Abstract
Concept and purpose
Virtually all water treatment facilities worldwide generate an enormous amount of water treatment residual (WTR) solids for which environmentally friendly end-use options are continually being sought as opposed to their landfilling. Aluminium-based WTR (Al-WTR) can offer huge benefits particularly for phosphorus (P) removal and biofilm attachment when used as media in engineered wetlands. However, potential environmental risks that may arise from the leaching out of its constituents must be properly evaluated before such reuse can be assured. This paper presents results of an assessment carried out to monitor and examine the leachability and leaching patterns of the constituents of an Al-WTR used as media in laboratory-scale engineered wetland systems.
Main features, materials and methods
Al-WTR was used as media in four different configurations of laboratory-scale engineered wetland systems treating agricultural wastewater. Selected metal levels were determined in the Al-WTR prior to being used while levels of total and dissolved concentration for the metals were monitored in the influent and effluent samples. The increase or decrease of these metals in the used Al-WTR and their potential for leaching were determined. Leached metal levels in the effluents were compared with relevant environmental quality standards to ascertain if they pose considerable risks.
Results
Aluminium, arsenic, iron, lead and manganese were leached into the treated effluent, but aluminium exhibited the least leaching potential relative to its initial content in the fresh Al-WTR. Levels of P increased from 0.13 mg-P/g (fresh Al-WTR) to 33.9–40.6 mg-P/g (used Al-WTR). Dissolved levels of lead and arsenic (except on one instance) were below the prescribed limits for discharge. However, total and dissolved levels of aluminium were in most cases above the prescribed limits for discharge, especially at the beginning of the experiments.
Conclusions, recommendations and perspectives
Overall, the study indicates that leaching is observed when Al-WTR is beneficially reused for enhanced P removal in engineered wetlands. In particular, levels of aluminium in the treated effluent beyond the prescribed limits of 0.2 mg/l were observed. However, since the results obtained indicate that aluminium leached is mostly associated with solids, a post-treatment unit which can further reduce the level of aluminium in the treated effluent by filtering out the solids could serve to mitigate this. In addition, plants used in such wetland systems can uptake metals and this can also be a potential solution to ameliorating such metal releases. Periodic monitoring is thus advised. Notwithstanding, the use of Al-WTR as a media in engineered wetlands can serve to greatly enhance the removal of P from wastewaters and also serve as support material for biofilm attachment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water treatment residual (WTR) refers to the inevitable by-product obtained as a result of the drinking water treatment processes in water treatment facilities worldwide. The disposal of WTRs has become an integral part of the operation and management of water treatment facilities due to stringent regulations. Several authors have shown that common options for managing WTRs are not optimal solutions and several alternative WTRs management methods have been highlighted with major emphasis placed on reusing or recycling WTRs (Bourgeois et al. 2004; Babatunde and Zhao 2007). Currently, there are over 11 ways in which WTRs are being reused (Babatunde and Zhao 2007), but there is yet to be any reuse option that can utilise the vast amount of WTRs generated daily worldwide. Therefore, the need for a continued research into reuse/recycle options for WTRs is continuing. A recent and growing trend in research is the possible reuse/recycle of WTR in wastewater treatment. In particular, it has been demonstrated that aluminium-based WTR (Al-WTR) which is derived from water treatment facilities that use aluminium salts as coagulant can be a good adsorbent material for a variety of contaminants in wastewaters including phosphorus (P) (Babatunde and Zhao 2007). Al-WTR is the most widely generated WTR worldwide, prompting increased concerns as regards its disposal and/or alternative beneficial reuses.
