Abstract
Fresh tobacco or the smoke resulting from waterpipe and cigarette contains large amounts of polycyclic aromatic hydrocarbons (PAHs), which consumption can cause releasing of these contaminants into the indoor air of cigarette and waterpipe cafés. This study was conducted to investigate the urinary concentrations of unmetabolized PAH compounds among the employed workers as well as the customers in waterpipe and cigarette cafés along with its association with oxidative stress factors plus kidney injury biomarkers. For this, 35 staffs and 35 customers in these cafés (as an exposed group (EG)), 20 staffs in non-smoking cafés (as 1st control group (CG-1)), and 20 of the public population (as 2nd control group 2 (CG-2)) were chosen and their urine specimens were collected. The results indicated that there is a significant difference between urinary concentration of ƩPAHs in the exposed and control groups (P value < 0.05). Also, “type of tobacco” can be considered as an influential and determining factor for the urinary levels of PAHs among the subjects. Considering the contribution of PAHs to the total toxic equivalents, benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DahA), and fluoranthene (Flrt) with 32.76%, 27.62%, and 18.65% claimed the largest share in waterpipe/cigarette cafés. The results also indicated a positive and significant relationship between some PAHs and oxidative stress biomarkers as well as uKIM-1 (biomarker for assessing and diagnosing glomerular damage) and TIMP-1 (biomarker of stress in primary steps of injury in tubular cell). Thus, it can be expressed that the workers of these smoking cafés are prone to the detrimental health impacts. Accordingly, proper policies and decisions should be taken to limit the activity of these cafés or proper protective strategies should be adopted to protect the health of exposed individuals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessing the adverse effects of tobacco smoking has changed into a hot research topic at academic and scientific centers worldwide, and many attempts are being progressively made to mitigate these negative, with around 6 million deaths attributed to this factor annually worldwide (Kassem et al. 2014, Rostami et al. 2019). With the research and regulatory attempts to diminish the health impacts of tobacco products being focused on cigarettes (Barnes 2011, Dobaradaran et al. 2019), similar attention should also be paid to other various products of tobacco. Waterpipe tobacco smoking (WTS) is presently a prevailing and worldwide health plight especially between the teenagers and used in different countries including East Mediterranean, Eastern Europe, and Western countries including the USA (Jawad et al. 2016, Odani et al. 2018). Estimation of prevalence of WTS indicated that out of 68 countries of the world, the largest rate of prevalence was observed among the adults in East Mediterranean countries, though the prevalence is almost equal among the youth in East Mediterranean and European countries (Jawad et al. 2018). A national study in the USA reported that 13% of 15–17-year-old people had a history of smoking waterpipe, and 2.9% smoked waterpipe over the past 30 days. This study also showed that 44.4% of the 18–24-year-old people had history of waterpipe smoking, and 18.2% of them were currently waterpipe smokers (Kasza et al. 2017). WTS is performed via a waterpipe apparatus (which is also recognized as Ghalyan, Nargile, Ghalun, Shisha, Hookah, or Argileh). Waterpipe consists of a water jar (bowl) (made of glass or clay, which is filled with water up to the half or two thirds), a body (a vertical tube placed inside the water jar), a head (in which tobacco is placed and foiled wrapped, with charcoal placed on the foil), and finally a flexible and soft pipe with mouthpiece (Rostami et al. 2019). The tobacco is heated and burnt by the burning charcoal in the waterpipe head, whereby the resulting smoke goes through water bowl, and then inhaled through the pipe by the smoker. Most waterpipe consumers use fruit-flavored tobaccos. Fruit-flavored tobacco, also recognized as Maassel, Mu’assel, or Moassel, is a composition, 30% of which is tobacco while the rest are natural or artificial flavorings, humectants (such as polyethylene glycol), and sweeteners (e.g., honey, sugars, and molasses) (Schubert et al. 2012). Smoking waterpipe has been related with higher risk of developing lung and mouth cancers, congestive cardiovascular diseases, and obstructive lung illnesses (Maziak et al. 2015, Kasza et al. 2017).
Extensive studies performed on fresh (unsmoked) WPT or the smoke resulting from tobacco or cigarette have indicated that tobacco contains large amounts of dangerous particular and gaseous pollutants including aromatic amines, alcohols, carbonyls, carbon oxide, heavy metals, BTEX (benzene, toluene, ethylbenzene, and xylene), aldehydes, phenol, and PAHs. Therefore, its consumption in waterpipe and cigarette cafés results in releasing of different contaminants into the internal air of these cafés (Heydari et al. 2019, Masjedi et al. 2019, Rostami et al. 2019, Heydari et al. 2020, Kashfi et al. 2022). Thus, the people working in these cafés are heavily exposed to the air polluted with different toxic and hazardous compounds. One group of these hazardous pollutants is PAHs, which have attracted extensive attention due to their bioavailability, as well as potential carcinogenic, mutagenic, and teratogenic properties (Mirzaei et al. 2015, Dobaradaran et al. 2019). Some of these compounds are also known as stable organic contaminants and endocrine disrupter chemicals (EDCs) (Oliveira et al. 2020a). Benzo(a)pyrene is classified as a definite carcinogen for human (group I) by the International Agency for Research on Cancer (IARC). It has also placed nine other compounds including naphthalene, benzo(j)fluoranthene, enzo(b)fluoranthene, benzo(b)fluoranthene, dibenzo(a,h)anthracene, dibenzo(a,l)pyrene, dibenz(ah)anthracene, benz(a)anthracene, and chrysene in groups 2A/2B (Cancer 2010). PAHs have lipophilic properties as well as octanol–water partitioning coefficient 3–8 (De Craemer et al. 2016). For this reason, PAHs can easily cross the cell membrane (either on skin or inside the body) through passive diffusion. The parent PAH compounds are not straight able to detriment DNA, but their impacts occur mostly via the changes in the expression of various genes resulting from the metabolization and activation of aryl hydrocarbon receptor (AhR), thereby indirectly generating inflammation, tumorigenesis, changing the hormonal routes, cell proliferation, and loss of cellular adhesion (Murphy et al. 2007, Shaban et al. 2013, Moorthy et al. 2015). In this regard, PAHs are metabolized by cytochrome P450 enzymes to generate active semiquinones in humans and animals’ body (Shaban et al. 2021, Lin et al. 2022) which are free radical intermediates and can go through redox cycling and generate reactive oxygen species (ROS). The ROS can then cause oxidative modification of DNA and lipids in the body (Chen et al. 2020, Shaban et al. 2020). The DNA adduct, 8-hydroxy-20-deoxyguanosine (8-OHdG), is a useful biomarker of DNA damage to assess human exposure to carcinogenic compounds and malondialdehyde (MDA) is an indicator for oxidative stress and lipid peroxidation (Huang et al. 2020, Al-Taie et al. 2021, Shaban et al. 2022).
