Abstract
Perfluorooctanoic acid (PFOA) is considered a persistent environmental pollutant. The aim of this study was to assess the potential toxicity of PFOA to earthworms (Eisenia fetida) in artificial soil. The activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione S-transferase (GST) as well as the contents of malondialdehyde (MDA) were measured after exposure to 0, 5, 10, 20, and 40 mg kg−1 PFOA in soils for 7, 14, 21, and 28 days. The results showed that SOD activity increased at 14 days and decreased from 21 to 28 days; MDA levels were highest in the treatment with 40 mg kg−1 PFOA after 28 days of exposure. In contrast, CAT and POD activities increased after 14–21 days of exposure and significantly decreased with long-term exposure (28 days). GST activity increased significantly from 14 to 28 days. Our results indicate that PFOA has biochemical effects on E. fetida, thereby contributing to our understanding of the ecological toxicity of PFOA on soil invertebrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyfluorinated chemicals (PFCs) are being increasingly used as surfactants in furniture, clothing, and various other industry sectors due to their unique chemical properties, such as high chemical stability and extremely low surface tension. They are also components of firefighting foam (Jensen and Leffers 2008). The most commonly PFCs used are perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) (Zhang et al. 2008). In 2001, the company 3M, one of the world’s leading manufacturers of PFCs, decided to voluntarily eliminate the production of perfluorooctane sulfonyl surfactants due to an increased detection of PFOS worldwide (Giesy and Kannan 2002). With the discontinuation of perfluorooctane sulfonate and its derivatives, PFOA gradually becomes the most produced and emitted perfluoroalkyl acid compound, with higher detection levels than PFOS. Between 1950 and 2000, an estimated 500 metric tons of PFOA existed in the environment (Lau et al. 2007). The Yangtze River was moderately contaminated with both chemicals: median concentration was 4.2 ng L−1 for PFOS and 5.4 ng L−1 for PFOA, and a remarkably high concentration of PFOA was found at two sampling sites of the Yangtze River (110.6 and 297.5 ng L−1) (Jin et al. 2009). Toxicological tests carried out in Italy and Greece detected PFOA in all plasma samples taken from different groups of adults and women of reproductive age (Vassiliadou et al. 2010; De Felip et al. 2015). Meanwhile, Jin et al. (2015) have observed that PFOA was still the major chemical in use at a main fluorochemical manufacturing park in China.
Perfluorooctanoic acid (C8HF15O2, PFOA) is a strong, organic acid, with a solid, crystalline form at room temperature. It is considered a persistent organic pollutant in the environment, majorly because it is difficult to be hydrolyzed, photolyzed, and degraded by microbial populations (Cui et al. 2012). According to Olsen et al. (2007), the half-life of PFOA in the human blood serum is as long as 3.8 years.
Perfluorooctanoic acid is widely distributed in aquatic environments, including groundwater and surface waters. Generally, PFOA contaminates aquatic bodies in one of the two following ways: (1) through industrial wastewater (Liu et al. 2016b); e.g., according to Wang et al. (2012), industrial waste discharged into the Haihe River and the Liaohe River could be the cause of the higher PFC levels in the two major Chinese cities: Tianjin and Liaoning. (2) Through long-distance migration from the atmospheric environment, PFOA in the atmosphere could eventually reach soils and water via wet and dry settlements (Liu et al. 2016b).
Recently, the presence of PFOA in soil and its impacts on the ecology have been receiving increased attention. For example, unusually high level of PFOS, PFOA, and other fluorine-containing compounds in agricultural soils in Decatur, Alaska, USA, has caused significant concerns (Renner 2009). Contamination with PFOA, especially in soils, has become the focus of a number of studies. For example, Zhang et al. (2013b) have reported decreased enzymatic activities in PFOA-polluted soils, He et al. (2016) have observed PFOA induced weight loss and bioaccumulation in earthworms, and Zhu (2015) has determined acute toxic effects of PFOA on Eisenia fetida: after 7 and 14 days of exposure in artificial soil, LD50 of earthworm was 964.85 and 932.79 mg kg−1, respectively. Despite much effort directed to the impact of PFOA on E. fetida, the relationship between the damage of the antioxidant enzymatic system of E. fetida and PFOA-polluted soils remains unknown.