Another new and novel way of reusing Al-WTR in wastewater treatment is utilising it as media in engineered wetland systems for wastewater treatment. Our previous studies have confirmed that Al-WTR can bring about significant improvements in P removal in engineered wetland systems due to its high P adsorption capacity (Yang et al. 2006a, b; Babatunde et al. 2008; Babatunde and Zhao 2009) and it can also successfully act as a biofilm carrier in such systems (Zhao et al. 2008, 2009a, b). This can bring about enhanced P reduction in wastewater during treatment in engineered wetlands. In addition, Al-WTR is an easily available by-product in towns, cities, and metropolis regions worldwide that utilise surface waters as a drinking water source. Any attempt to reduce P in wastewaters is valuable because inputs of P to rivers and lakes from point and diffuse sources continue to pose an environmental problem that is gaining increased attention. While there are a number of ways and technologies to remove P from wastewater, most involve the use of expensive chemicals and the generation of a secondary sludge. On the other hand, engineered wetland systems are fast becoming a preferred wastewater treatment system of choice for treating a diverse range of wastewaters due to their green appeal, low cost, ease of construction and operation, low environmental footprint and good performance in terms of organics removal. However, their performance in terms of nutrient removal (especially P) is often inconsistent and poor. Therefore, the possibility of using a residual waste material such as Al-WTR in an engineered wetland system to particularly enhance P removal from wastewaters before entry into water bodies is quite an attractive and strategic initiative.
This current work is part of a larger project being carried out for the past 5 years at the University College Dublin, Ireland towards developing a novel Al-WTR based engineered wetland system for wastewater treatment. Zhao et al. (2009a) gives a roadmap of the different phases leading to the development of the system. These have included physico-chemical characterization of the Al-WTR in relation to its use as the main media in CWs (Babatunde and Zhao 2009); static bottle adsorption tests using different P species to determine P adsorption behaviour, mechanism and capacity (Yang et al. 2006a, b; Zhao et al. 2007; Razali et al. 2007; Babatunde et al. 2008); determination of optimum configuration using six model laboratory-scale CWs (Babatunde 2007); single stage Al-WTR CWs tests (Zhao et al. 2008) and detailed investigations on: (a) forms and patterns of P retained in the Al-WTR after use (Babatunde et al. 2009), (b) P recovery from the Al-WTR after saturation (Zhao and Zhao 2009) and (c) ageing effect (for 18 months) of the Al-WTR on its P adsorption ability (Yang et al. 2008). Furthermore, short-term (Zhao et al. 2008) and long-term (Zhao et al. 2009b) laboratory tests of a multi-stage format of the CWs were carried out and the lifetime was also addressed. This current study is focused on addressing the possible release of substances from the Al-WTR into the treated effluent during its use as media in engineered wetland system since this is an environmental issue of priority.
Few studies have been conducted to address this concern. Cornwell et al. (1992) investigated the leaching of various metals from a WTR using toxicity characteristic leaching procedure.
It was reported that aluminium (Al) concentrations were particularly negligible for the 1.1 m of rainfall permeated through the wet residual solid columns over the study period. Similarly, Hsieh et al. (2000) investigated several factors that may affect the leaching of metals from WTR during reuse. It was concluded that pH and metal solubility can affect the release of metals in the WTR but the particle size has no significant effect on the metal leaching. In other studies, Hsieh et al. (2006) studied the leaching of metals and organic chemicals from WTR using a model monofill. Manganese (Mn) was reported to have the highest leaching capability, while the quantity of calcium (Ca) in the leachate was noted to increase appreciably with time. However, none of the studies evaluated the leaching tendency of WTRs in real reuse situations.
In this study, we seek to determine whether the constituents (particularly metals) of Al-WTR will leach out when it is being reused as a media in engineered wetland systems, particularly to enhance P removal. We also determined the levels of leached metals in the treated effluent. Four laboratory-scale engineered wetland systems using Al-WTR as main media for enhanced P removal were constructed and set up to treat wastewater from an animal research farm. The levels of specific elements/metals were monitored in the Al-WTR and in the influent and effluents of the four systems over a 25-week period. This paper presents the data obtained during the study and discusses the potential leaching and leachability of specific elements/metals from the Al-WTR during such reuse. The leaching patterns were also examined and the inferences made would provide real and useful information for further field application of the Al-WTR as a P-removing media for engineered wetlands.