It is expected that the parent PAHs would have less sensitivity compared to their metabolized counterparts in relation to changes in urinary concentration (creatinine level), since they are cleared from the body through kidneys through inactive diffusion rather than active excreting (Waidyanatha et al. 2003, De Craemer et al. 2016). Hence, they are better indicators compared to their metabolites for assessing individual exposure. Accordingly, parent PAHs have been utilized in numerous studies for assessing occupational and environmental exposure as biomarker (Campo et al. 2006, Campo et al. 2007, Rossella et al. 2009, De Craemer et al. 2016, Ratelle et al. 2020, Thai et al. 2020). Nevertheless, to the best of our knowledge, no study has evaluated the exposure of people working in cigarette or waterpipe cafés to PAHs through measuring the values of these pollutants in their urinary specimens as well as assessing their relationship with oxidative stress/inflammation biomarkers plus kidney injury biomarkers. Accordingly, this study was performed for the first time to explore (i) the values of unmetabolized PAHs in urinary specimens of people working in cigarette/waterpipe cafés and comparing them with the corresponding values among people working in non-smoking cafés as well as customers, (ii) the effect of influencing factors on urinary unmetabolized PAHs, and eventually (iii) the association between urinary levels of unmetabolized PAHs and oxidative stress/inflammation biomarkers plus kidney injury biomarkers.
Materials and methods
Design of study and selecting sampling sites
For this research, 35 smoking cafés were randomly selected out of 50 smoking cafés across the 2nd district of Tehran municipality. For this, the smoking cafés available in the area were listed in an Excel from 1 to 50, and then by using following randomization function in Excel, and 35 cases were selected (Eq. 1).
Thereafter, the 35 selected cafés were listed, and their address as well as information were collected for sampling. Twenty three out of these 35 cafés were those in which waterpipe would be smoked (hereafter referred to as WPC), while 12 were cafés that only cigarette would be smoked (CC). Moreover, 20 cafés were non-smoking which would be regarded as control group 1 (CG-1). One of the staffs and one customer would be chosen from each café, and their urine specimens would be collected.
Furthermore, the urine specimens of the 2nd control group (CG-2) consisted of 20 subjects from the Tehranian general people were collected. Note that the individuals with any kind of disease or illness were excluded from this research in order to avoid their probable effect on the outcomes; that is, all participants were healthy. Then, the necessary justification was given by the researchers to convince the owners and managers as well as staff and customers of the chosen cafés in order to acquire the sampling permission. Once the participants signed the written research informed agreement form, the sampling procedure was started.
Data collection and sampling
Before initiating the sampling, background information of each café was reported including services number given per day, type of tobacco consumed (traditional or fruit-flavored tobacco), and the distance between cafés and traffic using a checklist prepared by the researcher. The information of the sample donors, including gender, age, weight, height, BMI, smoking tobacco by the person, duration of working in these cafés, and application of personal protective equipment, is also provided in Table 1.
Then, the suitable method of specimen collection and the best way to prevent possible cross-contamination were trained to the participants. Next, two urine collection containers were given to each of the participants: one would be taken as the fasting specimen before beginning the working shift and the other after their working shift. The early morning specimens were collected in polyethylene 100-mL containers as before exposure samples (BE), while the specimens after the working shift would be collected as the after exposure (AE). The same procedure of sampling was performed for control group 1 whereas for control group 2, mid-morning urine specimens were collected. Also, samples were taken randomly from the customers when referring to the cafés. Once collected, the urine specimens were transferred to the laboratory in cold conditions (cold box with dry ice) immediately after labeling and coding. The specimens were properly packed, and kept in the fridge at − 20 °C until final analyses. The sampler of this work had been trained on sampling, packing, and transferring of bio-samples including urine, nail, and blood specimens.
Preparing samples and determining the concentration of unmetabolized PAHs
In order to prepare the samples to measure the unmetabolized PAHs, the method recommended by Campo et al. (Campo et al. 2014) was used with minor modification. In summary, 2 mL of urine specimens was poured inside silanized glass vials, and then spiked with 1 μL of internal standard solutions containing nine deuterated PAH compounds (including Anthracene (Ant), Benz[a]anthracene (BaA), Dibenz[a,h]anthracene (DahA), Fluoranthene (Flrt), Benzo[k]fluoranthene (BkF), Benzo[b]fluoranthene (BbF), Chrysene (Chr), Phenanthrene (Phen), Pyrene (Pyr), Benzo[a]pyrene (BaP), Benzo[ghi]perylene (BghiP), and Indeno[1,2,3-cd]pyrene (IndP)). Next, the analytes were extracted from the studied specimens by a 100-μm polydimethylsiloxane SPME fiber during 1 h at ~ 80–85 °C, and then desorbed during 10 min at 270 °C into the injection port of chromatography. The concentration of nine PAHs in the urine specimens was determined using gas chromatography-mass spectrometry (Agilent 7890 GC–5975MSD) with capillary column and an auto-injector. GC analysis was carried out with following analytical conditions: helium (as carrier gas) purity of 99.9999% at 1 mL·min−1 flow rate, injection point temperature 270 °C, oven temperature program: 5 min at 100 °C constant temperature, and then temperature elevation at 20 °C/min until reaching 340 °C, and then remaining at this temperature for 5 min. Mass spectrometer detection was done with following thermal and analytical conditions: the temperature of transfer line was 280 °C and it was 300 °C in ion source, operating in single ion monitoring mode.
