Abstract
Human exposure to carcinogenic polycyclic aromatic hydrocarbons (PAHs) in cigarette smoking might result in generation of reactive oxygen species (ROS) and induce formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG). This study was designed to examine whether levels of 8-OHdG are associated with levels of urinary metabolites of PAHs. Two groups (smokers and non-smokers) were recruited from college students in Guangzhou, China. Their urine samples were collected and analyzed for ten urinary mono-hydroxylated PAHs (OH-PAHs) and 8-OHdG by liquid chromatography equipped with tandem mass spectrometer (LC/MS/MS). Multiple linear regression analysis was performed to examine correlations between urinary levels of 8-OHdG and OH-PAHs. No significant difference was observed for creatinine-adjusted OH-PAHs between smokers and non-smokers. The levels of 8-OHdG between smokers and non-smokers were comparative. OH-PAH levels in this study were 2–50 times higher than those in populations from other countries and areas. The estimated daily intake (EDI; μg/day) of PAHs ranged from 0.02 to 371.4, which were far lower than the reference doses (RfDs) specified by U.S. Environmental Protection Agency (EPA). Though smoking was a main factor, which affected the PAH exposure, it was not a dominant factor in the exposure to PAHs of Guangzhou college students. The environmental exposure could not be ignored. The sum concentrations of OH-PAHs (∑OH-PAHs) had a dose-increase relationship with 8-OHdG both for smokers and non-smokers, especially for smokers. Though people in Guangzhou bore higher PAH hazards, the estimated environmental risk was still under safe ranges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants with two or more fused aromatic rings. They are also present in fossil fuels or formed by incomplete combustion of carbon-containing fuels. Human are exposed to PAHs by direction inhalation of polluted air, dietary intake, and dermal contact. Occupational exposure to PAHs, such as the workers in coke plant and aluminum and steel manufacture (Strickland and Kang 1999), could enhance the incidence of skin, lung, and bladder cancer (IARC 1983; Boström et al. 2002; Lloyd 1971; Roelofzen et al. 2012). For those non-occupational exposure populations, whose levels of exposure from smoking may be in the same range as exposure via diet, cigarette smoking is the major route of exposure to PAHs (Ding et al. 2005). Cigarette coke tar generates a large amount of PAHs (Huang and Ge 2006). Particularly, the high carcinogenic benzo[a]pyrene (B[a]P) could be formed and detected in cigarette tar (Kaiserman and Rickert 1992). There is substantial evidence that the increasing urinary levels of PAHs are associated with smoking. Generally, mono-hydroxylated PAHs (OH-PAHs) in human urine are used as the biomarkers to monitor the PAH exposure, characterize the possible exposure routes, and assess the PAH exposure risk (Scherer et al. 2000).
Carcinogenic PAHs could induce the generation of reactive oxygen species (ROS) (Palackal et al. 2002). The excessive oxidative modification of DNA and lipids in the body caused by ROS can damage macromolecules and interfere with DNA repair. Consequently, it will lead to aging and cancer (Valavanidis et al. 2009). The DNA-adduct, 8-hydroxy-2′-deoxyguanosine (8-OHdG), is a useful biomarker to DNA damage to assess human exposure to different carcinogenic compounds. Though many other physiological stressors and environmental contaminants may lead to DNA damage, it was reported that exposure of occupational population to PAHs could cause higher levels of urinary 8-OHdG (Han et al. 2010; Navasumrit et al. 2008; Kuang et al. 2013; Lee et al. 2012; Fan et al. 2012a).) Plasma benzo[a]pyrene-r-7,t-8,t-9,c-10-tetrahydotetrol-albumin adducts (BPDE-Alb) and sum concentrations of OH-PAHs (∑OH-PAHs) in 1,333 Chinese coke oven workers could result in significant dose-related increases in oxidative DNA and lipid damage (Kuang et al. 2013). However, strong correlation between urinary levels of 8-OHdG and levels of ∑OH-PAHs was not established in non-occupational population (Fan et al. 2012a).