2 Materials and methods
2.1 Al-WTR and effluent samples
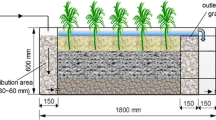
Al-WTR was obtained from the biggest water treatment plant in Ireland, located on the shores of a 5,000-acre Poulaphuca reservoir in Co. Kildare, Ireland. The plant uses aluminium sulphate as the primary coagulant and serves approximately one third of the entire Irish population, producing 230,000 m3 d−1 of potable water and generating 45–75 tonnes of Al-WTR daily. X-ray diffraction and scanning electron microscope–energy dispersive X-ray analyses both showed that the Al-WTR is mainly composed of amorphous aluminium and it has a high P adsorption capacity (Babatunde and Zhao 2009). Upon collection, the Al-WTR was transported to the laboratory and air-dried. Thereafter, the Al-WTR was ground to pass a 2-mm mesh sieve and then used as the main media in four laboratory-scale engineered wetland systems. The engineered wetlands were simulated in the laboratory using Pyrex tubes which were 900 mm in height and 95 mm in diameter.
Figure 1 shows a schematic diagram of the setup of the four systems labelled as 1, 2, 3 and 4 respectively. Gravel was used at the base of all the systems to act as support for the Al-WTR. Systems 2, 3 and 4 contained pea gravel up to varying depths as shown in Fig. 1.
Thus the proportions of pea gravel in the systems were 0%, 20%, 40% and 60% for systems 1, 2, 3 and 4, respectively. This translates to 100%, 80%, 60% and 40% by proportion of Al-WTR in the systems 1, 2, 3 and 4, respectively. At the time of the experiments, the pea gravels were used in the systems to examine their effect on improving wastewater flow and delaying clogging. Therefore, it should be noted that each individual configuration of the systems represents a potential configuration for the final field-scale system from the point of view of delayed clogging and P removal. We have examined the influence of the pea gravel and the different configurations on P removal and delayed clogging between the systems (Babatunde 2007). All the systems were fed from the same feed tank which contains wastewater collected from the animal research farm. The wastewater had a typical concentration (after settlement) of 322–510 mg/l (suspended solids), 720–1523 mg/l (chemical oxygen demand), 540–850 mg/l (5-day biochemical oxygen demand), 48–73 mg/l (phosphorus) and 6.7–7.4 (pH)
The wastewater was introduced into the systems in batches of six to eight daily feeding regimes and this was carried out using programmed peristaltic pumps. The designed hydraulic loading rate ranged between 1.23 and 1.86 m3/m2 d−1 across the systems. The experiment was conducted for 25 weeks. At the end of the experiments, used Al-WTR samples were collected from the within 10 cm of the topmost Al-WTR layer of the Al-WTR in the systems. The topmost layer is the Al-WTR layer that is first in contact with the wastewater passing through the system and which would reasonably be the most loaded Al-WTR layer. Samples were taken from within the first 10 cm of this layer. The sampling of the used Al-WTR was limited to the topmost layer of the Al-WTR in each system due to the high cost involved in analysing all the samples. Both the used Al-WTR samples collected and the fresh Al-WTR samples were stored in the refrigerator at ≈4°C until they were analysed according to standard recommendations (Pierzynski 2000). At the same time, samples from the feed tank and effluent samples from the systems were collected on three occasions during the 25-week operating period and specifically analysed for metals. The first set of samples were taken immediately after the commencement of operation (referred to as period 1), the second set was taken at about midway into the entire operational time (referred to as period 2), while the last set of samples were taken at the end of the experiments (referred to as period 3).