Quality assurance and quality control (QA/QC)
PAH compounds
Standard stock solution of PAHs was prepared at 500 mcg/L from labeled standards of PAH compounds. Then, working solutions were prepared within the concentration range 0.5–1000 ng/L, and then employed to prepare the calibration curves. The urine specimens were spiked with standards plus isotope-labeled standards, and then analyzed according to the analytical method mentioned above for real specimens. Through repeating measurements of low-level spiked standards of PAHs in urine, the signal-to-noise ratio (S/N) values were obtained. S/N equal to 3 was regarded as the limit of detection (LOD), while S/N of 9 was considered as the limit of quantification (Table 2). For all statistical treatments, the specimens with values lower than LOQ were considered with LOQ/2. The correlation coefficient (R2) of calibration curves was used as an index for assessing linearity. For precise assessment of the analyses, relative standard deviations (RSDs) were obtained for repeating analysis of standard solutions with different concentration levels. In addition, the intraday precision was assessed by comparing with prepared standard solutions six times within a day. Also, the inter-day precision was also evaluated by with prepared standard solution in six consecutive days. In order to ensure the accuracy of analysis, the spiked urine specimens were analyzed with four concentration levels of PAH standards (0.1, 5, 10, and 20), whereby the extent of recovery for each level was obtained.
Analysis of oxidative stress biomarkers
The collected urine specimens were analyzed in order to detect the values of 8-hydroxyguanosine (8-OHdG; as a biomarker for DNA oxidative stress) plus 8-isoprostane (as biomarker for lipid peroxidation) and malondialdehyde (MDA; universal oxidative stress biomarker). The urine specimens were hydrolyzed and affinity-purified by affinity chromatography before determining the value of isoprostane-8. For quantification of both biomarkers, commercial kits were used via enzyme-linked immunosorbent assay (ELISA) method. There were 96 microplates in each kit and the measurements were conducted according to the instruction recommended by the manufacturer. Every microplate had its particular working standard curve for measuring these target biomarkers (MDA, 8-isoprostane, and 8-OHdG). The limits of detection of these kits for measuring MDA, 8-isoprostane, and 8-OHdG were 1 ng/L, 2.7 pg/mL, and 0.5 ng/L, respectively. Each ELISA measurement was replicated for urine specimens and it was observed that the result variations (during duplicates) were lower than 15%. However, the current research has been done to survey the impact of PAH concentrations on the levels of target biomarkers (8-OHdG, 8-isoprostane, and MDA). Thus, it is necessary to avoid possible systematic errors in this approach.
Statistical analyses
Data analyses of this study were carried out by Excel 2019 and SPSS software (version 21.0; SPSS Inc.). The normality of data distribution associated with each of the factors was evaluated using Shapiro–Wilk test. Differences of the urinary concentrations of PAHs between the exposed (WPC and CC) and control groups (CG-1 and CG-2), as well as between AE and BE values, were explored through non-parametric Mann–Whitney U test. The concentration of pollutants was considered without creatinine adjustment. Creatinine concentration depends not only on muscle mass but also on the extent of urine discharge (urine dilution factor). So, creatinine adjustment might cause mistakes for estimating exposure and human risk outcome. Two-sided P value < 0.05 was considered as statistical significance level.
Results and discussion
General characteristics of the subjects
In this research, a 70-member group (35 of staff and 35 customers) in smoking cafés was recruited as the exposed group. Additionally, 1st control group (CG-1) and control group 2 (CG-2) consisted of 20 staff in non-smoking cafés and 20 of the general population, respectively, were chosen as non-exposed groups. The general (e.g., demographic and lifestyle) features of these people are given in Table 1 (according to the checklist information). As seen, all of the studied individuals were male, and the average age of the groups of staff and customers of smoking cafés were 34 and 35 years, respectively, and for CG-1 and CG-2 were also 32 and 35 years, respectively. The age of individuals in CG-1 was slightly lower than the other groups. No significant difference was found between exposed and control groups regarding weight, height, and BMI. Based on WHO categorization, most of the cases had minor overweight (~ 60%), normal weight (30%), and only ~ 10% were overweight. In addition, most of the subjects in the exposed groups had a low socioeconomic and academic status. There was a considerable difference (P value < 0.05) among the staffs of cigarette-smoking and CG-1 group regarding the periods of occupation. The period of occupation in workers of non-smoking cafés (CG_1) was longer. Most of the staff and customers in smoking cafés used fruit-flavored tobacco. Badly, very few number (about 5%) in cafés utilized PPE equipment.
Urinary concentration of PAHs among the exposed and control groups
Urine is the most practical matrix for assessing exposure to cigarette and tobacco pollutants, as it is a non-invasive biological fluid that can be obtained easily. In addition, this matrix accumulates higher levels of some biological markers in it compared to other biological fluids (Torres et al. 2018), and is known as the most well-known human fluid for assessing PAH biomarkers’ exposure. Thus, detection of PAHs and their metabolites in urine is suitable for evaluating exposure of various groups to PAHs, as the internal concentration of these contaminants covers all origins and routes of exposure (Oliveira et al. 2020b, Fernández et al. 2021). In this study, the concentration of 12 PAHs was determined in the urine specimens collected from exposed (staff and customers of tobacco/cigarette smoking cafés) and two control groups (CG-1 and CG-2) with the relevant statistical findings depicted in Fig. 1 and Table 3. Among these PAHs in the specimens, only Ant, Pyr, and BghiP were not detectable in the CG-2 and CG-1, while Flrt and D[ah]A could not be detected in the CG-1 customer group (< LOQ).
