES preparation was carried out as follows: ChCl and Lac were well mixed with different molar ratio, and then the solvent was heated in an oil bath at 60 °C for 60 min to form a transparent homogeneous liquid. The separation of DES-Lignin was based on previous literature method (Li et al. 2017). In each group of experiment, 2.5 g of extractive-free powder and DESs were mixed with a solid/liquid ratio of 1/30 (g/g) in round-bottomed flask, followed by heating in an oil bath at 120 °C and stirring at 300 rpm for 12 h. After the treatment procedure, the mixture was filtered with a G2 funnel to separate the DESs soluble and the solid residue, followed by rinsing the solid residue with deionized water until the solid fraction recovered was a lighter shade. The surplus eutectic solvent which remained in filtrate was removed by rotary evaporator, and then deionized water was added to 500 mL to precipitate for 12 h. Finally, the DES-Lignin was obtained by filtration and freeze-drying for 24 h. Every sample was tested at least three times and the average value was taken and reported.
Solid residue yield was calculated as follows:
DES-Lignin yield was calculated as follows:
Lignin extracted by DESs
To evaluate the efficiency of the treatment, choline chloride/lactic acid (ChCl/Lac) was applied to produce DES-Lignin under the conditions of 120 °C with different molar ratios and time. The DES-Lignin yield and solid residue yield were analyzed and are shown in Fig. 1a, b. As shown in Fig. 1a, the amount of lignin yield increased with increasing molar ratio, while the DES-Lignin yield reached a maximum at a mole ratio of 1:10. The yield of lignin and solid residue was 61.23 and 51.26%, respectively. With excessive lactic acid ratio, the yield of lignin showed a downward trend. As shown in Fig. 1b, with the extension of extraction time, the lignin yield increased with increasing time. While the solid residue showed an opposite trend in the treatment. When the reaction time extended to 12 h, the yield of lignin and solid residue was 81.73 and 42.58%, respectively. As extraction time prolonged, the lignin yield showed a slight increase and the solid residue decreased. Thus, a treatment with a molar ratio of 1:10 and extraction time of 12 h was sufficient for high lignin yield.
Characterization of lignin
The chemical compositions of lignin samples extracted from Cunninghamia lanceolata were determined using the typical procedure of National Renewable Energy Laboratory (Sluiter et al. 2008). The molecular weight distribution of the lignin samples was determined by gel permeation chromatography (Agilent, Santa Clara, CA, USA). FTIR spectra were collected by a Bruker ALPHA FTIR spectrophotometer (Karlsruhe, Germany). Lignin and oven-dried KBr were pressed into transparent films with a ratio of 1/100 mg. The spectra were collected in a range of 500–4000 cm−1 at 4 cm−1 resolution, and 64 scans. The 13C-NMR spectra were collected on a Bruker AVANCE III HD 400 MHz. Approximately, 80 mg of oven-dried lignin sample was fully dissolved in 0.5 ml of dimethyl sulfoxide-d6 and then injected in an NMR tube and analyzed for 2 h. 2D-NMR spectra were analyzed using an Advance II 400 MHz spectrometer (Brucker Daltonic Inc, Bremen, Germany). 80 mg of oven-dried sample was dissolved in 0.5 ml of dimethyl sulfoxide-d6 and then injected in an NMR tube and analyzed for 12 h at 25 °C. The thermal stability was investigated using a thermal gravimetric analyzer (TGA) (DTG-60, Shimadzu, Japan).
Assessment of antioxidant capacity
Evaluation of the antioxidant capacity of DES-Lignin was carried out by ultraviolet spectrophotometry and involving the consumption of the free radical originating from DPPH in ethanol solution at different concentrations of lignin (Li et al. 2012; Lu et al. 2012). The desired lignin was fully dissolved in dioxane/H2O (v/v), and afterward the concentration of DPPPH was adjusted to 25 mg/ml with ethanol. The lignin solution was mixed to DPPH solution (1/1, v/v) and reacted in a dark place at 25 °C for 30 min. After the treatment procedure, the absorbance of the solution was measured at 517 nm using an ultraviolet spectrophotometer. The DPPH radical inhibitory activity was calculated as follows:
A0: the absorbance of sample without lignin; A1: the absorbance of lignin.