2.2 Analytical procedures and methods
To obtain the total metals in the Al-WTR samples, an Anton Paar MULTIWAVE microwave sample preparation system was used to digest the samples. The metals analysed were selected based on their relative presence in the Al-WTR as observed in the initial analysis obtained from the plant operators. The use of microwave-enhanced acid digestion of solid samples for elemental analysis is now well established as a routine sample preparation method (Jin et al. 1999), and it is noted to be a rapid and efficient method of sample decomposition prior to the determination of metals (Robache et al. 2000). The low-volume microwave digestion allows the determination of analytes in small samples (<0.1 g), thereby avoiding reduction in method sensitivity (Sandroni et al. 2003). Approximately 0.025 g of the Al-WTR samples (fresh and used) was weighed into clean trifluoromethylene vessels followed by the addition of 4 ml HNO3 + 200 µl HF + 4 ml H2O. A built-in computer programme was used to specify the decomposition programme, control the MULTIWAVE and hold a library of sample data. When decomposition is complete, the sample is transferred to a volumetric flask and the volume is made up to 15 ml. These were then sent out to a certified laboratory for total metal analysis. Total and dissolved metal analysis on samples from the feed tank (influent) and samples from the systems (effluent) was also done through the contracted certified laboratory using ICP (IRIS) and ICP-MS for the total and dissolved metals respectively. The method detection limits are included in Table 1. All sample handling/preservation and classification were done according to standard methods (APHA-AWWA-WEF 1998).
2.3 Leaching potential
An attempt was made in this study to quantify the relationship between quantities of elemental/metals in the effluents and their corresponding quantity in the fresh Al-WTR. Following a similar work done by Hsieh et al. (2006), the leaching potential of the respective constituents of the Al-WTR was determined as a ratio of the mass of the constituent in the leachate at time t, to the corresponding mass of the constituent present in the unused Al-WTR using Eq. (1). This gives a sort of indication as to the relative potential for leaching (leachability) for the respective constituents in the Al-WTR.
where G is determined by Eq. (2)
and Cabs is given by Eq. (3)
C abs (mg/l) is absolute concentration of the constituent in the effluent, C f (mg/g) is the concentration of the constituent in the unused Al-WTR and S is the solids content. C eff and C inf represent the respective concentration of the constituent in the effluent and influent in milligrammes per litre. The value of G was computed to be 0.075 for a solids content of 0.57.
3 Results and discussion
3.1 Changes in the element/metal concentration of Al-WTR used as media in engineered wetlands
Figure 2 shows the elemental/metal concentration in the Al-WTR, before and after use in the four engineered wetland systems. It should be quickly pointed out that the level of metals in the fresh Al-WTR, particularly those of lead (Pb) and zinc (Zn) are below their typical levels in uncontaminated soils (Elliot et al. 1990; Elliot and Dempsey 1991). The metal levels are also in range with those reported for WTRs by other authors (DeWolfe 2006; Makris and O’Connor 2007). Al had the highest proportion by composition in the Al-WTR. Al (expressed as Al2O3) accounted for 8.1% of the sampled Al-WTR. In separate reviews on WTRs, Babatunde and Zhao (2007) reported a mean value of 29.7% Al for Al-WTR (which is equivalent to 56.1% Al expressed as Al2O3), while from Makris and O’Connor (2007), a range of 2.9% to 57% Al as Al2O3 can be inferred. Thus the Al-WTR samples used in this study can be suggested to be in the lower end of the range of Al concentrations in such WTRs.
The concentrations of elements/metals in the Al-WTR before and after use give an idea of whether they are leached or not. By comparing the concentrations of elements/metals in the fresh and used Al-WTR samples as shown in Fig. 2, it can be observed that the concentrations of Al, arsenic (As), iron (Fe), Pb and Mn were all decreased. On the other hand, the concentration of P, Ca, Mg, Zn and titanium (Ti) were increased. Leaching of constituents from WTR has been reported in literature (Cornwell et al. 1992), although simulated conditions were used. Hsieh et al. (2006) noted that the amount of metals retained in a WTR depends on the form of metals and the anion group that they are associated with, while Elliot et al. (1990) noted that the extent to which metals are solubilised depends on a variety of factors. Thus, the release or otherwise of any metal will be specific to the metal and several complex and interacting processes.