The mean level of ΣPAHs in the urine specimens gathered from the workers of WPC (1206.38 ± 551.09 ng/L) and CC (850.37 ± 206.91 ng/L) was determined 15.46 and 10.9 times as large as that of CG-1 (77.68 ± 19.79 ng/L), 86.14 and 60.71 times as large as that of CG-2 (13.56 ± 8.96 ng/L) specimens. Based on the results, ΣPAH concentration was significantly different among the exposed (WPC and CC) and control (CG-1 and CG-2) groups regarding (P value < 0.05). This comparison among exposed and control groups indicates that the activities associated with tobacco smoking aggravate the level and severity of exposure (Zhang et al. 2021). This observation indicated that participants who worked within high-polluted cafés could be easily affected by smoking-related airborne PAHs (Adesina et al. 2021). Actually, the considerably higher (P < 0.05) urinary PAHs in cases from smoking cafés could be due to higher exposure through inhalation, ingestion, and/or dermal contact (Rostami et al. 2019). PAH compounds are ubiquitous in ambient air, contained in cigarette smoke with high volumes in vehicular traffic, and is elevated in indoor air when tobacco-related products (e.g., traditional/fruit flavored tobacco, cigarette, and snus) are smoked (Bortey-Sam et al. 2017, Rostami et al. 2019). Zhang et al. also reported that the level of PAH metabolites was larger in the specimens prepared from smokers compared to non-smokers (Zhang et al. 2021). The study results suggest the fact that although PAHs are not considered specific makers of tobacco smoke, they are found at high concentrations in smoking environments, and thus in addition to workers, it also influences the people who referred to these areas for the aims other than smoking tobacco or cigarette such as tea drinking, and generally spend about 1 h of their time in these places (Torres et al. 2018, Masjedi et al. 2019). In this regard, Kim et al. also said that the urinary level of nine PAH biomarkers was higher in non-smoking children (6–18 years) exposed to tobacco smoke compared to non-exposed children (Kim et al. 2014). Meanwhile, since workers spend more time in these cafés, they would have greater exposure to these pollutants (Gurung et al. 2016, Masjedi et al. 2021). Thus, working in cigarette and waterpipe cafés is considered as one of the major routes of exposure to different contaminants including PAHs (Swami et al. 2009). Comparison between the workers and customers in this study also confirmed this fact and indicated that the average level of PAHs in the specimens collected from the workers of WPC, CC, and CG-1 groups was 3.46, 3.31, and 2.79 times this concentration obtained in the specimens from the customers of these groups. In a research conducted by Rostami et al. (Rostami et al. 2019), the findings indicated that the mean level of PAHs in the indoor air of smoking cafés was 5522.1 ± 594.8 ng/m3. It was 5915.2 ± 650.5, 2404 ± 271.4, and 7304.9 ± 846.0 ng/m3 for cafés with fruit-flavored tobacco, regular tobacco, and the both, respectively (Rostami et al. 2019).
In addition, PAH concentration was higher in subjects of WPC in comparison with the concentration among subjects of CC. The findings of other studies also suggested that in waterpipe cafés as compared to cigarette cafés, the extent of exposure to high molecular weight PAHs was higher while it was lower for low molecular weight PAHs (Farley et al. 2018). Our previous study also revealed that the heavy metal level was larger in waterpipe cafés in comparison with cigarette counterparts (Heydari et al. 2019, Masjedi et al. 2019, Masjedi et al. 2021). The tobacco of WP is heated by burning charcoal, and thus it burns at a temperature lower than that of cigarette, with its resulting smoke being different, whereby the PAHs produced by it are several times those of cigarette (Shihadeh et al. 2015). Meanwhile, the individuals who sit in WP cafés are exposed to considerable concentration of the air contaminants which is dangerous for human (Zaidi et al. 2011, Cobb et al. 2013, Rostami et al. 2019).
The results related to comparing the concentration of PAHs in urinary specimens of AE and BE of exposed and CG-1 are displayed in Fig. 2. PAH concentration in the post-shift specimens (AE) of workers in WPC, CC, and CG-1 groups was 1.41, 1.5, and 1.61 times the concentration in the pre-shift specimens (BE). The results of statistical test also suggested noticeable difference among BE and AE concentrations for BghiP, BkF, DahA, IndP, and Pyr in the WPC-exposed group (P value < 0.05) as well as BbF, BghiP, BkF, Chr, DahA, and Pehn between BE and AE specimens in the exposed group CC (P value < 0.05). However, no significant difference was seen for other PAHs (P value > 0.05). Among the CG-1 worker, from among all PAHs, a significant difference was seen only between BE and AE concentrations of BaA, BkF, Phen, and Pyr (P value < 0.05). The difference between PAH urinary concentration before and the shift can reflect the level of daily exposure. Lack of significant difference between AE and BE concentrations of some PAHs can be due to differences in their absorption and excretion kinetics (Campo et al. 2006). The maximum and minimum PAH concentrations in AE specimens in the two exposed groups were related to Phen an Indp, while in the control group 1 (CG-1), they were related to Phen and BghiP, respectively. The urinary PAH concentration in this study has been higher than the concentration reported for road pavers (RP) and construction workers (Campo et al. 2006), as well as non-smoking children exposed to tobacco smoke (Kim et al. 2014).
Investigating the effect of influential factors
In order to explore the influence of environmental, occupational, and lifestyle parameters of subjects on the urinary levels of PAHs, multiple regression (MLR) test was utilized, with the findings reported in Table 4. The selected factors included age, BMI, level of education, services number provided to customers in each shift, occupational background, type of tobacco, distance with street traffic, personal protective equipment, and urinary creatinine level. In this analysis, the relationship observed between the urinary levels of observed PAHs and the mentioned factors is evaluated according to the regression coefficient and P value. As observed in Table 4, “service number provided in each shift,” “type of tobacco,” “distance between cafés and traffic,” and “use of personal protective equipment” have been determining and influential variables on urinary concentration of PAHs. Creatinine was also found as a remarkable predictor for urinary Phen, Ant, Pyr, Chr, BkF, and BghiP, but not for other urinary PAHs. No considerable correlation was found between the urinary concentration of PAHs and other investigated factors such as working duration in the café, education level, BMI, and age.