Results and discussion
Purity analysis of the lignin
To investigate the purity of the DES-Lignin and MWL, the chemical composition of acid soluble lignin (ASL), acid insoluble lignin (AIL) and other main chemical compositions were determined and are listed in Table 2. The results indicated that 0.86% of glucose (Glu), 0.34% of xylose (Xyl), and 0.21% of arabinose (Arab) were retained in DES-Lignin. In contrast, 1.23% of Glu, 0.76% of Xyl, and 0.23% of Arab were retained in MWL. The ash of two lignin samples was 0.41 and 0.43%, respectively. The lignin purity analysis showed that the two lignin samples both had a high purity (> 90%). The purity of MWL was 92.17%. However, the DES-lignin showed a higher purity (94.18%) and less carbohydrate than MWL. Therefore, high-purity lignin can successfully be selectively fractionated by deep eutectic solvents under the conditions of 120 °C for 12 h.
Molecular weight of lignin
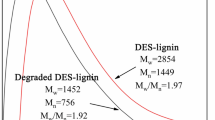
During isolation and extraction process, depolymerization of lignin occurred in the treatment. Lignin macromolecules were degraded into lower molecules under the action of eutectic solvents (Lou et al. 2019). The number-average molecular weights (Mn) and weight-average molecular weights (Mw) for the two lignin samples are shown in Table 3. The Mw and Mn of DES-Lignin were 1967 and 1261 g mol−1, much lower than that of MWL of 5817 and 4308 g mol−1, respectively. Furthermore, the polydispersity index (PI) of DES-Lignin and MWL was 1.56 and 1.35, respectively. Polydispersity of the DES-Lignin was slightly higher than that of MWL, but both lignin samples had a narrow molecular weight distribution (PI < 2.0) that could provide convenience for the downstream application.
FTIR spectra analysis
FTIR is a basic and effective method to analysis different functional groups of lignin (Bauer et al. 2012). A comparison between DES-Lignin and MWL samples showed that the common features and particular vibrations of the fingerprint region were quite similar. It indicated that the DES treatment process had no obvious negative effect on the main functional groups, and the obtained lignin structure was rather complete. The infrared spectra of two lignin samples are illustrated in Fig. 2, and the bands were based on previous literature reports (Liu et al. 2018a; Sun et al. 2014; Xu et al. 2019). The absorbance peaks at 1642 cm−1 were assigned to carboxyl group and unconjugated carbonyl stretching. The typical lignin pattern bands at 1506, and 1456 cm−1 correspond to vibrations of aromatic rings. The absorbance band at 1455 and 1424 cm−1 was assigned to methoxyl groups (Wang et al. 2020). The absorbance peak at 1365 cm−1 corresponds to the presence of syringyl and non-condensed guaiacyl ring breathing with a C–O stretch, and the peak at 1263 cm−1 corresponds to C = O stretch bending signals of guaiacyl ring. The signals corresponding to G-type units (1263 cm–1) presented a higher intensity than those of the S-type units (1365 cm–1), so the spectrum of lignins displayed classical features of softwood lignin of the G,S type (Liu et al. 2018b).
13C-NMR analysis
To investigate the structural features of lignin, the linkages in the structure of DES-Lignin and MWL were analyzed by 13C-NMR technology. The 13C-NMR spectra of samples are presented in Fig. 3, and the positions of signals are listed in Table 4. The lack of peaks between 90 and 100 ppm demonstrated the absence of carbohydrates in lignin samples and this also verified the above chemical compositions analysis. The aromatics carbons of lignin signal emerged at 102–160 ppm in the 13C-NMR spectrum (Capanema et al. 2005). The strong intensity signals of the G units emerged at 150–149.5 ppm (C-3 etherified), 147 ppm (C-4 etherified), 134.5 (C-1), 104 ppm (C-6) and 105 ppm (C-2). The S units were observed by peaks at 154–152 ppm (C-3 C-5 etherified) and 147–148 ppm (C-3 C-5 non-etherified), 125.5 ppm(C-4), and 105–104 ppm (C-2 C-6) (Jiang et al. 2018). The signals of the G and S units of lignin samples were clearly detected in the 13C-NMR spectra, which elucidated the backbone structures of isolated lignin known as G, S-type (Xu et al. 2006). In addition, the peaks of β–O–4 were proven by the signals at 73 ppm and 61 ppm which correspond to Cα, Cβ and Cγ, respectively. The trace caused by –OCH3 can be proven by the signals at 56.4 ppm in lignin samples. At the same time, two peaks emerged at 53.8 and 53.3 ppm, which were attributed to β–β and β-5′ structures.