A number of studies have focused on the toxicity of PFOA, especially hepatotoxicity, which has been demonstrated in experiments using rodents and fish (Cui et al. 2012). Liu et al. (2007) have shown that PFOA can induce primary, cultured Tilapia (Oreochromis niloticus) hepatocytes to produce oxidative stress and induce apoptosis with involvement of caspases: significant induction of reactive oxygen species (ROS) accompanied by increases in activities of superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR) was found, while activities of glutathione peroxidase (GPx) and glutathione S-transferase (GST) were decreased. Chen et al. (2017) have found that PFOA can inhibit luteal function via oxidative stress and apoptosis in pregnant mice: PFOA administration inhibited activities of SOD and CAT, increased generation of hydrogen peroxide and malondialdehyde (MDA), and downregulated level of Bcl-2 and upregulated p53 and BAX proteins. Yang (2010) also found that PFOA may induce peroxisomal fatty acid oxidation and impose the oxidative stress through the alteration of cellular oxidative homeostasis in the liver of male Japanese medaka (Oryzias latipes): a significant inhibition of CAT activity at high doses with no changes of SOD or GPx activities in the liver. In a similar study, Panaretakis et al. (2001) indicated that PFOA induces the production of ROS and thereby impacts the mitochondrion-mediated pathway in human HepG-2 cells. Jantzen et al. (2017) have shown that chronic, low-dose exposure of zebrafish to PFOA significantly altered normal development, survival, and fecundity.
In this study, we investigated the damages to the antioxidant system (SOD, CAT, peroxidase (POD)) and GST of E. fetida caused by exposure to PFOA in artificial soil under standard laboratory conditions. The main goal of this study was to enhance our understanding of the effects of PFOA on E. fetida and to provide a basis for ecological risk assessment and early warning indicators of soils contaminated with PFOA.
Material and methods
Chemicals and earthworms
Perfluorooctanoic acid (98% purity) was purchased from Beijing Bailingwei Technology Co., Ltd. (Beijing). Other chemicals used in this study were also of analytical grade and purchased from local commercial sources. Glassware was meticulously cleaned to reduce any background of PFOA contamination. All chromic acid-washed glassware was placed in an oven at 300 °C overnight, after cooling until use.
Earthworms (E. fetida) were provided by Shijiazhuang Zhongxiang earthworm breeding professional cooperatives. We selected vigorous earthworms with obvious reproductive bands, between 250 and 350 mg, and 2 months old. The worms were precultured in an incubator at 20 ± 1 °C, with 75 ± 2% humidity, for 7 days prior to the experiments.
PFOA exposure
To evaluate the toxic effects of PFOA on earthworms (E. fetida), we conducted artificial soil tests according to the OECD normal method (OECD 1984). The artificial soil was mixed with 10% sphagnum peat, 20% kaolin clay (more than 50% of kaolin), and 70% industrial quartz sand (containing 50% or more of fine particles of 0.05–0.2 mm). Soil pH was adjusted to 6.0 ± 0.5 by adding calcium carbonate. The artificial soil was air-dried, passed through a 2-mm nylon sieve, and divided into 500 g portions. For the toxicity tests, we used PFOA concentrations of 0, 5, 10, 20, and 40 mg PFOA kg−1 soil−1. The PFOA was dissolved in distilled water and thoroughly mixed into the artificial soil to obtain the different concentrations. All soils were rehydrated to 35% moisture and kept for 1 day to equilibrate. The artificial soil was transferred to 1000-mL beakers, with each beaker containing 500 g of soil. Each concentration gradient consisted of three replicates.
The earthworms were cultivated for 24 h in untreated artificial soil and then placed in the PFOA-contaminated artificial soil. Ten worms with uniform body lengths and weights were randomly divided into the five treatment groups, with three replicates per treatment. The beakers were sealed with a plastic film containing holes for respiration and placed in an artificial light incubator at 20 ± 1 °C, 75 ± 2% humidity, and a photoperiod of 12-h light/12-h dark.
Preparation of earthworm extracts
A single earthworm was collected from each replicate beaker on the 7th, 14th, 21st, and 28th day after PFOA application and then washed and weighed. Subsequently, the earthworms were individually placed into a 25-mL glass homogenizer and 0.05 M phosphate buffer (pH 7.8) was added; the mass volume of earthworm weight and phosphate buffer was 1:10 (g:mL). The worms were homogenized under ice-cold conditions and subsequently transferred to 15-mL centrifuge tubes. Centrifugation was performed at 10,000 r min−1 for 20 min at 4 °C, and the supernatant was stored at −20 °C until analysis.