It is observed that the urinary levels of PAHs have had a positive and meaningful relationship with the type of tobacco (P value < 0.01). For more precise assessment of disassociation, the urinary levels of PAHs (ΣPAHs) of staff working in smoking cafés were compared between traditional and fruit-flavored tobacco, as shown in Fig. 3. As observed, ΣPAH values have been 12,458.10 and 33,457.26 ng/L in the specimens collected from the cafés serving traditional and fruit-flavored tobacco, respectively. Again, regarding customers, the urinary levels of ΣPAHs have been 1390.33 and 5134.67 ng/L in cafés with traditional and aromatic (fruit-flavored) tobacco, respectively. High levels of organic substances, flavorings, essences, and other various additives added to fruit-flavored tobaccos during the manufacturing process (Farley et al. 2018) result in emission of large concentration of different air contaminants (including PAHs) into the inside air of these cafés. In turn, the individuals working in these places are exposed to larger levels of these contaminants (Heydari et al. 2019, Rostami et al. 2019). Hence, the higher concentration of PAHs in urine specimens of workers and customers of aromatic (fruit-flavored) cafés could be attributed to this above-mentioned justification. As a confirmation, it should be noted that Naddafi et al. (Naddafi et al. 2019) reported larger amounts of PAHs, aldehydes (e.g., formaldehyde, acetaldehyde) particles, into the indoor air of cafes serving fruit-flavored tobacco in contrast to those serving traditional product. Masjedi et al. (Masjedi et al. 2019) also reported that the concentrations of contaminants such as nicotine, carbon monoxide (CO), formaldehyde, and particulate matters were higher in the indoor air of cafes offering fruit-flavored tobacco. In addition, smoking flavored tobacco is an enjoyable pastime for young people because of the sweet and pleasant taste of its smoke as well as being more burn retarding. Thus, they tend to stay more in these places and so are exposed to a larger concentration of these contaminants (Fazlzadeh et al. 2015, Rostami et al. 2019).
The urinary concentration of PAHs also showed a positive and considerable correlation (P value < 0.05) with the “service number in each shift” and “distance between café and traffic.” The number of services offered in the working shift of cafes means “active hookah/cigarette heads” number per day. As the number of these heads increases in the inside air of cafes, the extent of tobacco smoke emission into the indoor air also grows. This in turn results in higher concentrations of the produced pollutants which expose the staff and customers of these cafes (Heydari et al. 2019, Masjedi et al. 2019, Rostami et al. 2019). The regional traffic density in which the participants lived has also been an influential factor on the urinary values of PAHs measured (Table 4). These observations have been well in agreement with the studies reporting that the extent of exposure to air pollutants was higher for those living in proximity to high-traffic roads as compared to those living farther away from roads (Delgado-Saborit et al. 2011; Phuc and Oanh 2018).
A significant but reverse correlation was seen between urinary levels of PAHs and “use of PPE” (e.g., N95 mask) (P value < 0.05). This could be attributed to the fact that the utilized filters and masks have a suitable efficiency in inhibiting entrance of liquid and solid particles. Since most PAHs are emitted across the air in a particular form (Gysel et al. 2018), use of this personal protective equipment results in decreased exposure of staff as well as cigarette and waterpipe cafés to these particular pollutants. This in turn leads to lowered concentration of these pollutants (PAHs) in the urine specimens of people.
Relative carcinogenic strength
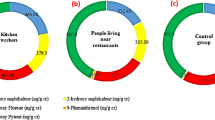
Seven compounds including BaA, Chr, BbK, BbF, BaP, IndP, and DahA are categorized as carcinogenic compounds, with BaP being also used as an indicator for carcinogenic activity of PAHs (Adesina et al. 2021). In this study, BaP urinary concentration varied between 0.05 and 124 ng/L, and its median concentration among the subjects in WPC, CC, CG-1, and CG-2 groups was 8.25, 12.15, 2.17, and 0.05 ng/L. The median total concentration of PAHs in WPC, CC, CG-1, and CG-2 groups was 22.96, 20.16, 2.12, and 0.09 ng/L, and the median concentration of Σ carcinogenic PAHs in these groups was 18.71, 18.16, 2.78, and 0.08 ng/L, respectively.
For all studied PAHs, assuming a toxic equivalency factor of 1 for BaP as the reference compound (TEF = 1), the toxic equivalents (TEQ) were calculated through multiplying the median urinary concentration of each by the TEF of their corresponding factor. Then, by comparing their TEQs, their relative carcinogenicity was investigated, with the results provided in Table 5. The total toxicity equivalent was obtained through summing up all TEQs. The TEF values were considered based on the studies (Nisbet and Lagoy 1992, De Craemer et al. 2016). The total TEQ related to groups CC, WPC, CG-1, and CG-2 was 33.31, 28.38, 3.17, and 0.38 ng/L.
In WPC, CC, and CG-1, the largest TEQ was related to BaP with 8.25, 12.15, and 2.17 ng/L, while in CG-2, the maximum level was related to BbF with 0.22 ng/L. In both WPC- and CC-exposed groups, the highest TEQ following BaP was related to DahA and Flrt, the minimum to Ant and Pyr. BaP, DahA, and Flrt overall claimed about 75% of total TEQ in WPC cafes, and 83% of total TEQ in CC cafes. To prioritize toxins, in addition to the internal concentration, the contribution percentage of each toxin should also be measured in total PAH toxicity. Also, the relationship between urinary concentrations and total toxicity effect of compounds introduced into the body should be determined. This is because unmetabolized PAH congeners excreted through the urine are different for each compound. Meanwhile, TEF is applicable for determining the carcinogenicity resulting from total exposure, while here only PAH concentration in urine has been measured (De Craemer et al. 2016). Adesina et al. reported the highest TEQ in all specimens for BaP within 0.134 ± 0.57 ng/L, followed by Naph, BaA, BbF, and BkF. In that study, total TEQ in the studied cafes lied within 0.474–1.049 ng/L (Adesina et al. 2021). In the report by Samara et al., again in all specimens, BaP, BbF, BaA, Ant, and Chr claimed the largest share in total TEQ (Samara et al. 2021). In another study, it was reported that BaP, Flt, and NAP claimed 54, 20, and 10% and totally 84% of total TEQ 0.3867 (De Craemer et al. 2016).