2D-NMR analysis
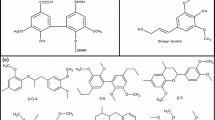
2D-NMR is an effective technology to enhance the understanding of lignin structure. Therefore, the DES-Lignin and MWL fractions were further analyzed by 2D-NMR for more complete structural information. The sidechain (δC/δH 50–90/2.5–5.5) and the aromatic (δC/δH 90–150/5.5–8.0) regions of the HSQC NMR spectra of these two lignin fractions are shown in Fig. 4 and the main lignin structure units are shown in Fig. 5. In the side chain region, the signals were mainly generated from methoxy group and β–O–4′ structure (substructure A) in the lignin fractions (Zikeli et al. 2016). The Cα–Hα corresponding to β–O–4′ substructures appeared at δC/δH71.8/4.81 for structures linked to G lignin units. The Cβ–Hβ correlations confirmed at δC/δH 83.9/4.18 and δC/δH 85.6/4.12 for β–O–4 structures linked to G and S units, respectively. Additionally, Cβ–Hβ and Cγ–Hγ correlations in the resinol substructures B were observed at δC/δH 53.3/3.15, and 71.5/4.23 and 3.83, respectively. The spirodienone substructure C was confirmed by the Cβ–Hβ and Cγ–Hγ correlation at δC/δH of 53.5/3.40, and 63.8/3.50.
As for the aromatic regions of the lignin, the S lignin units presented a rather weak signal for the C2,6–H2,6 correlation at δC/δH 104.1/6.78 (S2,6), Likewise, the signal of C2,6–H2,6 was observed at δC/δH 106.8/7.16 and 7.51 (S′2,6) (Hu et al. 2011). However, the G lignin units presented a higher intensity signal for the C2–H2 (δC/δH 111.6/7.02, G2), C5–H5 (δC/δH 116.1/6.85, G5), and C6–H6 (δC/δH 120.6/6.88, G6). Furthermore, the C2,6–H2 linkages in p-coumarate substructures were verified by the signals at δC/δH 130.0/7.46 (PB2,6). Therefore, G lignin occupied a major position in the separated lignin.
The main structures identified by 2D-NMR spectra of lignin: (A) β-aryl-ether units (β–O–4); (B) resinol substructures (β–β′); (C) phenylcoumaran substructures (β-5′); (G) guaiacyl unit; (S) syringyl unit; (S′) oxidized syringyl units with a ketone at Cα; (PB) p-hydroxybenzoate substructures.
Thermal stability analysis
Lignin pyrolysis is considered to be one of the most feasible methods to convert lignin into bioenergy and industrial chemicals (Manara et al. 2014). Results of the thermogravimetric (TG) and differential thermogravimetric (DTG) analysis are shown in Fig. 6. The TG represents the relationship between the weight loss of lignin samples and the degradation temperature, and DTG shows the corresponding weight loss rate of each obtained sample. The first phase of weight loss before 160 °C was due to moisture evaporation and loss of some organic constituents (Sun et al. 2013). The second phase between 160 and 500 °C was the main degradation stage, where a sharp downward curve of TG emerged with increasing temperature, which can be attributed to the cleavage of the interunit linkages such as β–O–4 and β–β of lignin and the evaporation of monomer phenol (Tejado et al. 2007). The maximum rate of mass loss occurred at 376 °C. As for pyrolysis stage phase, the TG curve of lignin degraded slowly until it remained steady when the temperature raised above 500 °C. The quality loss of lignin was mainly attributed to the degradation of branched chain and aromatic structure to alcohols and aldehydes. At the final temperature, the residue solid yields of DES-Lignin were 38.25%, and 40.63% of MWL.
Antioxidant activity against DPPH radical
Evaluation of the antioxidant activity of lignin is based on free radical scavenging ability test (Dizhbite et al. 2004; Sun et al. 2014), and the curves of antioxidant activity against DPPH and radical scavenging index (RSI) value are shown in Fig. 7. The 50% inhibition percentage (IP) concentration of lignin was calculated and defined as IC50. As presented in Fig. 7, all lignin samples showed strong scavenging effect on DPPH radicals. In the present study, when the reaction time was extended to 12 h, the antioxidant activity of DES-Lignin started to be higher than that of commercial antioxidants (butylated hydroxyanisole, BHA). The IC50 values of treatment time of about 12 h, 16 h, 20 h, 24 h were 0.174, 0.163, 0.157 and 0.147, as compared to 0.180 for BHA, respectively. The data suggested that the lignin samples had higher antioxidant activity than BHA. Lignin with a higher content of condensed G-OH showed excellent antioxidant activity (Sun et al. 2018). The results showed that the type of phenolic hydroxyl groups played a pivotal part in antioxidant activity of lignin. DES-lignin with excellent antioxidant properties could be used as natural preservative for food or cosmetics.