China is becoming the world's largest tobacco consumer and producer of cigarettes. With the economic development, the tobacco consumption increases by 7 % per year. Smoking is harmful to human health and leads to an increasing mortality caused by respiratory disease (Johnston et al. 2012). Negative effects of smoke on young adults are even greater due to their higher metabolism and ventilation rates. According to statistical data, the smokers cover 8.39 % among all students of Guangzhou universities, of which 18.28 % for male and 2.46 % for female (Li et al. 2007). The health effects caused by cigarette should be investigated and assessed among college students.
In order to investigate the potential carcinogenic effects from PAH exposure, 19 male smokers and 34 non-smokers, which came from one university in Guangzhou, China, were recruited. 8-OHdG and ten urinary OH-PAHs, including 1-hydroxypyrene (1-OHP), 1-, 2-hydroxynaphthalene (1-OHN, 2-OHN), 2-, 3-hydroxyfluorene (2-, 3-OHF), and 1-, 2-, 3-, 4-, 9-hydroxyphenanthrene (1-, 2-, 3-, 4-, 9-OHPhe), were simultaneously determined by using liquid chromatography equipped with tandem mass spectrometer (LC/MS/MS) in negative electrospray ionization (ESI) mode. The relationship between 8-OHdG levels and individual OH-PAH as well as the concentrations of ∑OH-PAHs caused by smoking were investigated. Urinary PAH levels were used to estimate daily intakes of PAHs and assess the environmental risks of these students.
Materials and methods
Studied subjects and sample collection
Nineteen male college students (23–26 years old, 165–178 cm in height, and 50–75 kg in weight) were recruited from a university in Guangzhou, China. Their average numbers of cigarette smoking was 7.1 per day. Thirty-four college students (18 males and 16 females, 20–24 years old, 155–180 cm in height, and 40–75 kg in weight) from the same university were recruited as the control group. They are not smokers. Each participant was interviewed by a trained recruiter and required to fill a consent form and answer a questionnaire regarding their name, gender, age, dietary habits, health status, and cigarette and alcohol consumption.
Due to the main route of PAH exposure was from diet consumption, which accounts for 75 % in China (Li et al. 2005; Zhang et al. 2014), all recruited students were required to have lightly cooked or fresh food 3 days before urine sampling, so that the PAH variations from food intakes could be reduced.
Subject recruitment and the urine sample collection were completed in Nov, 2011. The urine sample was each collected in a screw-cap-sealed plastic bottle and shipped to the lab in 2 h. They were stored at −20 °C in the lab until chemical analysis.
Reagents and materials
8-OHdG, 2-OHN (purity 99 %), and 3-OHF were from Sigma (St. Louis, MO, USA). 2-OHF (purity 98 %), 9-OHPhe, and 1-OHP (purity 98 %) were from Aldrich (St. Louis, MO, USA). 1-OHN was from Fluka (purity 99 %, St. Louis, MO, USA). 1-OHPhe (purity 99 %), 2-OHPhe (purity 99.6 %), and 4-OHPhe (50 μg/mL in acetonitrile) were from Dr. Ehrenstorfer (Augsburg, Germany). 3-OHPhe (purity 98 %, 50.0 μg/mL in toluene), 13C6-3-OHPhe (purity 95 %, 50.0 μg/mL in acetonitrile), and 15 N5-8-hydroxy-2′-deoxyguano-sine (15 N5-8-OHdG, 15 N5 purity 98 % and 95 % chemical purity) were from Cambridge Isotope Lab (Andover, MA, USA). D8-2-OHN and D9-1-OHP were from C-D-N Isotope Inc. (Quebec, Canada). D9-2-OHF was from Santa Cruz Biotech. Inc. (Santa Cruz, CA, USA). β-Glucuronidase/arylsulphatase was from Sigma (St. Louis, MO, USA). Methanol (LC-MS Chromasolv, ≥99.9 %) was from Fluka (St. Louis, MO, USA). Water was supplied by Milli-Q water purification system. Glacial acetic acid (HAC), sodium acetate (NaAC), and KH2PO4 (high-performance liquid chromatography (HPLC) grade) were from Fisher Scientific (Houston, TX, USA). All other reagents were of analytical grade and used without further purification. The Bond Elut C18 solid-phase extraction (SPE) cartridge (500 and 6 mL) was obtained from Varian (Santa Clara, CA, USA). An XPerra-C-18 column (5 μm, 4.6 × 250 mm, Waters, USA) was used to measure OH-PAHs and 8-OHdG simultaneously.