SOD activity
SOD activity was determined by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) chloride, as described by Song et al. (2009), with slight modifications. The reaction mixture (3 mL) contained 50 mM phosphate buffer (pH 7.8), 100 μM ethylene diamine tetra acetic acid disodium salt (EDTA-Na2), 130 mM methionine, 750 μM NBT, 20 μM riboflavin, and 50 μL enzyme extract. Riboflavin was added last, and the tubes were shaken and illuminated with 4000-lx fluorescent tubes. The reaction was allowed to proceed for 30 min; subsequently, the lights were switched off and the tubes were covered with a black cloth. Absorbance of the reaction mixture was read at 560 nm. One unit (U) of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the NBT photoreduction rate; the result was expressed as units per milligram protein.
POD activity
POD activity was determined according to the method established by Kochba et al. (1977), with slight modifications. The reaction mixture was prepared by adding 28 μL guaiacol and 19 μL of 30% hydrogen peroxide (H2O2) to 50 mL phosphate buffer (100 mM, pH = 6.0). Subsequently, 20 μL of the supernatant was added to 3 mL of the reaction mixture; absorbance was measured spectrophotometrically at 470 nm every 30 s, with a total of six readings.
CAT activity
CAT activity was determined according to the method established by Mueller et al. (1997), with slight modification. At room temperature, the reference cell contained 10 μL enzyme extract and 3.0 mL phosphate buffer I; the sample cell contained 10 μL enzyme extract and 3.0 mL H2O2-phosphate buffer II. Phosphate buffer I was deionized water dissolved in 3.522 g KH2PO4 and 14.612 g Na2HPO4·12H2O, with the volume brought up to 1 L. The H2O2-phosphate buffer II, 160 μL H2O2 (30%, w/v), was diluted to 100 mL with phosphate buffer I. The reference and sample cells were measured every 5 s at 0 to 60 s, using a 250-nm, 1-cm quartz cuvette to determine enzyme activity.
GST activity
GST activity was determined according to the method of Habig et al. (1974). Various substrates were used in assays to further characterize GSTs, using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The assays were carried out by monitoring the appearance of the conjugated complex of CDNB and GSH at 340 nm. The homogenization buffer contained 75.6 mL of 0.2 M Na2HPO4 and 14.4 mL of 0.2 M NaH2PO4 solution; subsequently, 20 mL glycerol, 0.0585 g EDTA, 0.0031 g dithiothreitol, and 2 mL of 100 mM PMSF solution were added; pH was adjusted to 7.5. The mixture was stirred with a glass rod and brought to a volume of 200 mL.
Protein content
Determination of the protein content was necessary to calculate SOD, CAT, and POD activities. Enzyme assay are mostly based on the protein content change. The protein content was determined according to the method established by Bradford (1976). Bovine serum albumin (BSA) was used as the standard. The absorbance was measured at 595 nm. The standard curve was plotted with the protein concentration (mg mL−1) as the abscissa and the absorbance as the ordinate. Take 0.1 mL enzyme solution and determine the absorbance by the above method.
MDA content
MDA content is usually measured by the addition of thiobarbituric acid and subsequent spectrophotometry (Esterbacer and Zollner 1989). This method is frequently referred to as the thiobarbituric acid-reactive substance (TBARS) assay (Lykkesfeldt and Svendsen 2007). The reaction mixture (4.4 mL) contained 0.2 mL of 8.1% SDS, 0.2 mL of 100 mM phosphate buffer (pH 7.8), 1.5 mL of 20% acetate buffer, 1.5 mL of 0.5% barbituric acid solution, and 1 mL deionized water. The control and sample treatment solution were heated up to 95 °C in a water bath for 1 h and cooled immediately, and absorption was measured at 532 nm on a spectrophotometer. Malondialdehyde was calculated using the molar extinction coefficient: e = 1.56 × 105 M−1 cm−1 (Gonzalez Flecha et al. 1991); MDA (nmol mg protein−1) = (OD / (e × c).