By comparing the results of analyses of fresh and used Al-WTR from systems 1, 2, 3 and 4 as shown in Fig. 2, it can be seen that there was consistency in the elements/metals that showed increase or decrease in concentration. However, the magnitudes of the increase or decrease in concentration were not the same and do not reflect the differences in the proportion of Al-WTR in the systems. For instance, system 1 had the highest proportion of Al-WTR (100%), but the increase or decrease in the concentration of the elements/metals was not always the highest. Similarly, although system 4 had the least proportion of Al-WTR in its composition, it had the same magnitude of decrease for Pb as the other systems, while the decrease in Fe was more in the system than in system 2. This indicates that the total mass of Al-WTR in the systems is not a uniform indicator of the magnitude of increase or decrease in elemental metal concentration and also the release of metals into the effluent. It also implies that certain factors and conditions, which are inherent in each reactor, do contribute to the release or otherwise of metals from the reactors
The pH levels of the influent and effluents of the four systems did not reveal any marked difference and it ranged from 6.7 to 7.4 and 6.8 to 7.2 for the influent and effluent, respectively. In addition, the pH did not vary greatly across the four systems. It is therefore likely that other inherent conditions that are specific to each of the systems might have influenced the increase or decrease in the concentration of the elements/metals. From Fig. 2, it can also be seen that across the systems, the relative decrease (between the fresh and used Al-WTR) in As concentration was the highest, followed by Mn and then Al. The concentration of Pb was also decreased. The release of Mn might suggest a reducing environment. On the other hand, the increase in P was highest across the systems, followed by Ca, Zn, Mg and Ti. The huge increase in P concentration in the used Al-WTR attests to the high P adsorption capacity of the Al-WTR. In our previous studies, it was shown that Al-WTR has a preferential adsorption for P (Yang et al. 2006a, b) and the adsorption capacity of the Al-WTR can range from 10.2 to 31.9 mg-P/g (determined using the Langmuir adsorption isotherm; Babatunde and Zhao 2009).
In all the samples analysed, the percentage of increase in P was between 1 and 3 orders of magnitude higher than the increases in Ca, Zn and Mg highlighting the capacity and preference of the Al-WTR for P adsorption. The increases in Ca, Zn and Mg also show the adsorption ability of the Al-WTR for other ions. Elliot et al. (1990) remarked in their study that freshly precipitated hydrous oxides have a large capacity to occlude, coprecipitate and sorb divalent metal ions from the surrounding aqueous media, despite an unfavourable surface charge. Overall, these results indicate a simultaneous uptake and release of substances from the Al-WTR matrix when used as a media in an engineered wetland system. The uptake of certain elements from the wastewater is desirable and beneficial, in particular P. However, the potential release of some metals from the Al-WTR is not totally desirable, but caution is needed before any conclusion can be made and the release pathway needs to be established.
3.2 Monitoring of levels of elements/metals from the leachate of the Al-WTR-based engineered wetland systems
Table 1 shows the levels of total and dissolved metals in the influent and effluent of the four systems across the three periods. Any increase in the level of the metals in the effluents may suggest the leaching of the metal from the Al-WTR into the effluent, while decrease in the level of any metal can equally suggest uptake of the metal by the Al-WTR. In most cases, the levels of dissolved metal concentration were compared with prescribed limits. This is because metal levels associated with solids can be removed/reduced by including an in-line filtration unit in the system design. Therefore, by checking the level of dissolved metals against the prescribed limits, recommendations can be made as to whether further treatment unit will be required. From Table 1, it can be observed that the level of Al (both total and dissolved) in the effluents was always higher than that in the influent (feed) in all the cases except on one occasion. This indicates that there was some release of Al into the treated wastewater during passage through the systems. Although the influent wastewater had some background Al concentration, this is quite small, compared to the level in the effluents. In addition, based on the level of total and dissolved concentration of Al in the influent, it can be inferred that most of the Al in the influent is probably associated with the solids. The results also indicate that the relative proportion of dissolved Al to the total Al in the effluent decreased over the periods.
For instance, from Table 1, the proportion of dissolved Al to total Al for system 1 decreased from 1.2 during the first period to 0.4 and 0.7 during the second and third period, respectively. This may suggest that Al leached to a great extent at the beginning of the experiment. Over the three periods, levels of dissolved Al monitored in the effluent from all the systems ranged from 58 to 1106 μg/l. The prescribed limit for Al for discharge into all waters is 200 μg/l (EPA 1997; UK Technical Advisory Group 2008). This limit was exceeded in about 66.7% of all of the sampling cases with most of the exceedances occurred during the early stages of the experiment.