Urinary creatinine was determined by using Jaffee method to normalize the urinary concentrations of targets compounds (Taussky 1954).
Sample preparation and instrumental analysis
Samples were prepared and analyzed as described previously (Fan et al. 2012b) with a slight modification. Briefly, 2-mL urine sample was spiked at levels of 10–50 μg/L with a mixture of isotope-labeled internal standards, and then 3 mL sodium acetate buffer and 10 μL β-glucuronidase/sulfatase enzyme were added for overnight incubation at 37 °C. After that, the target analytes were extracted by using SPE cartridges. The extracts were concentrated to 100 μL in methanol and analyzed by a 20A HPLC (Shimadzu, Japan)/API Q-Trap 5500 mass spectrometer (AB, SCIEX, USA). HPLC column was used to separate OH-PAHs and 8-OHdG simultaneously. Ten OH-PAHs, including 1-, 2-OHN, 2-, 3-OHF, 1-, 2-, 3-, 4-, 9-OHPhe, and 1-OHP, were quantified by the optimized LC/MS/MS conditions (Table 1). 1-OHP and 8-OHdG were quantified by their own isotope-labeled internal standards.1-OHN and 2-OHN were quantified by D8-2-OHN; 2-OHF and 3-OHF were quantified by D9-2-OHF; 1-, 2-, 3-, 4-, 9-hydroxyphenanthrenes were quantified by 13C6-3-OHPhe. Water with 0.1 % (v/v) acetic acid and methanol were used as mobile phase A and B, respectively. The gradient elution program was set as follows: 60 % B was initially held for 5 min, and then ramped to 78 % B over 18 min, and then to 85 % B over 2 min, and finally to 100 % B. The total run time was 30 min. The flow rate was set at 0.6 mL/min, and the column temperature was held at 40 °C.
QA/QC and statistics method
A diluted urine pool spiked with low levels of analytes was analyzed, and a signal-to-noise ratio (three) of LC/MS/MS response of each analyte was used to estimate method detection limits (MDLs). MDLs for each analyte were in a range of 0.006–0.059 ng/L (Table 1). The method accuracy and precision was evaluated by replicate analysis of matrix spikes at three levels of 0.75–7.5, 0.375–3.75, and 0.15–1.5 μg/L for OH-PAHs and 8-OHdG. Recoveries were 80–120 % with relative standard deviations (RSDs) of 10–20 %. Matrix spiked with internal standard was used to check the matrix interference, and no interference from matrix was found. Prior to daily instrumental analysis, the lowest calibration standard prepared in the diluted urine pool was analyzed. The LC/MS/MS response of each analyte in this solution was compared with the previous one to confirm acceptable LC resolution and MS sensitivity. If neither of deviations exceeded 15 %, the instrumental performance (resolution and sensitivity) was considered acceptable for the analysis of a batch of samples. Two methanol blanks were run following the analysis of the highest levels of calibration standards and QC samples to examine and avoid the potential carryovers. In order to evaluate the precision and accuracy of the real sample analysis, 15 % of the real urine samples in each batch were analyzed in duplicates and the relative percentage difference (RPD) was in a range of less than ±20 %.
SPSS program (IBM, version 20.0) was used to statistically analyze data. For target compounds with concentrations below the MDLs in urine, we directly used MDLs for statistical analysis rather than using zero. As to descriptive analysis (geometric mean, median, SD, range), results are presented micrograms per gram creatinine (μg/g crt.) All the concentrations were decimal log-transformed to assure normal distribution. Paired sample t test (two tailed) was carried out to detect significant differences of the geometric means between urinary PAH metabolites and 8-OHdG. Spearman's correlations (two tailed) were used to test the associations between different variables. The level of significance was set as p < 0.05. Multiple linear regression analysis was performed to examine correlations between 8-OHdG levels and ∑OH-PAHs.
