Abstract
In the southeastern Peruvian Amazon, artisanal and small-scale gold mining (ASGM) is estimated to have released up to 300 tonnes of mercury (Hg) to the environment between 1995 and 2007 alone, and is claimed to be responsible for Hg concentrations above international thresholds for aquatic wildlife species. Here, we examined whether Hg concentrations in bat populations are potentially related to regional ASGM-Hg releases. We determined Hg concentrations in the fur of bats collected at three different distances from the major ASGM areas in Peru. Our findings from 204 individuals of 32 species indicate that Hg concentrations in bat fur mainly resulted from differences in feeding habits, because Hg concentrations were significantly higher in omnivorous bats than in frugivorous bats. At least in two species, populations living in ASGM-affected sites harbored higher Hg concentrations than did populations in unaffected sites. Because Hg concentrations reflect Hg dietary exposure, Hg emissions from amalgam roasting sites appear to deposit locally and enter the terrestrial food web. Although our study demonstrates that ASGM activities (and Hg point sources) increase Hg exposure in wildlife, the overall Hg concentrations reported here are relatively low. The measured Hg concentrations were below the toxicity threshold at which adverse neurological effects have been reported in rodents and mink (>10 µg g−1), and were in the range of Hg concentrations in the fur of bats from nonpoint source affected sites in other latitudes. This study emphasizes the importance of considering feeding habits when evaluating Hg concentrations in bats and other vertebrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artisanal and small-scale gold mining (ASGM) is currently the largest global anthropogenic source of mercury (Hg) (UNEP 2013). Gold miners use metallic Hg (Hg0) for extracting gold particles from alluvial ores by amalgamation. However, because of the lack of efficient recovery techniques, Hg0 is released into soils, tailing, and water systems during the amalgamation process and into the atmosphere during the roasting of the amalgam. Metallic Hg has a relatively long atmospheric residence time, between 6 months and 2 years, resulting in its capacity to travel great distances before being deposited in terrestrial and aquatic ecosystems (Driscoll et al. 2013; Engstrom et al. 2014; Lamborg et al. 2002).
The main concern about Hg lies in its toxicity and adverse health effects. Under anoxic conditions, for example, atmospheric-deposited or waterborne Hg is methylated and produces a potent neurotoxin that readily enters the food-web and accumulates in living organism (Eckley and Hintelmann 2006; Ullrich et al. 2001). Because methyl-Hg is able to penetrate the blood-brain barrier and biomagnify along the trophic web, top predators—especially fish-eaters—are at a high risk of Hg dietary exposure. Mercury-associated neurochemical changes and functional behavioral deficits have been demonstrated both in humans and other mammals, such as rodents, otters, mink, and bats (Basu et al. 2005; Bose-O’Reilly et al. 2016; Burton et al. 1977; Nam et al. 2012; Wobeser et al. 1976).
In Latin America, ASGM is an ever-growing activity since the 1970s, when the second gold rush started. In the southeastern Peruvian Amazon (within the Tropical Andes biodiversity hotspot; Myers et al. (2000)), the Madre de Dios region accounts for 70% of the country’s artisanal gold production (Brooks et al. 2007). Between 1999 and 2012 alone, the land use for ASGM in Madre de Dios increased by 400% in this region (Asner et al. 2013). Although the extent of Hg losses from gold mining activities is still unclear, it is estimated that between ~160 and ~300 tonnes Hg were emitted into the atmosphere between 1995 and 2007 (based on a Hg-to-gold ratio of 1:1 and 2:1 for gold extraction process with and without retorts, respectively) (Mosquera et al. 2009).
Based on these estimates of Hg losses, Hg concentrations in environmental and fish samples from southeastern Peru that were above international thresholds have been associated to Hg releases from regional gold mining activities (CAMEP 2013; Deza Arroyo 1996; Diringer et al. 2015; Gutleb et al. 2002, 1997; Roach and Busch 2004). The direct link between Hg releases from gold mining activities and Hg concentrations in environmental samples and wildlife has not been unambiguously demonstrated (Laperche et al. 2014; Roulet et al. 1998). On one side, the pathway by which Hg0 enters the aquatic trophic web (i.e., its conversion into methyl-Hg) and the factors controlling the accumulation of Hg throughout the food web are still not fully understood. Laboratory experiments suggest that oxidation and dissolution of Hg0 is enhanced in oxygenated environments and in the presence of dissolved organic matter or manganese oxide (Meech et al. 1998; Melamed et al. 2000; Miller et al. 2015). This pathway may be limited in some rivers of the Madre de Dios region because the content of organic matter is low and bottom sediments are exclusively oxic due to high water current (Moreno-Brush et al. 2016). On the other side, Hg concentrations that have been determined in regional environmental samples resemble those from unpolluted areas (Beal et al. 2013; Diringer et al. 2015; Moreno-Brush et al. 2016). Furthermore, we lack Hg baseline data obtained from wildlife living in unpolluted areas of this region with which to compare the current Hg concentrations in wildlife of polluted areas.