Conclusion
In this work, Cunninghamia lanceolata was subjected to DESs to isolate high-purity lignin and evaluate its antioxidant activity. Due to the mild treatment process, the extracted lignin had a relatively complete structure, and the backbone characteristics were not significantly modified. The result showed that the yield of isolated DES-Lignin is 81.73% under optimal conditions (120 °C, 1:10, 12 h). At the same time, DES-Lignin had a low molecular weight (Mw 1967 g/mol) and higher purity of 94.18% than MWL (92.17%). It was observed that the lignin had similar structural characteristics to MWL, and the main interaction units were mainly β–O–4 aryl ether. Moreover, DES-Lignin showed excellent performance in scavenging free radicals, indicating that it could develop to a kind of potential natural antioxidant as a preservative for food and cosmetics.
References
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147. https://doi.org/10.1021/ja048266j
Bauer S, Sorek H, Mitchell VD, Ibáñez AB, Wemmer DE (2012) Characterization of Miscanthus giganteus Lignin isolated by ethanol organosolv process under reflux condition. J Agr Food Chem 60:8203–8212. https://doi.org/10.1021/jf302409d
Capanema EA, Balakshin MY, Kadla JF (2005) Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic resonance spectroscopy. J Agr Food Chem 53:9639–9649. https://doi.org/10.1021/jf0515330
Del Río JC, Lino AG, Colodette JL, Lima CF, Gutiérrez A, Martínez ÁT, Lu F, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenerg 81:322–338. https://doi.org/10.1016/j.biombioe.2015.07.006
Dizhbite T, Telysheva G, Jurkjane V, Viesturs U (2004) Characterization of the radical scavenging activity of lignins natural antioxidants. Bioresour Technol 95:309–317. https://doi.org/10.1016/j.biortech.2004.02.024
Dwivedi P, Alavalapati JRR, Lal P (2009) Cellulosic ethanol production in the United States: conversion technologies, current production status, economics, and emerging developments. Energy Sustain Dev 13:174–182. https://doi.org/10.1016/j.esd.2009.06.003
Hu G, Cateto C, Pu Y, Samuel R, Ragauskas AJ (2011) Structural characterization of switchgrass lignin after ethanol organosolv pretreatment. Energ Fuel 26:740–745. https://doi.org/10.1021/ef201477p
Huang F, Singh PM, Ragauskas AJ (2011) Characterization of Milled Wood Lignin (MWL) in loblolly pine stem wood, residue, and bark. J Agr Food Chem 59:12910–12916. https://doi.org/10.1021/jf202701b
Inkrod C, Raita M, Champreda V, Laosiripojana N (2018) Characteristics of lignin extracted from different lignocellulosic materials via organosolv fractionation. Bioenerg Res 11:277–290. https://doi.org/10.1007/s12155-018-9895-2
Jiang B, Zhang Y, Guo T, Zhao H, Jin Y (2018) Structural characterization of lignin and lignin-carbohydrate complex (LCC) from ginkgo shells (Ginkgo biloba L) by comprehensive NMR spectroscopy. Polymers 10:736. https://doi.org/10.3390/polym10070736
Li M, Sun S, Xu F, Sun R (2012) Microwave-assisted organic acid extraction of lignin from bamboo: structure and antioxidant activity investigation. Food Chem 134:1392–1398. https://doi.org/10.1016/j.foodchem.2012.03.037
Li T, Lyu G, Liu Y, Lou R, Lucia L, Yang G, Chen J, Saeed H (2017) Deep eutectic solvents (DESs) for the isolation of willow lignin (Salix matsudana cv. Zhuliu). Int J Mol Sci 18:2266. https://doi.org/10.3390/ijms18112266
Liu C, Si C, Wang G, Jia H, Ma L (2018a) A novel and efficient process for lignin fractionation in biomass-derived glycerol-ethanol solvent system. Ind Crop Prod 111:201–211. https://doi.org/10.1016/j.indcrop.2017.10.005
Liu C, Wang X, Lin F, Zhang H, Xiao R (2018b) Structural elucidation of industrial bioethanol residual lignin from corn stalk: a potential source of vinyl phenolics. Fuel Process Technol 169:50–57. https://doi.org/10.1016/j.fuproc.2017.09.008
Liu J, Qi L, Yang G, Xue Y, He M, Lucia LA, Chen J (2020) Enhancement of lignin extraction of poplar by treatment of deep eutectic solvent with low halogen content. Polymers 12:1599. https://doi.org/10.3390/polym12071599
Lou R, Ma R, Lin K, Ahamed A, Zhang X (2019) Facile extraction of wheat straw by deep eutectic solvent (DES) to produce lignin nanoparticles. ACS Sustain Chem Eng 7:10248–10256. https://doi.org/10.1021/acssuschemeng.8b05816