Results and discussion
Profiles of urinary PAHs and 8-OHdG levels between two groups
In this study, two pairs of isomers, namely, 3-OHPhe and 2-OHPhe and 1-OHPhe and 9-OHPhe, were co-eluted congeners. As showed in Table 2, their concentrations were presented as 2-OHPhe + 3-OHPhe (sum concentrations of 3-OHPhe and 2-OHPhe) and 1-OHPhe + 9-OHPhe (sum concentrations of 1-OHPhe and 9-OHPhe). Most analytes in urine samples could be determined in this method, with the exception of 2-OHN in 3 samples, 1-OHN in 11 samples, 2-OHF in 5 samples, 3-OHF in 2 samples, and 2- + 3-OHPhe in 3 samples, which were below MDLs (showed in Table 1). Geometric means (GMs) of 8-OHdG and OH-PAHs in the unit of micrograms per liter for smokers were significantly higher than those for non-smokers (t test, p < 0.05). It suggested that smoking caused higher urinary levels of PAHs and potential carcinogenic risk. Our results were in agreement with reported studies (Angerer and Heudorf 2001; Ichiba et al. 2006). GM of 1-OHP for non-smokers was significantly lower than that for smokers (p = 0.000). When the data was adjusted by urinary creatinine, GMs of 1-OHN, 2-OHN, and 4-OHPhe and ∑OH-PAHs for smokers were slightly higher than those for non-smokers (p > 0.05) (Table 2). GMs of 2-OHF, 3-OHF, 2- + 3-OHPhe, 1- + 9-OHPhe, and 8-OHdG were even comparative between the smokers and the non-smokers (p > 0.05). PAH exposure may be from diet and inhalations (Li et al. 2005; Zhang et al. 2014). Though the dietary intake was controlled in our study, intakes of PAHs from inhalation of polluted air and the cigarette smoking could not be excluded. Guangzhou ranked as the third city with the highest PAH pollutions among 14 cities of China. Particularly, PAHs in air were 2–15 times higher in winter than in summer (Wang et al. 2006). Additionally, all recruited students lived in the different areas of the university. Those who lived within high-traffic areas would be easily affected by traffic-related airborne PAHs (Naeveàez et al. 2008; Fan et al. 2012a, b). For occupational or non-occupational populations who were exposed to high levels of PAHs in their ambient environment, effects of smoking on urinary PAH levels were insignificant (Kuang et al. 2013; Lee et al. 2012). The smokers in our study averagely smoked seven cigarettes per day. The amount of smoking was less than that as a smoker, defined as 20 cigarettes per day (Wang et al. 2008). Based on the data of this study and the published results, we deduced that urinary PAH levels were from smoking and from exposure to ambient environment. Smoking was not the dominant factor for those whose daily cigarette smoking numbers was less than 20 in Guangzhou. Our finding is consistent with those in previous studies, which showed the level of biomarkers (OH-PAHs) in urine is affected by environmental exposure (Fan et al. 2012a; Yoon et al. 2012).
Relationship between 8-OHdG concentrations and PAHs exposure
There existed no significant differences for levels of 8-OHdG between non-smokers (10.50 μg/g crt.) and smoker (10.45 μg/g crt.), respectively (p > 0.05). It indicated that the two groups had the similar mutagenic and carcinogenic burdens. If data from the two groups were combined and processed statistically, 8-OHdG correlated well with 1-OHN (r = 0.315 p < 0.05), 2-OHN (r = 0.420, p < 0.01), 1-OHP (r = 0.587, p < 0.01), 2-OHF (r = 0.682, p < 0.01), 3-OHF (r = 0.552, p < 0.01), 2- + 3-OHPhe (r = 0.433, p < 0.01), and 1- + 9-OHPhe (r = 0.444, p < 0.01), respectively (Table 3). Moreover, there were significant and positive correlations between 1-OHN and 2-OHN, 2-OHF and 1-OHP, 3-OHF and 1-OHP, 2-OHF and 3-OHF, 2-OHF and 2- + 3-OHPhe, and 2-OHF and 1- + 9-OHPhe as well as 2- + 3-OHPhe and 1- + 9-OHPhe (coefficients above 0.500 and p < 0.05). These results suggested that 2-OHF and 1-OHP were the suitable biomarkers to indicate PAHs exposure without a necessity to examine other biomarkers of PAHs for both smokers and non-smokers (Chetiyanukornkul et al. 2004).