Statistical analysis
The relationships between PFOA concentration and SOD, POD, CAT, and GST activity and MDA content were tested by analysis of variance (ANOVA). Significance level was set at p < 0.05; values represent mean ± SD. All analyses were performed using the software package SPSS (Standard version 13.0, SPSS Inc.).
Results
SOD activity
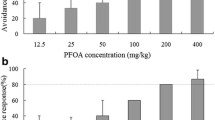
Figure 1 shows that SOD activity changed with PFOA concentration and exposure time. SOD activity in earthworms showed a trend of activation at the beginning and an inhibition towards the end. In comparison with the control, SOD activity was significantly increased on day 14. After 21 to 28 days of exposure, SOD activity significantly decreased, reaching the highest inhibition rate in the 40 mg kg−1 treatment group as the concentration increased.
CAT activity
The CAT activity changed with PFOA concentration and exposure duration (Fig. 2). It was significantly inhibited after 7 days of exposure. In comparison with the control, CAT activity of earthworms significantly increased from days 14 to 21. After 14 days of exposure, the highest activation rate was achieved in the 20 mg kg−1 treatment group. After 28 days of exposure, CAT activity decreased with increasing concentration.
POD activity
Changes in the activity of POD are presented in Fig. 3. After 7 days of exposure, POD activity was inhibited, although the difference to the control group was not significant. The POD activity significantly increased from 14 to 21 days of exposure, reaching maximum activation rates in the 20 mg kg−1 treatment group compared to the control. After 28 days of exposure, POD activity was inhibited by increasing PFOA concentrations, with the highest inhibition rates in the 40 mg kg−1 treatment.
GST activity
Figure 4 shows the changes in GST activity in the different treatments. Overall, GST activity was significantly stimulated by the treatments, compared to the control group, except at 7 days of exposure. After 28 days of exposure, GST activity significantly increased, except in the 20 and 40 mg kg−1 treatment groups.
Protein content
The effect of different PFOA doses on the concentration of soluble protein in earthworms is shown in Fig. 5. After 7 days, compared with the control group, soluble protein content was not significantly different. After 21 days of exposure, soluble protein content increased with increasing concentration, with a significant increase in the treatment with 20 mg kg−1 PFOA. After 28 days of exposure, compared to the control group, soluble protein content was significantly decreased, except in 5 and 10 mg kg−1.
MDA content
The effect of different PFOA doses on the concentration of MDA in earthworms is shown in Fig. 6. Levels of MDA increased with exposure time and PFOA concentration. After 14 days, MDA content was significantly increased in the treatment with 20 mg kg−1 PFOA. After 21 days of exposure, the MDA content increased with increasing concentration, with a significant increase in the treatment with 40 mg kg−1 PFOA. After 28 days of exposure, compared to the control group, MDA was significantly increased in all treatment groups, reaching maximum levels in the treatment with 40 mg kg−1.
Discussion
The present study investigated the effect of PFOA in soil on enzyme activities in earthworms. Our results show that enzymatic activity is impacted by different concentrations of PFOA and exposure times. The molecular response of the organisms can be used as an early warning index to assess the potential adverse effects of pollutants on the environment (Gao et al. 2007). In soil environments, earthworms are an important component of the ecosystem and have an enrichment effect on organic pollutants. Because of their relatively large size, a number of parameters in the earthworm life cycle can be easily measured, making earthworms important indicators of environmental pollution (Thompson 1971).
Enzymatic systems include SOD, CAT, POD, GSH-Px and other antioxidant enzymes, while nonenzymatic systems include GSH and other nonenzymatic substances. SOD, CAT, POD and GSH-Px activities are often used as biomarkers to indicate ROS production and are involved in ROS detoxification (Wen et al. 2011; Caverzan et al. 2016). There is a positive correlation between CAT and SOD, which operate together (Flnkel and Holbrook 2000). Superoxide dismutase dismutates superoxide radical (O2 −·) to H2O2 and oxygen. However, hydrogen peroxide is also toxic to cells and has to be further detoxified by catalase and peroxidases to water and oxygen (Singh et al. 2006).