The level of As (total) in the effluent was relatively stable over time. However, although the level of dissolved As changed over time in the effluent, the result suggests that most of the As in the effluent was held in the solid phase. Notwithstanding, the level of dissolved As in the effluent ranged from 3 to 63 μg/l. Except on one occasion, the dissolved As levels were all below the prescribed limits of 50 μg/l for discharge into freshwaters (EPA 1997; UK Technical Advisory Group 2008). Ca in the influent wastewater was mostly in the soluble form, probably associated with CaCO3. However, from Table 1, it can be seen that there was a huge reduction in the level of Ca in the effluent exiting all the systems as compared to the Ca levels in the influent. It was also noted that there was a corresponding huge increase in the concentration of Ca in the used Al-WTR samples from all the systems as shown in Fig. 2. This implies that the Ca ions were removed from solution, possibly by adsorption/precipitation on the Al-WTR. The level of total Fe in the influent was in most cases, higher than the level in the effluent, but the inverse was mostly the case for dissolved Fe. It can be suggested that most of the Fe is held in the solid phase in the influent and possibly filtered out onto the Al-WTR. Hence, the level in the effluent is mostly lower than the level in the influent. The higher level of dissolved Fe in the effluent than in the influent may however suggest some release/dissolution of Fe held in the solid phase in the influent into the effluent. It should however be noted that at all times, the concentration of Fe in the effluent were very well below the prescribed limits of 1.0 mg/l for discharge into all waters (EPA 1997; UK Technical Advisory Group 2008).
The concentration of dissolved Pb in the effluent ranged from <1 to 5 μg/l and this is clearly below the prescribed limit of 50 μg/l for discharge into freshwaters (EPA 1997; UK Technical Advisory Group 2008). Mg levels were decreased in the effluents but increased in the used Al-WTR samples. This has potential benefits as the increase in the Mg level of the Al-WTR can increase its micronutrient value. To some extent, Mn may have leached from the Al-WTR into the effluent samples. This is however a bit difficult to ascertain, since the influent also contain some level of Mn which was higher than the level in the effluent in some instances. There were also decreases in the level of Zn (both total and dissolved) and this can be explained by the ability of the Al-WTR to further adsorb Zn from the aqueous solution, based on the increase in Zn concentration in the Al-WTR as shown in Fig. 2.
3.3 Leaching potential
The leaching potential (L.P) covering ten elements/metal for the systems over the three periods is shown in Table 2. In calculating the L.P, only values of dissolved concentration were used, thus excluding metals/constituent associated with the solid phase. Analysing across the periods, it can be seen that Mn has the highest L.P during the first period, while Zn had the highest L.P in the second (excluding system 3) and third period (excluding system 4). Using system 1 as a typical case, the pattern of L.P in decreasing order was Mn > Ti > Al > Fe in the first period, Zn > As > Al in the second period and Zn > As > Pb > Fe > Al in the third period (note that only constituents that appear to be leached during each period of assessment were ranked. Constituents are deemed leached if the level of the respective metal is higher in the effluent than in the influent). It can be observed that relatively, Al had the lowest L.P (for the elements that leached).