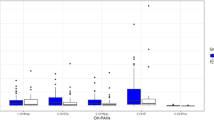
When ∑OH-PAHs was transformed into natural logarithms, the multiple regression analysis showed that 8-OHdG levels has significant dose-related increase with enhanced urinary ∑OH-PAHs for smokers (r = 0.804, p < 0.01) and non-smokers (r = 0.493, p < 0.01) (Fig. 1), respectively. It suggests that PAH exposure caused by smoking would lead to more DNA damage. Besides 8-OHdG, malondialdehyde (MDA) in urine was also used as the oxidative stress of DNA damage to indicate environmental contaminants. Though many researches confirmed that higher PAH exposure would lead to higher DNA damage, the results about their associations were different for different populations. The study on children and adults in Korea showed that statistical significance of MDA was only found in female adults (Yoon et al. 2012). Our previous study also suggested that higher urinary PAH exposure resulted in higher 8-OHdG levels (Fan et al. 2012a). An association between occupational PAHs exposure from fine particulate matter (PM2.5) of diesel exhaust particles at an inspection station and an increased excretion of urinary 8-OHdG in inspectors was found. But the study on coke oven workers by Kuang et al. (2013) strongly suggested that there existed dose-response relationships between ∑OH-PAHs and DNA damage, including oxidative stress (8-OHdG) and PAH-albumin adduct (BPDE-Alb). It was in accordance with our study. All these results indicated that 8-OHdG was a suitable biomarker to indicate the mutagenic and carcinogenic hazards induced by PAHs both for occupational and non-occupational populations.
Assessment of environmental risk and estimated daily intakes of PAHs by using biomonitoring data
Compared with non-occupational populations from different countries and areas (showed in Table 4), exposure levels of naphthalene, fluorene, and phenanthrene in our study were comparative with those from America, China, and Korea (CDC 2009; Yue et al. 2009; Kim et al. 2000; Gündel et al. 1996). However, 1-OHP concentrations were 2–50 times higher than other reports (Ofori-Adjei et al. 2009; CDC 2009; Yue et al. 2009; Kim et al. 2000; Gündel et al. 1996; Hecht et al. 2004), suggesting that people in Guangzhou were exposed to higher PAHs and with more potential hazards.
Air and food monitoring data were often used to assess the environmental risks of PAHs. However, due to spot variations and the individual differences of metabolism, external exposure data often resulted in bigger deviations. Internal exposure could reflect the sum of exposures from food, air, and dermal contacts accurately. Hence, the assessment model using biomonitoring data could better assess the potential health risk. On the basis of distribution analysis of all the variables in the following equation, we estimated the daily intake (EDI; μg/day) of PAHs as showed in Eq. 1 (Guo et al. 2013).
where EDI is the sum of PAH daily intake (μg/day), C is the individual OH-PAH concentration (μg/L) in urine, V is the urine volume of human daily excretion (L/day) (assumed a volume of 2 L), M 1 and M 2 are the molecular weights of parent PAHs and its corresponding metabolites (g/mol), respectively, and f is the ratio of OH-PAH excreted in urine relative to the total exposure dose (values of 100, 6.8, 60, and 11 % were used for naphthalene, pyrene, fluorene, and phenanthrene, respectively (Li et al. 2012).
The EDIs of PAHs (μg/day) for smokers ranged from 7.18 to 154 for naphthalene, from 48.9 to 172 for pyrene, from 3.91 to 162 for phenanthrene, and from 1.30 to 24.1 for fluorene, respectively. For non-smokers, the EDIs of PAHs (μg/day) ranged from 1.53 to 54.6 for naphthalene, from 66.7 to 371 for pyrene, from 13.8 to 361 for phenanthrene, and from 2.68 to 35.9 for fluorene, respectively. The intakes of pyrene were significantly higher than those of naphthalene, fluorene, and phenanthrene (p < 0.05). The reference doses (RfDs) of the U.S. Environmental Protection Agency (EPA) derived from chronic oral exposure for naphthalene, fluorene, and pyrene were 20, 40, and 30 μg/kg/day, respectively (EPA 2013). Assumed that the weight of an adult was 65 kg, then, the tolerable daily intakes of naphthalene, fluorene, and pyrene were 1,300, 2,600, and 1,950 μg/day, respectively. As showed in Fig. 2, the results indicate that the estimated exposure doses for all PAH compounds from our study were far below their RfDs of the U.S. EPA, even for 1-OHP.