SOD plays an important role in protecting cells against oxygen free radicals by decomposing the superoxide radical (O2 −) into H2O2 and oxygen (Anju et al. 2013). In this study, after 7 days of PFOA exposure, SOD values were not significantly increased. Our results are in agreement with the findings of Liu et al. (2015), who found that in earthworms exposed to certain concentrations of bromadiolone, the SOD activity had slight change in most groups at 7 days. However, after 14 days, SOD was significantly higher in 5–40 mg kg−1 compared to the control group. From 21 to 28 days of exposure, SOD was significantly inhibited, indicating that PFOA causes earthworms to produce active radicals such as peroxides or hydrogen peroxides. Increased SOD levels, induced by ROS, prevent the organisms from damage. With increasing PFOA concentrations, SOD activities decreased, indicating that the antioxidant defense system could not tolerate excessive ROS levels induced by PFOA stress, leading to cell dysfunction. Oxidative stress after prolonged exposure to contaminants can quickly and easily destroy the balance between O2 − formation and removal under normal physiological conditions maintained by SOD (Du et al. 2015a). Wang et al. (2017) also found that in earthworms exposed to dimethomorph, the SOD activity at higher doses was significantly inhibited on days 14 and 28.
H2O2 is the main metabolite of the SOD catalysis process; it is cytotoxic and is further removed by CAT and POD (Zhang et al. 2013a). POD catalyzes the oxidation of various organic and inorganic compounds, such as hydrogen peroxide or related compounds (Dunford and Stillman 1976). In our study, POD activity was significantly increased from 14 to 21 days of exposure; after 28 days, POD activity decreased with increasing PFOA concentration, with the highest inhibition in the 40 mg kg−1 treatment. Similar findings have been observed previously (Xu et al. 2013). Towards the end of our study, POD activity in the treatments with high PFOA dosages and long exposure times was significantly lower compared with the control group. This may have been due to a decreased enzyme protein synthesis or irreversible inactivation of enzyme proteins due to increased production of free radicals (Jafari 2007).
CAT plays an important role in the antioxidant system. It protects cells from damage by converting H2O2 to water and oxidizing it to molecular oxygen (Zamocky et al. 2008). Changes in CAT activity in cells reflect changes in oxidative stress induced by contaminants (Cao et al. 2013). In our study, the CAT activity in earthworms was inhibited after PFOA-treated groups during early exposure. Similar results have been reported by Xu et al. (2013) after exposure to PFOS; CAT activities in earthworms were significantly lower from 2 to 7 days of exposure. From 14 to 21 days, CAT activity significantly increased, indicating that earthworms have the ability to resist oxidative stress. In other words, increased H2O2 levels will lead to higher CAT activity in earthworms. Imre et al. (1984) found that in 5-month-old mice fed with low-dose aqueous H2O2, CAT activity in the liver increased significantly. After exposure for 28 days, CAT activity was inhibited compared with the control group, indicating that after long-term exposure to a high dose of PFOA, the excessive ROS levels destroyed the antioxidant enzyme system of earthworms, thereby inhibiting CAT.
GST is a family of multifunctional enzymes involved in cell detoxification and excretion of physiologically and exogenous substances (Wilce and Parker 1994) and has a wide range of functions, such as the removal of reactive oxygen species and the regeneration of S-thiolated protein (both of which are the consequence of oxidative stress), catalysis of the combination of endogenous ligands, and catalysis of metabolic reactions unrelated to detoxification (Sheehan et al. 2001). In the potential biomarker, earthworm GST enzymes respond to toxin exposure (LaCourse et al. 2009). Based on our results, GST activity in earthworms was significantly inhibited after 7 days of exposure to PFOA. Similarly, Wang et al. (2016) have reported that GST activity in E. fetida was inhibited at the early exposure to different concentrations of imidaclothiz, possibly as GST is also one of the pathways in cellular oxidative stress reaction (Leiers et al. 2003). Inhibition of GST activity might result from changes in enzyme synthesis and inactivation of GSH and glutathione (Zhang et al. 2017). In our study, PFOA could stimulate GST activity from 14 to 28 days, where an increase in GST activity indicated that GST has detoxification and antioxidant capacity. However, after 28 days of exposure, GST activity was significantly increased, except in the 20–40 mg kg−1 treatment. Zhang et al. (2015) showed that GST activity in E. fetida was inhibited after 28 days of exposure to high concentrations of spirotetramat. Aly and Schröder (2007) have pointed out that herbicides may, to a certain extent, be detoxified by earthworms; however, they are also potent stress factors. High doses or prolonged exposure might negatively affect earthworms and limit their viability. Thus, decreased GST activities may be due to the impacts of high concentrations of PFOA on earthworm biosynthesis. However, this hypothesis needs to be tested in further studies.