The DNA/lipid oxidative damage and kidney injury
Several researches have shown that exposure to PAHs can cause development of oxidative stress. Thus, in this study Pearson correlation’s test was employed to assess the correlation among exposure to these compounds and oxidative stress indicators (MDA, 8-isoprostane, and 8-OHdG) among the staff working in cigarette and waterpipe cafés, with the findings presented in Table 6 and Fig. 4. As shown, some PAHs have had a positive and significant correlation with oxidative stress biomarkers. For example, the relationship between urinary concentration of Phen, Flrt, Pyr, Chr, BbF, BaP, DahA, and BghiP and 8-OHdG biomarker has been positive and significant. Also, the urinary concentration of Ant, Pyr, BkF, and PI has had a positive and meaningful association with 8-isoprostane (P value < 0.01). Eventually, the relationship between the urinary concentration of Pyr, Chr, BbF, BaP, and DahA and MDA has also been positive and significant (P value < 0.01). Other associations between the pollutants and oxidative stress biomarkers have also been positive but insignificant. Similarly, the correlation among exposure to PAHs and DNA oxidative damage has been observed in various in vitro and in vivo studies (Kuang et al. 2013, Sun et al. 2017, Yu et al. 2021). Numerous researches have showed that PAHs cause redox cycling reactions, which can produce free radicals and other active species including superoxide anion and nitric oxide radicals in the human body and other biological systems and thus have high health risk potential (Lou et al. 2019). Although a higher precise investigation on the mechanism of elevated oxidative stress through exposure to these pollutants is needed, generally the positive correlation among the urinary levels of PAHs and oxidative stress biomarkers among the staff working in smoking cafés could indicate serious health risks for these individuals.
This research also for the first time dealt with exploring the association among exposure to various PAHs as well as biomarkers of kidney injury among the staff working in cigarette and waterpipe cafes. For this purpose, using Pearson correlation test, the relationship between the concentration of PAHs in urine specimens and levels of uCyst-C, uOPN, uNGA, uTIMP-1, and uKIM-1 was assessed, with the findings presented in Table 6 and Fig. 4. As shown, a notable correlation has been observed among urinary concentration of PAHs and kidney injury biomarkers (in some cases). For example, the relationship between uKIM-1 and Pyr (r = 0.327), BbF (r = 0.335), and DahA (r = 0.331) has been positive and significant (P < 0.01 for all associations). Pre-clinical studies suggested that KIM-1 can be an significant indicator for kidney injury (Vaidya et al. 2010). They introduced uKIM-1 as a valuable biomarker for assessing and diagnosing glomerular destruction. Acute kidney injury as well as ischemic damage resulting in proximal tubular injury in the nephron can cause a rise in uKIM-1 (Zhao et al. 2011). Other studies have also reported that this elevation (elevation of uKIM-1) can be due to exposure to environmental contaminants such as PAHs or PM (Aztatzi-Aguilar et al. 2016, Singh et al. 2018). In this study, it was also observed that exposure of workers in cigarette and waterpipe cafes to PAHs can lead to elevation of uKIM-1 biomarker.
In addition, a positive and considerable association was observed between uTIMP-1 and Ant (r = 0.323, P < 0.01) plus DahA (r = 0.333, P < 0.01). In previous studies, it has been reported that TIMP-1, as a biomarker of cellular stress in primary stages of tubular cell injury, develops in response to some stresses (such as exposure to pollutants) (Liu et al. 2016). Another research observed that in patients with nephritis lupus, the level of uTIMP-1 was significantly larger in comparison with the healthy control group (Jiang et al. 2010). Therefore, this biomarker can also be an indicator for assessing kidney injury. However, no association was observed among exposure to PAHs and urinary level of the other kidney injury biomarkers.
As mentioned earlier, this is the first study exploring the exposure of staff working in waterpipe plus cigarette cafés to PAHs as well as its impact on kidney injury biomarkers and oxidative stress factors. In this regard, it is noteworthy that in addition to PAH compounds, other contaminants (e.g., toxic metals, particulate matters, carbon oxide, phthalate esters, formaldehyde, volatile organic compounds, BTEX compounds) can also emit into these cafés causing increasing of the urine-contained oxidative stress plus kidney injury biomarkers in these individuals. Therefore, more studies should perform more precise evaluation of exposure to toxins and their mix in individuals exposed to cigarette and waterpipe cafés; the influence on their health should also be checked. The limitations of this project include a small sample size and cross-sectional initial design. Thus, the real impact of exposure to various pollutants on health markers (kidney injury plus oxidative stress biomarkers, respiratory impacts, etc.) should be explored using a comprehensive design with a higher sample size.
Conclusion
This is the primary research to survey the urinary levels of PAHs among the workers/customers of cigarette and tobacco cafés as well as its association with oxidative stress factors plus kidney injury biomarkers. The results demonstrated that there was a considerable difference in terms of ΣPAHs between exposed and control groups. According to the results, working in cigarette and waterpipe cafés can be considered as one of the main origins of exposure to PAHs because of spending long hours in these cafés. Meanwhile, the individuals in WP were found to be exposed to larger amounts of PAHs compared to CC; in these cafés, again the type of tobacco has considerable impact on the urinary levels of PAHs. Specifically, PAH concentration in the urine specimens of staff and customers of cafés serving fruit-flavored tobacco was larger than those serving traditional tobacco. A significant and positive association was also found among the urinary level of PAHs and service number in each shift as well as distance between café and traffic. The results also suggested a positive and significant relationship between some PAHs and oxidative stress biomarkers. uKIM-1 as a biomarker for assessing and diagnosing glomerular damage showed a positive and significant relationship with urinary concentration of Pyr, BbF, and DahA, while TIMP-1, a biomarker of cellular stress in primary stages of tubular cell damage, revealed a positive and significant relationship with urinary concentration of Ant and DahA. Thus, it is recommended to conduct more precise studies on determining the relationship among the level of these contaminants in inside air of cafés and their urinary concentrations. Accordingly, the necessary policies and decisions should be made to restrict the activity of these cafés or to adopt proper protective strategies to keep the public health.
Data availability
The datasets used in this study are available from the corresponding author on reasonable request.