Conclusion
In conclusions, after controlling for dietary intakes of PAHs, our study indicated that there was no significant difference between smoking and non-smoking students. Compared with previous reports, these college students in Guangzhou bore more urinary PAHs, especially for pyrene. 1-OHP levels in our study were 2–50 times higher than those from USA, Germany, Ghana, and Korea. EDIs were far below the RfDs recommended by U.S. EPA. The reasons might be because that PAH exposures in Guangzhou were also affected by ambient environment, and smoking was not the dominant factor for PAHs intakes. Though no significant difference for urinary 8-OHdG was found between the two groups, to our best knowledge, it was the first time for us to find that 8-OHdG showed dose-related increase to urinary PAH concentrations both for non-smokers and smokers. More subjects would be recruited to confirm and solidify the finding.
References
Angerer J, Heudorf U (2001) Urinary monohydroxylated phenanthrene and hydroxypyrene: the effects of smoking habits and changes induced by smoking on monoxygenase-mediated metabolism. Int Arch Occup Environ Health 74:177–183
Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C (2002) Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 110(3):451–488
CDC (Centers for Disease Control and Prevention), National Center for Health Statistic (2009) Fouth national report on human exposure to environmental chemicals, Atlanta. Available from http://www.cdc.gov.nchs/nhanes.htm
Chetiyanukornkul T, Toriba A, Kizu R, Hayakawa K (2004) Urinary 2-hydroxyfluorene and 1-hydroxypyrene levels in smokers and nonsmokers in Japan and Thailand. Polycycl Aromat Compd 24:467–474
Ding YS, Trommel JS, Yan XJ (2005) Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environ Sci Technol 39(2):471–478
Fan R, Wang D, Mao C, Ou S, Lian Z, Huang S et al (2012a) Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2’-deoxyguanasine in Guangzhou, China. Environ Int 42:53–58
Fan R, Wang D, Ramage R, She J (2012b) Fast and simultaneous determination of urinary 8-hydroxy-2`-deoxyguanosine and ten monohydroxylated polycyclic aromatic hydrocarbons by liquid chromatography/tandem mass spectrometry. Chem Res Toxicol 25(2):491–499
Gündel J, Mannschreck C, Büttner K (1996) Urinary levels of 1-hydroxypyrene, 1-, 2-, 3-, and 4-hydroxyphenanthrene in females living in an industrial area of Germany. Arch Environ Contam Toxicol 31(4):585–590
Guo Y, Senthilkumar K, Alomirah H, Moon HB, Minh TB, Mohd MA et al (2013) Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol 47(6):2932–2938
Han YY, Donovan M, Sung FC (2010) Increased urinary 8-hydroxy-2’-deoxyguanosine excretion in long-distance bus drivers in Taiwan. Chemosphere 79:942–948
Hecht SS, Carmella SG, Le KA (2004) Effects of reduced cigarette smoking on levels of 1-hydroxypyrene in urine. Cancer Epidemiol Biomarkers Prev 13(5):834–842
Huang SH, Ge XM (2006) Content of polycyclic aromatic hydrocarbons (PAHs) in mainstream smoke of Chinese cigarettes. J Environ Health 23(1):46–48
IARC (International Agency for Research on Cancer) (1983) Polynueclear Aromatic Compounds. Part I. Chemical, environmental and experimental data: IARC monograph on the evaluation of carcinogenic risk of chemical to man, vol 32. France, Lyon
Ichiba M, Matsumoto A, Kondoh T, Horita M, Tomokuni K (2006) Decreasing urinary PAH metabolites and 7-methylguanine after smoking cessation. Int Arch Occup Environ Health 79:545–549
Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2012) Monitoring the future national results on adolescent drug use: overview of key findings, 2011. Institute for Social Research, The University of Michigan, Ann Arbor
Kaiserman MJ, Rickert WS (1992) Carcinogens in tobacco smoke: benzo [a] pyrene from Canadian cigarettes and cigarette tobacco. Am J Public Health 82(7):1023–1026