Earthworm biomarkers include the decrease of protein content and enzyme activities in response to agrochemicals (Mosleh et al. 2003). Protein content also can be used as a biomarker in response to organic pollutants. In our study, after 21 days of exposure, soluble protein content increased with increasing concentration, with a significant increase in the treatment with 20 mg kg−1 PFOA. The increase in protein contents of earthworms might be due to increased synthesis of metabolic enzymes and stress proteins. Our results are in agreement with the findings of Tripathi et al. (2010), who observed that in earthworms exposed to carbofuran, the protein content increased significantly in all treatment groups of three species earthworms. With longer exposure times, compared to the control group, soluble protein content was significantly decreased, except in 5 and 10 mg kg−1. With the decrease in protein content of earthworms at higher doses PFOA, to overcome the stress situation, earthworms require high energy and this energy demand may have led to the simulation of protein catabolism (Ribeiro et al. 2001).
MDA is a major product of the oxidation reaction between free radicals and unsaturated fatty acids in cellular membranes (Du et al. 2015b). It is commonly used as a measure of lipid hydroperoxides, which has led to the term “lipid peroxidation” (Lykkesfeldt and Svendsen 2007). Malondialdehyde may cause some degree of cell damage, and the MDA assay has been found to be one of the better predictors of oxidative damage, which is believed to be the most reliable biomarker of lipid oxidation (Morrow 2000). In our study, MDA contents changed slightly during early exposure (7–14 days). With longer exposure times, MDA contents significantly increased in all treatment groups and reached a maximum accumulation at 40 mg kg−1; the other treatments (5, 10, and 20 mg kg−1) showed no obvious changes. This pattern may be due to the successful defense of earthworm antioxidant enzymes and detoxification enzymes under low PFOA doses. However, with increased PFOA doses and exposure times, the antioxidant enzymes cannot cope with the excessive free radicals, leading to lipid peroxidation and increased MDA contents. Our results are in agreement with the findings of Liu et al. 2016a), who observed that in earthworms exposed to ionic liquid [omim]PF6, the MDA content increased significantly at higher doses rather than at lower doses.
Conclusion
In the present study, several indicators were used to assess the potential toxicity of PFOA to earthworms (E. fetida) in artificial soils. These results provide a theoretical basis to explain the physiological mechanism of organic pollutant accumulation. We found that PFOA was causing the increased antioxidant and detoxification activities. The activity of SOD was stimulated at 14 days of exposure time, while activities of CAT, POD, and GST were significantly increased from 14 to 21 days. However, with longer exposure times, SOD, CAT, and POD activities decreased. Compared with the controls, MDA contents changed slightly during early exposure (7–14 days); with longer exposure times, MDA contents significantly increased in all treatment groups. Overall, our results show that PFOA has a potential biochemical toxic effect on E. fetida.
References
Aly MAS, Schröder P (2007) Effect of herbicides on glutathione S-transferases in the earthworm, Eisenia fetida. Environ Sci Pollut Res 15:143–149
Anju A, Jeswin J, Thomas PC, Paulton MP, Vijayan KK (2013) Molecular cloning, characterization and expression analysis of cytoplasmic Cu/Zn-superoxide dismutase (SOD) from pearl oyster Pinctada fucata. Fish Shellfish Immunol 34:946–950
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao X, Yang C, Liu J, Hui X, Yang W, Li S, Tian Y, Cai L (2013) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia fetida) induced by flumorph. Appl Biochem Biotechnol 172:2276–2285
Caverzan A, Casassola A, Patussi Brammer S (2016): Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. Abiotic and Biotic Stress in Plants- Recent Advances and Future. Intech. doi:10.5772/61368