References
Adesina OA et al (2021) Indoor levels of polycyclic aromatic hydrocarbons (PAHs) from environment tobacco smoke of public bars. Ecotoxicol Environ Saf 208:111604
Al-Taie A et al (2021) 8-Hydroxydeoxyguanosine: a valuable predictor of oxidative DNA damage in cancer and diabetes mellitus. Elsevier, Cancer, pp 179–187
Aztatzi-Aguilar O et al (2016) Early kidney damage induced by subchronic exposure to PM2.5 in rats. Part Fibre Toxicol 13(1):1–20
Barnes RL (2011) Regulating the disposal of cigarette butts as toxic hazardous waste. Tob Control 20(Suppl 1):i45–i48
Bortey-Sam N et al (2017) Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (PAHs) in Kumasi, Ghana. Environ Pollut 228:311–320
Campo L et al (2006) Biological monitoring of exposure to polycyclic aromatic hydrocarbons by determination of unmetabolized compounds in urine. Toxicol Lett 162(2):132–138
Campo L et al (2007) Unmetabolized polycyclic aromatic hydrocarbons in urine as biomarkers of low exposure in asphalt workers. J Occup Environ Hyg 4(sup1):100–110
Campo L et al (2014) Urinary carcinogenic 4–6 ring polycyclic aromatic hydrocarbons in coke oven workers and in subjects belonging to the general population: role of occupational and environmental exposure. Int J Hyg Environ Health 217(2–3):231–238
Cancer IAFRO (2010) Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Press, Lyon
Chen Z et al (2020) Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Biol Med 152:116–141
Cobb CO et al (2013) Indoor air quality in Virginia waterpipe cafes. Tob Control 22(5):338–343
De Craemer S et al (2016) Investigating unmetabolized polycyclic aromatic hydrocarbons in adolescents’ urine as biomarkers of environmental exposure. Chemosphere 155:48–56
Delgado-Saborit JM, Aquilina NJ, Meddings C, Baker S, Harrison RM (2011) Relationship of personal exposure to volatile organic compounds to home, work and fixed site outdoor concentrations. Sci Total Environ 409:478–488
Dobaradaran S et al (2019) Cigarette butts: an overlooked source of PAHs in the environment? Environ Pollut 249:932–939
Farley SM et al (2018) Flavour chemicals in a sample of non-cigarette tobacco products without explicit flavour names sold in New York City in 2015. Tob Control 27(2):170–176
Fazlzadeh M et al (2015) Concentrations of carbon monoxide in indoor and outdoor air of Ghalyun cafes. Atmos Pollut Res 6(4):550–555
Fernández SF et al (2021) Children’s exposure to polycyclic aromatic hydrocarbons in the Valencian Region (Spain): urinary levels, predictors of exposure and risk assessment. Environ Int 153:106535
Gurung G et al (2016) Effects of shisha smoking on carbon monoxide and PM2.5 concentrations in the indoor and outdoor microenvironment of shisha premises. Sci Total Environ 548:340–346
Gysel N et al (2018) Chemical speciation, including polycyclic aromatic hydrocarbons (PAHs), and toxicity of particles emitted from meat cooking operations. Sci Total Environ 633:1429–1436
Heydari G et al (2019) Levels and health risk assessments of particulate matters (PM2.5 and PM10) in indoor/outdoor air of waterpipe cafés in Tehran, Iran. Environ Sci Pollut Res 26(7):7205–7215
Heydari G et al (2020) Load characteristics and inhalation risk assessment of benzene series (BTEX) pollutant in indoor air of Ghalyan and/or cigarette cafes compared to smoking-free cafes. Environ Pollut Bioavail 32(1):26–35
Huang Y-D et al (2020) Non-linear relationships between seasonal exposure to polycyclic aromatic hydrocarbons and urinary 8-hydroxy-2′-deoxyguanosine levels among Chinese young students. Chemosphere 251:126352
Jawad M et al (2016) Waterpipe tobacco smoking prevalence and correlates in 25 Eastern Mediterranean and Eastern European countries: cross-sectional analysis of the Global Youth Tobacco Survey. Nicotine Tob Res 18(4):395–402
Jawad M et al (2018) The prevalence and trends of waterpipe tobacco smoking: a systematic review. PloS one 13(2):0192191
Jiang Z et al (2010) Relationships between MMP-2, MMP-9, TIMP-1 and TIMP-2 levels and their pathogenesis in patients with lupus nephritis. Rheumatol Int 30(9):1219–1226
Kashfi FS et al (2022) Occurrence and exposure assessment of microplastics in indoor dusts of buildings with different applications in Bushehr and Shiraz cities, Iran. Sci Total Environ 829:154651
Kassem NO et al (2014) Benzene uptake in Hookah smokers and non-smokers attending Hookah social events: regulatory implications. Cancer Epidemiol Prev Biomark 23(12):2793–2809
Kasza KA et al (2017) Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 376(4):342–353
Kim H-W et al (2014) Synergistic interaction between polycyclic aromatic hydrocarbons and environmental tobacco smoke on the risk of obesity in children and adolescents: the US National Health and Nutrition Examination Survey 2003–2008. Environ Res 135:354–360
Kuang D et al (2013) Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol 47(13):7446–7456
Lin X-Y et al (2022) Polycyclic aromatic hydrocarbon exposure and DNA oxidative damage of workers in workshops of a petrochemical group. Chemosphere 303:135076
Liu X et al (2016) Early predictors of acute kidney injury: a narrative review. Kidney Blood Press Res 41(5):680–700
Lou X-Y et al (2019) Urinary metabolites of polycyclic aromatic hydrocarbons in pregnant women and their association with a biomarker of oxidative stress. Environ Sci Pollut Res 26(26):27281–27290
Masjedi MR et al (2019) Air pollutants associated with smoking in indoor/outdoor of waterpipe cafés in Tehran, Iran: concentrations, affecting factors and health risk assessment. Sci Rep 9(1):1–11
Masjedi MR et al (2021) Use of toenail-bounded heavy metals to characterize occupational exposure and oxidative stress in workers of waterpipe/cigarette cafés. Environ Geochem Health 43(5):1783–1797
Maziak W et al (2015) The global epidemiology of waterpipe smoking. Tob Control 24(Suppl 1):i3–i12
Mirzaei N et al (2015) The statistical analysis of seasonal and time variations on trend of important air pollutants (SO2, O3, NOx, CO, PM10) in western Iran: a case study. Int J Pharm Technol 7(3):9610–9622
Moorthy B et al (2015) Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145(1):5–15