To contribute to the understanding of Hg exposure associated with gold mining of wildlife in southeastern Peru, we determined Hg concentrations in the most abundant bat species (Chiroptera) in the region. Research and public attention have so far focused on Hg exposure of regional fish and fish-eating wildlife species; however, vertebrate species that do not live on a fish-based diet have rarely been examined. Bats may serve as good Hg bioindicator because of their unique life history and biology (Hickey et al. 2001; Jones et al. 2009; Wada et al. 2010; Yates et al. 2014; Zukal et al. 2015). They are widely distributed and cover both low and high trophic levels due to their different feeding habits. Bats may also be more susceptible to Hg bioaccumulation than other similarly sized mammals because of their relatively long life expectancy, i.e., bats may accumulate Hg with age. Furthermore, bats are also more susceptible to Hg biomagnification than other mammals due to their high metabolic rate and their high food intake in relation to their body mass. Insectivorous bats, for example, can consume up to 100% of their body mass daily (Hickey and Fenton 1996). Most previous studies that have investigated Hg concentrations in bats have focused in temperate regions, mostly in North America and on insectivorous species (Karouna-Renier et al. 2014; Little et al. 2015a, b; Miura et al. 1978; Nam et al. 2012; O’Shea et al. 2001; Wada et al. 2010; Yates et al. 2014). To our knowledge, Hg concentrations in tropical bats are only described for Malaysia (Syaripuddin et al. 2014).
We analyzed total Hg concentrations in fur of bat species that differed in feeding habits and sampled at three sites varying in distance to the major ASGM areas in southeastern Peru. If Hg derived from gold mining is enriched in bats, it should be reflected in the fur—a proxy for methyl-Hg content in blood and internal organs (Yates et al. 2014). Higher Hg concentrations have been reported from bats captured near historical Hg-contaminated sites, compared to bats from nonpoint-source affected sites (Karouna-Renier et al. 2014; Nam et al. 2012). We hypothesize two scenarios: (1) If ASGM-related Hg emissions are deposited locally, close to the amalgam roasting sites, we would find a spatial pattern of exposure, i.e., bats living in the proximity of Hg point source emissions should contain higher Hg concentrations than those in distant, less affected sites. (2) If ASGM-related Hg emissions are not deposited locally but are subject to long-range atmospheric transport (i.e., beyond the lowland Amazon of southeastern Peru), bats throughout the study region would present similar Hg concentrations. We further explored associations between Hg concentrations in fur and intrinsic specific traits, i.e., feeding habit, sex, reproductive status, and body size, to identify ecological and physiological factors affecting Hg accumulation in the examined bat species. If variations in Hg concentrations mainly reflect Hg dietary exposure, and Hg releases from gold mining activities indeed biomagnify throughout the local terrestrial food web, bat species of higher trophic levels would show higher Hg concentrations than bat species of lower trophic levels. Similarly, if Hg accumulates in bats with age, adult bats would present higher Hg concentrations than juveniles.
Materials and methods
Study area and sample collection
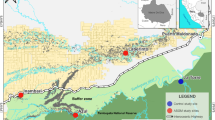
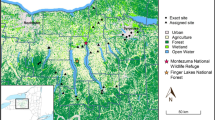
Bat capturing and sampling were conducted in the Madre de Dios region, at three different sites varying in distance to the major regional ASGM mines, between November 2014 and July 2015 (Fig. 1). The site closest to the gold mining activities is Santa Rita (SAN) (1), located ~26 km East from mine Huepetuhe—the country’s largest ASGM mine (Brooks et al. 2007)—and ~15 km West from mine La Pampa. Gold mining by sluicing and dredging is observed at the sampling site, although in smaller proportion than in Huepetuhe and La Pampa. The site Malinowski (MAL) (2) is located ~78 km East of Santa Rita, within the forest of the buffer zone of the Tambopata National Reserve, where illegal ASGM also occurs. The site Tahuamanu (TAH) (3) is located furthest and upwind from the gold mining activities (~190 km North from Malinowski) and is assumed to be unaffected by gold mining-related Hg emissions.