It can also be seen that the leaching of As was observed over time, while the intensity of leaching of Mn decreased over time. Similar, but slightly different patterns were also observed for the other systems. For instance, the pattern for system 2 was Mn > Ti > Fe > Al in the first period, Zn > As > Pb > Al in the second period and Zn > As > Pb > Fe > Al in the third period. In this case, it can also be observed that Al had the lowest L.P across all the periods while there was an increase in the leaching intensity of As observed over time. The intensity of leaching of Mn similarly decreased over time. The slight differences in the L.P for the different metals in the systems may be due to differences in the prevailing conditions in the systems. However, it is very useful to note that, relative to the initial level of constituents in the fresh Al-WTR, Al had a relatively low L.P as compared to the L.P for all the other metals that were leached. This is in agreement with the findings of other authors (Cornwell et al. 1992; Hsieh et al. 2006), which showed that Al leached out in very small quantities from WTRs. It can thus be reasonably inferred that, although Al did leach out from the Al-WTR used in this laboratory-scale study, the relative quantity leached out is small. It should however be stressed that due to its high content in the fresh Al-WTR, the concentrations of Al observed in the effluent were above the limit values allowed for disposal in majority of the cases. Due to the fact that leaching was observed for certain metals (particularly Al, As and Pb), periodic monitoring and evaluation on a case by case basis is highly recommended. However, being that the leachates are also associated with solids, a post-treatment unit to philtre out the solids and lower the metal concentrations in the final treated effluent can be used. Furthermore, the metal concentrations in the effluents can further be reduced through metal uptake by the constructed wetland plants and this has been demonstrated by Bounheng et al. (2006).
4 Conclusions
This study examined the leachability and leaching patterns of elements/metals from engineered wetland systems setup using an aluminium-based water treatment residual as the media. Results reveal that aluminium, arsenic, iron, lead and manganese leached out in varying quantities, while phosphorus, calcium, magnesium, zinc and titanium were adsorbed. Phosphorus increased from 0.13 mg-P/g in the fresh Al-WTR to 33.9–40.6 mg-P/g in the used Al-WTR. Dissolved levels of lead and arsenic in effluents range from <1 to 5 μg/l and 3 to 63 μg/l, respectively, and these were below the prescribed limits of 50 μg/l (except arsenic on one instance) for their respective discharge into freshwaters. However, dissolved levels of aluminium ranged from 58 to 1,106 μg/l with about 66.7% of samples above prescribed limit of 200 μg/l for aluminium. While the use of the aluminium-based water treatment residual as the media in engineered wetland showed great promise particularly for phosphorus removal, periodic metals monitoring is recommended and in particular, a post-treatment unit may be required to reduce aluminium levels in the treated effluent.
References
APHA-AWWA-WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Babatunde AO (2007) The development of an alum sludge based tidal flow constructed wetland for optimizing phosphorus and organic matter removal from wastewaters, PhD dissertation, University College Dublin, Ireland
Babatunde AO, Zhao YQ (2007) Constructive approaches towards water treatment works sludge management: an international review of beneficial re-uses. Crit Rev Environ Sci Tech 37:129–164
Babatunde AO, Zhao YQ (2009) Forms, patterns and extractability of phosphorus retained in alum sludge used as substrate in laboratory-scale constructed wetland systems. Chem Eng J 152:8–13
Babatunde AO, Zhao YQ, Yang Y, Kearney P (2008) Reuse of dewatered aluminium-coagulated water treatment residual to immobilize phosphorus: batch and column trials using a condensed phosphate. Chem Eng J 136:108–115
Babatunde AO, Zhao YQ, Burke AM, Morris MA, Hanrahan JP (2009) Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ Pollut 157:2830–2836
Bounheng S, Kazunori N, Munehiro M, Nobuo C, Osamu N (2006) Phragmites australis: a novel biosorbent for the removal of heavy metals from aqueous solution. Water Res 40:2295–2302
Bourgeois JC, Walsh ME, Gagnon GA (2004) Comparison of process options for treatment of water treatment residual streams. Environ Eng Sci 3:477–484