Chen Y, Zhou L, Xu J, Zhang L, Li M, Xie X, Xie Y, Luo D, Zhang D, Yu X, Yang B, Kuang H (2017) Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol 69:159–166
Cui Y, Bai C, Xu T, Wang X, Chen Y, Jin D (2012) PFOA-induced developmental toxicity, behavior change and DNA damage in zebrafish embryos. Asian J Ecotoxicol 7:241–250
De Felip E et al (2015) Current exposure of Italian women of reproductive age to PFOS and PFOA: a human biomonitoring study. Chemosphere 137:1–8
Du L, Li G, Liu M, Li Y, Yin S, Zhao J (2015a) Biomarker responses in earthworms (Eisenia fetida) to soils contaminated with di-n-butyl phthalates. Environ Sci Pollut Res Int 22:4660–4669
Du L, Li G, Liu M, Li Y, Yin S, Zhao J, Zhang X (2015b) Evaluation of DNA damage and antioxidant system induced by di-n-butyl phthalates exposure in earthworms (Eisenia fetida). Ecotoxicol Environ Saf 115:75–82
Dunford HB, Stillman JS (1976) On the function and mechantism of action of feroxidases. Coord Chem Rev 19:187–251
Esterbacer H, Zollner H (1989) Methods for determination of aldehydic lipid peroxidation products. Free Radic Biol Med 7:197–203
Flnkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Gao Y, Sun Z, Sun X, Bao Y (2007) Toxic effect of olaquindox antibiotic on Eisenia fetida. Eur J Soil Biol 43:S252–S255
Giesy JP, Kannan K (2002) Perfluorochemical surfactants in the environment. Environ Sci Technol 36(7):146A–152A
Gonzalez Flecha BS, Repetto M, Evelson P, Boveris A (1991) Inhibition of microsomal lipid peroxidation by α-tocopherol and α-tocopherol acetate. Xenobiotica 21:1013–1022
Habig WH, Pabst MJ, Jakob WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
He W, Megharaj M, Naidu R (2016) Toxicity of perfluorooctanoic acid towards earthworm and enzymatic activities in soil. Environ Monit Assess 188:424
Imre S, Toth F, Fachet J (1984) Superoxide dismutase, catalase and lipid peroxidation in liver of young mice of different ages. Mech Ageing Dev 28:297–304
Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231:30–39
Jantzen CE, Toor F, Annunziato KA, Cooper KR (2017) Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod Toxicol 69:34–42
Jensen AA, Leffers H (2008) Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31:161–169
Jin YH, Liu W, Sato I, Nakayama SF, Sasaki K, Saito N, Tsuda S (2009) PFOS and PFOA in environmental and tap water in China. Chemosphere 77:605–611
Jin H, Zhang Y, Zhu L, Martin JW (2015) Isomer profiles of perfluoroalkyl substances in water and soil surrounding a chinese fluorochemical manufacturing park. Environ Sci Technol 49:4946–4954
Kochba J, Lavee S, S-R P (1977) Differences in peroxidase activity and isoenzymes in embryogenic and non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol 18:463–467
LaCourse EJ, Hernandez-Viadel M, Jefferies JR, Svendsen C, Spurgeon DJ, Barrett J, Morgan AJ, Kille P, Brophy PM (2009) Glutathione transferase (GST) as a candidate molecular-based biomarker for soil toxin exposure in the earthworm Lumbricus rubellus. Environ Pollut 157:2459–2469
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Leiers B, Kampkötter A, Grevelding CG, Link CD, Johnson TE, Henkle-Dührsen K (2003) A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic Biol Med 34:1405–1415
Liu C, Yu K, Shi X, Wang J, Lam PK, Wu RS, Zhou B (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat Toxicol 82:135–143
Liu J, Xiong K, Ye X, Zhang J, Yang Y, Ji L (2015) Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida. Chemosphere 135:250–256
Liu X, Zhang S, Wang J, Wang J, Shao Y, Zhu L (2016a) Biochemical responses and DNA damage in earthworms (Eisenia fetida) induced by ionic liquid [omim]PF6. Environ Sci Pollut Res Int 23:6836–6844
Liu Z, Lu Y, Wang P, Wang T, Liu S, Johnson AC, Sweetman AJ, Baninla Y (2016b) Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in Central and Eastern China. Sci Total Environ. doi:10.1016/j.scitotenv.2016.12.085
Lykkesfeldt J, Svendsen O (2007) Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J 173:502–511
Morrow JD (2000) The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev 32:377–385
Mosleh YY, Paris-Palacios S, Couderchet M, Vernet G (2003) Effects of the herbicide isoproturon on survival, growth rate, and protein content of mature earthworms (Lumbricus terrestris L.) and its fate in the soil. Appl Soil Ecol 23:69–77