Kim H, Cho SH, Kang JW (2000) Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int Arch Occup Environ Health 74(1):59–62
Kuang D, Zhang W, Deng Q, Zhang X, Huang K, Guan L (2013) Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 47:7446–7456
Lee MW, Chen ML, Lung CSC, Tsai CJ, Lai SCF, Yang SC et al (2012) Increase of urinary concentrations of 8-hydroxy-2’-deoxyguanosine in diesel exhaust emission inspector exposed to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 85:273–282
Li X, Li B, Tao S, Guo M, Cao J, Wang X (2005) Population exposure to PAHs in Tianjin area. Chin Acta Scientiae Circumstantiae 25(7):989–993
Li JL, Xiao C, Liu BC (2007) Survey on smoking status of undergraduates in Guangzhou university. Chin J Health Edu 23(4):294–295
Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M et al (2012) Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol 25(7):1452–1461
Lloyd JW (1971) Long-term mortality study of steelworkers: V. respiratory cancer in coke plant workers. J Occup Environ Med 13(2):53–68
Naeveàez R, Lorihoepner N, Chilirud S, Yan B, Garfinkel R, Whyatt R (2008) Spatial and temporal trends of polycyclic aromatic hydrocarbons and other traffic-related airborne pollutants in New York city. Environ Sci Technol 42:7330–7335
Navasumrit P, Arayasiri M, Hiang OMT (2008) Potential health effects of exposure to carcinogenic compounds in incense smoke in temple workers. Chem Biol Interact 173(1):19–31
Ofori-Adjei D, Johnson NM, Afriyie-Gyawu E, Huebner H (2009) PAH exposure in a Ghanaian population at high risk for aflatoxicosis. Sci Total Environ 407:1886–1891
Palackal NT, Lee SH, Harvey RG (2002) Activation of polycyclic aromatic hydrocarbontrans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J Biol Chem 277(27):24799–24808
Roelofzen JHJ, van der Valk PGM, Godschalk R (2012) DNA adducts in skin biopsies and 1-hydroxypyrene in urine of psoriasis patients and healthy volunteers following treatment with coal tar. Toxicol Lett 213(1):39–44
Scherer G, Frank S, Riedel K (2000) Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiol Biomarkers Prev 9(4):373–380
Strickland P, Kang D (1999) Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicol Lett 108(2):191–199
Taussky HH (1954) A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 208(2):853–862
U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS). http://www.epa.gov/IRIS/index.html. Accessed Nov 2013
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C 27(2):120–139
Wang G, Kawamura K, Lee S, Ho K, Cao J (2006) Molecular, seasonal, and spatial distributions of organic aerosols from fourteen Chinese cities. Environ Sci Technol 40:4619–4625
Wang H, Yu M, Hu R, Wang L, Gong W (2008) Survey of smoking middle school students in Zhejiang. Chin Dis Surveill 23(2):114–116
Yoon HS, Lee KM, Lee KH, Kim S, Choi K, Kang D (2012) Polycyclic aromatic hydrocarbon (1-OHPG and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. Int J Hyg Environ Health 215:458–464
Yue Q, Wang DC, Yu ZQ, FAN RF (2009) Investigation of polycyclic aromatic hydrocarbons exposure levels in some middle school students in a city in southern China. Chin J Environ Health 26(5):385–387
Zhang Y, Ding J, Shen G, Zhong J, Wang C, Wei S (2014) Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites: a controlled case study in Beijing, China. Environ Pollut 184:515–522
Acknowledgments
This work was supported by grants from the Ph.D. Programs Foundation of Ministry of Education of China (no. 20124407120005) and Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (no. 693, 2013).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, J., Fan, R., Lu, S. et al. Exposure to polycyclic aromatic hydrocarbons could cause their oxidative DNA damage: a case study for college students in Guangzhou, China. Environ Sci Pollut Res 22, 1770–1777 (2015). https://doi.org/10.1007/s11356-014-2769-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2769-z