Cornwell DA, Vandermeyden C, Dillow G, Wang M (1992) Landfilling of water treatment plant coagulant sludges. Project No. 512, AWWA Research Foundation. Denver, Colorado, USA
DeWolfe J (2006) Water residuals to reduce soil phosphorus, AWWA research foundation
Elliot HA, Dempsey BA (1991) Agronomic effects of land application of water treatment sludges. J Am WaterWorks Assoc 4:126–131
Elliot HA, Dempsey BA, Maille PJ (1990) Content and fractionation of heavy metals in water treatment sludges. J Environ Qual 19:330–334
EPA (1997) Environmental quality objectives and environmental quality standards: the aquatic environment—a discussion document. Environmental Protection Agency (EPA), Ardcavan, Wexford, Ireland
Hsieh H, Tian P, Raghu D (2000) Leaching of metals from water treatment plant residuals. Pract Period Hazard Toxic Radioact Waste Manag 4:134–139
Hsieh H, Raghu D, Tian P (2006) Leaching studies on a model monofill of water treatment plant residuals. Environ Eng Sci 23:230–238
Jin Q, Liang F, Zhang H, Zhao L, Huan Y, Song D (1999) Microwave technique in analytical chemistry. Trends Anal Chem 18:479–484
Makris KC, O’Connor GA (2007) Beneficial utilization of drinking-water treatment residuals as contaminant-mitigating agents. In: Sarkar D, Datta R, Hannigan R (eds) Concepts and applications in environmental geochemistry. Elsevier, Amsterdam, pp 609–635
Pierzynski GM (2000) In: Pierzynski GM (ed) Methods of soil phosphorus analysis for soils, sediments, residuals and waters. Southern Cooperative series bulletin No. 396
Razali M, Zhao YQ, Bruen M (2007) Effectiveness of a drinking-water treatment sludge in removing different phosphorus species from aqueous solution. Sep Purif Technol 55:300–306
Robache A, Mathe F, Galloo JC, Guillermo R (2000) Multi-element analysis by inductively coupled plasma optical emission spectrometry of airborne particulate matter collected with a low-pressure cascade impactor. Analyst 125:1855–1859
Sandroni V, Clare MM, Donovan A (2003) Microwave digestion of sediment, soils and urban particulate matter for trace metals analysis. Talanta 60:715–723
UK Technical Advisory Group on the Water Framework Directive (2008). Proposals for environmental quality standards for Annex VIII substances. Final report SR1-2007
Yang Y, Tomlinson D, Kennedy S, Zhao YQ (2006a) Dewatered alum sludge: a potential adsorbent for phosphorus removal. Water Sci Technol 54:207–213
Yang Y, Zhao YQ, Babatunde AO, Wang L, Ren YX, Han Y (2006b) Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep Purif Technol 51:193–200
Yang Y, Zhao YQ, Kearney P (2008) Influence of ageing on the structure and phosphate adsorption capacity of dewatered alum sludge. Chem Eng J 145:276–284
Zhao XH, Zhao YQ (2009) Investigation of phosphorus desorption from P-saturated alum sludge used as a substrate in constructed wetland. Sep Purif Technol 66:71–75
Zhao YQ, Razali M, Babatunde AO, Yang Y, Bruen M (2007) Reuse of aluminium-based water treatment sludge to immobilize a wide range of phosphorus contamination: equilibrium study with different isotherm models. Sep Sci Technol 42:2705–2721
Zhao YQ, Babatunde AO, Razali M, Harty F (2008) Use of dewatered alum sludge as a substrate in reed bed treatment systems for wastewater treatment. J Environ Sci Health A Tox Hazard Subst Environ Eng 43:105–110
Zhao YQ, Babatunde AO, Zhao XH, Li WC (2009a) Development of alum sludge-based constructed wetland: an innovative and cost-effective system for wastewater treatment. J Environ Sci Health A Tox Hazard Subst Environ Eng 44:827–832
Zhao YQ, Zhao XH, Babatunde AO (2009b) Use of dewatered alum sludge as main substrate in treatment reed bed receiving agricultural wastewater: long-term trial. Bioresour Technol 100:644–648
Acknowledgements
Financial support obtained from the Environmental Protection Agency Ireland (grant no: 2005-ET-MS-38-M3) is hereby acknowledged. Mr. Patrick Kearney, Section head technician, Water and Effluents Laboratory, UCD and Dr Dave Healy of University College Cork are also thanked for their assistances.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Babatunde, A.O., Zhao, Y.Q. Leachability and leaching patterns from aluminium-based water treatment residual used as media in laboratory-scale engineered wetlands. Environ Sci Pollut Res 17, 1314–1322 (2010). https://doi.org/10.1007/s11356-010-0311-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0311-5