Lu Q, Zhu M, Zu Y, Liu W, Yang L, Zhang Y, Zhao X, Zhang X, Zhang X, Li W (2012) Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem 135:63–67. https://doi.org/10.1016/j.foodchem.2012.04.070
Lynam JG, Kumar N, Wong MJ (2017) Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresource Technol 238:684–689. https://doi.org/10.1016/j.biortech.2017.04.079
Manara P, Zabaniotou A, Vanderghem C, Richel A (2014) Lignin extraction from Mediterranean agro-wastes: Impact of pretreatment conditions on lignin chemical structure and thermal degradation behavior. Catal Today 223:25–34. https://doi.org/10.1016/j.cattod.2013.10.065
Rencoret J, Prinsen P, Gutiérrez A, Martnez ÁT, del Río JC (2015) Isolation and structural characterization of the milled wood lignin, dioxane lignin, and cellulolytic lignin preparations from brewer’s spent grain. J Agr Food Chem 63:603–613. https://doi.org/10.1021/jf505808c
Salanti A, Zoia L, Orlandi M, Zanini F, Elegir G (2010) Structural characterization and antioxidant activity evaluation of lignins from rice husk. J Agr Food Chem 58:10049–10055. https://doi.org/10.1021/jf102188k
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure. National renewable laboratory. Anal pro 1617:1–16
Sun S, Li M, Yuan T, Xu F, Sun R (2013) Effect of ionic liquid/organic solvent pretreatment on the enzymatic hydrolysis of corncob for bioethanol production. Part 1: structural characterization of the lignins. Ind Crop Prod 43:570–577. https://doi.org/10.1016/j.indcrop.2012.07.074
Sun S, Wen J, Ma M, Sun R, Jones GL (2014) Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind Crop Prod 56:128–136. https://doi.org/10.1016/j.indcrop.2014.02.031
Sun S, Liu F, Zhang L, Fan X (2018) One-step process based on the order of hydrothermal and alkaline treatment for producing lignin with high yield and antioxidant activity. Ind Crop Prod 119:260–266. https://doi.org/10.1016/j.indcrop.2018.04.030
Tejado A, Peña C, Labidi J, Echeverria JM, Mondragon I (2007) Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresource Technol 98:1655–1663. https://doi.org/10.1016/j.biortech.2006.05.042
Wang Z, Hong S, Wen J, Ma C, Tang L, Jiang H, Chen J, Li S, Shen X, Yuan T (2019) Lewis acid-facilitated deep eutectic solvent (DES) pretreatment for producing high-purity and Antioxidative Lignin. ACS Sustain Chem Eng 8:1050–1057. https://doi.org/10.1039/C8GC03064B
Wang H, Liu Z, Hui L, Ma L, Zheng X, Li J, Zhang Y (2020) Understanding the structural changes of lignin in poplar following steam explosion pretreatment. Holzforschung 74:275–285. https://doi.org/10.1515/hf-2019-0087
Xu F, Sun J, Sun R, Fowler P, Baird MS (2006) Comparative study of Organosolv lignins from wheat straw. Ind Crop Prod 23:180–193. https://doi.org/10.1016/j.indcrop.2005.05.008
Xu M, Wang C, Lyu G, Zhong L, Yang L, Wang Z, Qin C, Ji X, Yang G, Chen J, Xu F (2019) Structural characterization and antioxidant activity of milled wood lignin from xylose residue and corncob. Polymers 11:2092. https://doi.org/10.3390/polym11122092
Yang M, Zhang X, Cheng G (2019) A two-stage pretreatment using dilute sodium hydroxide solution followed by an ionic liquid at low temperatures: toward construction of lignin-first biomass pretreatment. Bioresour Technol Rep 7:100286. https://doi.org/10.1016/j.biteb.2019.100286
You TT, Mao JZ, Yuan TQ, Wen JL, Xu F (2013) Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J Agric Food Chem 61:5361–5370. https://doi.org/10.1021/jf401277v
Zikeli F, Ters T, Fackler K, Srebotnik E, Li J (2016) Wheat straw lignin fractionation and characterization as lignin-carbohydrate complexes. Ind Crop Prod 85:309–317. https://doi.org/10.1016/j.indcrop.2016.03.012
Acknowledgements
This work was financially supported by the National Key Research and Development Plan (Grant No.2017YFB0307901).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y., Liu, Z., Ma, L. et al. Structural characterization and evaluation of the antioxidant activity of DES-Lignin isolated from Cunninghamia lanceolata. Wood Sci Technol 55, 1041–1055 (2021). https://doi.org/10.1007/s00226-021-01300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-021-01300-x