Mueller S, Riedel H-D, Stremmed W (1997) Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal Biochem 245:55–60
OECD (1984) Test 207: earthworm, acute toxicity tests, OECD guideline for testing of chemicals. Organization for Economic Cooperation and Development, Paris
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR (2007) Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305
Panaretakis T, Shabalina IG, Grander D, Shoshan MC, DePierre JW (2001) Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol 173:56–64
Renner R (2009) EPA finds record PFOS, PFOA levels in Alabama grazing fields. Environ Sci and Technol:1245–1246
Ribeiro S, Sousa JP, Nogueira AJ, Soares AM (2001) Effect of endosulfan and parathion on energy reserves and physiological parameters of the terrestrial isopod Porcellio dilatatus. Ecotoxicol Environ Saf 49:131–138
Sheehan D, Meade G, Dowd VMFACA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem Soc 360:1–16
Singh S, Eapen S, D’Souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H (2009) DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem 41:905–909
Thompson AR (1971) Effects of nine insecticides on the numbers and biomass of earthworms in pasture. Bull Environ Contam Toxicol 5:577–586
Tripathi G, Kachhwaha N, Dabi I (2010) Comparative studies on carbofuran-induced changes in some cytoplasmic and mitochondrial enzymes and proteins of epigeic, anecic and endogeic earthworms. Pestic Biochem Physiol 96:30–35
Vassiliadou I, Costopoulou D, Ferderigou A, Leondiadis L (2010) Levels of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in blood samples from different groups of adults living in Greece. Chemosphere 80:1199–1206
Wang T, Khim JS, Chen C, Naile JE, Lu Y, Kannan K, Park J, Luo W, Jiao W, Hu W, Giesy JP (2012) Perfluorinated compounds in surface waters from Northern China: comparison to level of industrialization. Environ Int 42:37–46
Wang J, Wang J, Wang G, Zhu L, Wang J (2016) DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere 144:510–517
Wang C, Zhang Q, Wang F, Liang W (2017) Toxicological effects of dimethomorph on soil enzymatic activity and soil earthworm (Eisenia fetida). Chemosphere 169:316–323
Wen Y, Chen H, Shen C, Zhao M, Liu W (2011) Enantioselectivity tuning of chiral herbicide dichlorprop by copper: roles of reactive oxygen species. Environ Sci Technol 45:4778–4784
Wilce MCJ, Parker MW (1994) Structure and function of glutathione S-transferases. Biochim Biophys Acta 1205:1–18
Xu D, Li C, Wen Y, Liu W (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ Pollut 174:121–127
Yang JH (2010) Perfluorooctanoic acid induces peroxisomal fatty acid oxidation and cytokine expression in the liver of male Japanese medaka (Oryzias latipes). Chemosphere 81:548–552
Zamocky M, Furtmuller PG, Obinger C (2008) Evolution of catalases from bacteria to humans. Antioxid Redox Signal 10:1527–1548
Zhang H, Shi Z, Liu Y, Wei Y, Dai J (2008) Lipid homeostasis and oxidative stress in the liver of male rats exposed to perfluorododecanoic acid. Toxicol Appl Pharmacol 227:16–25
Zhang Q, Zhu L, Wang J, Xie H, Wang J, Han Y, Yang J (2013a) Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ Sci Pollut Res Int 20:201–208
Zhang W, Lin K-F, Yang S-S, Zhang M (2013b) Enzyme activities in perfluorooctanoic acid (PFOA)-polluted soils. Pedosphere 23:120–127
Zhang Q, Zhang G, Yin P, Lv Y, Yuan S, Chen J, Wei B, Wang C (2015) Toxicological effects of soil contaminated with spirotetramat to the earthworm Eisenia fetida. Chemosphere 139:138–145
Zhang Y, Zhang L, Feng L, Mao L, Jiang H (2017) Oxidative stress of imidaclothiz on earthworm Eisenia fetida. Comp Biochem Physiol C Toxicol Pharmacol 191:1–6
Zhu J (2015) Acute toxic effects of PFOA on Eisenia fetida. J Shanghai Jiaotong Univ (Agric Sci) 33:19–22 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Zhao, Y., Li, G., Qi, D. et al. Biomarker responses of earthworms (Eisenia fetida) to soils contaminated with perfluorooctanoic acid. Environ Sci Pollut Res 24, 22073–22081 (2017). https://doi.org/10.1007/s11356-017-9776-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9776-9