Murphy KA et al (2007) The intersection between the aryl hydrocarbon receptor (AhR)- and retinoic acid-signaling pathways. Vitam Horm 75:33–67
Naddafi K et al (2019) Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: measuring exposures and assessing health effects. Build Environ 165:106392
Nisbet IC, Lagoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16(3):290–300
Odani S et al (2018) State-specific prevalence of tobacco product use among adults—United States, 2014–2015. Morb Mortal Wkly Rep 67(3):97
Oliveira M et al (2020a) Firefighters exposure to fire emissions: impact on levels of biomarkers of exposure to polycyclic aromatic hydrocarbons and genotoxic/oxidative-effects. J Hazard Mater 383:121179
Oliveira M et al (2020b) Exposure of nursing mothers to polycyclic aromatic hydrocarbons: levels of un-metabolized and metabolized compounds in breast milk, major sources of exposure and infants’ health risks. Environ Pollut 266:115243
Phuc NH, Oanh NTK (2018) Determining factors for levels of volatile organic compounds measured in different microenvironments of a heavy traffic urban area. Sci Total Environ 409:478–488
Ratelle M et al (2020) Polycyclic aromatic hydrocarbons (PAHs) levels in urine samples collected in a subarctic region of the Northwest Territories, Canada. Environ Res 182:109112
Rossella F et al (2009) Urinary polycyclic aromatic hydrocarbons and monohydroxy metabolites as biomarkers of exposure in coke oven workers. Occup Environ Med 66(8):509–516
Rostami R et al (2019) Exposure and risk assessment of PAHs in indoor air of waterpipe cafés in Ardebil, Iran. Build Environ 155:47–57
Samara F et al (2021) Midwakh: assessment of levels of carcinogenic polycyclic aromatic hydrocarbons and nicotine in dokha tobacco smoke. J Anal Toxicol 46(3):295–302
Schubert J et al (2012) Waterpipe smoking: the role of humectants in the release of toxic carbonyls. Arch Toxicol 86(8):1309–1316
Shaban NZ et al (2013) Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem 141(3):1587–1596
Shaban NZ et al (2020) The synergistic hepatoprotective potential of Beta vulgaris juice and 2,3-dimercaptosuccinic acid in lead-intoxicated rats via improving the hepatic oxidative and inflammatory stress. BMC Complement Med Ther 20(1):1–15
Shaban NZ et al (2021) Synergistic protective effect of Beta vulgaris with meso-2,3-dimercaptosuccinic acid against lead-induced neurotoxicity in male rats. Sci Rep 11(1):1–18
Shaban NZ et al (2022) Protective and therapeutic role of mango pulp and eprosartan drug and their anti-synergistic effects against thioacetamide-induced hepatotoxicity in male rats. Environ Sci Pollut Res:1–15
Shihadeh A et al (2015) Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control 24(Suppl 1):i22–i30
Singh A et al (2018) Association between PAHs biomarkers and kidney injury biomarkers among kitchen workers with microalbuminuria: a cross-sectional pilot study. Clin Chim Acta 487:349–356
Sun H et al (2017) Dose-response relationship between urinary polycyclic aromatic hydrocarbons metabolites and urinary 8-hydroxy-2′-deoxyguanosine in a Chinese general population. Chemosphere 174:506–514
Swami K et al (2009) Trace metals analysis of legal and counterfeit cigarette tobacco samples using inductively coupled plasma mass spectrometry and cold vapor atomic absorption spectrometry. Spectrosc Lett 42(8):479–490
Thai PK et al (2020) Analysis of urinary metabolites of polycyclic aromatic hydrocarbons and cotinine in pooled urine samples to determine the exposure to PAHs in an Australian population. Environ Res 182:109048
Torres S et al (2018) Biomarkers of exposure to secondhand and thirdhand tobacco smoke: recent advances and future perspectives. Int J Environ Res Public Health 15(12):2693
Vaidya VS et al (2010) Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28(5):478–485
Waidyanatha S et al (2003) Determination of polycyclic aromatic hydrocarbons in urine of coke oven workers by headspace solid phase microextraction and gas chromatography–mass spectrometry. Chem Biol Interact 145(2):165–174
Yu Y et al (2021) Co-exposure to polycyclic aromatic hydrocarbons and phthalates and their associations with oxidative stress damage in school children from South China. J Hazard Mater 401:123390
Zaidi S et al (2011) Second-hand smoke in indoor hospitality venues in Pakistan. Int J Tuberc Lung Dis 15(7):972–977
Zhang Y-J et al (2021) Polycyclic aromatic hydrocarbon exposure, oxidative potential in dust, and their relationships to oxidative stress in human body: a case study in the indoor environment of Guangzhou, South China. Environ Int 149:106405
Zhao X et al (2011) Glomerular expression of kidney injury molecule-1 and podocytopenia in diabetic glomerulopathy. Am J Nephrol 34(3):268–280
Acknowledgements
We thank Tobacco Control Research Center (TCRC), Iranian Anti-Tobacco Association, for their help in some part of this study.
Funding
The Iran National Science Foundation (INSF) provided financial support through grant number 99001111.
Author information
Authors and Affiliations
Contributions
The research was designed by H. Arfaeinia and M. R. Masjedi. Sampling, experiment, and data collection were done by H. Arfaeinia. M. R. Masjedi, D. Ranjbar Vakil Abadi, and M. Tahmasbizadeh performed the statistical analysis. D. Ranjbar Vakil Abadi, M. Tahmasbizadeh, B. Ramavandi, and Y. Poureshgh wrote the manuscript. The final manuscript was investigated and approved by all the authors.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors declare they have given consent to publish this article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dariush Ranjbar Vakil Abadi and Masoumeh Tahmasbizadeh should be considered as joint first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abadi, D.R.V., Tahmasbizadeh, M., Arfaeinia, H. et al. Biomonitoring of unmetabolized polycyclic aromatic hydrocarbons (PAHs) in urine of waterpipe/cigarette café workers. Environ Sci Pollut Res 30, 22728–22742 (2023). https://doi.org/10.1007/s11356-022-23822-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23822-y