Study area in Madre de Dios region, southeastern Amazon of Peru. Shown are the three sampling sites (circles) and the area with artisanal and small-scale gold mining (ASGM) concessions. Huepetuhe is the largest ASGM mine in Peru. Puerto Maldonado is the capital city of Madre de Dios, where most regional gold shops are located. The layer of the mining concessions was downloaded from the website of the Peruvian Geological, Mining, and Metallurgical Institute (INGEMMET). Predominant wind direction is from the northeast
Bats were captured using mist nets (12 × 2.5 m) that were set up in the understory along presumed transit paths between roosting and foraging areas. Nets were opened for 6 h at dusk for 8–10 consecutive nights. Field excursions were conducted 10 nights before and 10 nights after new moon to reduce the influence of lunarphobic behavior of bats (Morrison 1978; Usman et al. 1980). Fur samples (~5–90 mg) were cut from the mid-dorsal region of captured specimens using stainless steel scissors and were stored in sterile cryovials until laboratory analysis. Scissors were cleaned with water and ethanol between samples to avoid cross-contamination. Captured bats were identified in the field using a morphological identification field key (Díaz et al. 2011). Field data taken included age (juvenile, sub-adult, adult; based on the form of the fourth metacarpal-phalangeal joint (Kunz and Anthony 1982)), sex, body mass (measured with pesola scales), and forearm length (measured with an electronic vernier scale). Feeding habits of each species were determined from the literature (Carrera 2003; Fleming 1988; Kalko et al. 1996; Moya et al. 2008). All bats were released near the place of capture immediately after processing, except those which could not be identified using the available field key. Non-identifiable bats (n = 4) were sacrificed and taken to the Universidad Nacional San Antonio Abad del Cusco for identification by means of skull morphology. Capture and handling procedures were conducted under the research permit (N° 006-2015-SERNANP-JEF) obtained from the Peruvian authorities of the national protected areas (Sernanp).
Mercury analyses
Mercury analyses were performed at the TU Braunschweig, Germany. Mercury concentration in fur samples was determined by thermal decomposition followed by pre-concentration of Hg on a gold trap and CVAAS Hg detection using a Milestone DMA-80 analyzer (US EPA Method 7473). In order to avoid problems caused by static electricity, fur samples were analyzed inside small glass tubes (previously washed with Milli-Q water and concentrated HNO3). Analytical quality was ascertained by standard reference materials—NCS-DC73322 (590 ± 50 ng g−1, mean ± standard deviation), Canmet LKSD-4 (190 ± 19 ng g−1), and DORM-4 (410 ± 55 ng g−1)—and by sample replicates, which were included with about every ten samples. The average measured concentration for NCS-DC73322 (n = 21) was 609 ± 24 ng g−1, for LKSD-4 (n = 34) it was 183 ± 17 ng g−1, and for DORM-4 (n = 9) it was 414 ± 29 ng g−1. Replicate analyses of 17 randomly selected samples were always within ±11% RSD.
The Milestone DMA-80 analyzer is calibrated in absolute nanograms of Hg. Therefore, its limit of quantification (LOQ)—calculated from a specific calibration (according to DIN 32645 or Neitzel 2002)—is equally expressed in nanograms. We report the Hg concentrations of samples that fell below the DMA-80 LOQ (about ~40% of the total analyzed samples) as <LOQ-values (µg g−1). LOQ-values expressed in concentration units were calculated by dividing the LOQ of the DMA-80 (in nanograms) and the sample weight (in grams).
Statistical analyses
As our data were not normally distributed, based on a post-hoc Anderson-Darling test, we used non-parametric tests for all statistical analyses. Due to the high number (~40%) of <LOQ-values in our dataset, descriptive statistics were calculated using a non-parametric Kaplan-Meier survival-analysis method, following Helsel (2012). For statistical comparison of Hg concentrations at genus and species level, the data were ranked (all <LOQ-values were assigned a value of 1) and analyzed using a Kruskal-Wallis analysis of variance (KW-ANOVA) on ranks with a 0.05 significance level. If groups were found to be significantly different, we used a Mann–Whitney U-test for pairwise comparison. Because of the small number of group comparisons done throughout the study (up to six comparisons) and the very few values within some of the groups, we did not adjust p-values of the the Mann–Whitney U-test for multiple comparisons. All statistical analyses were done using OriginPro software, version 9.0.0 (www.originlab.com).
Results
During the field campaigns of 2014 and 2015, a total of 204 fur samples were obtained from 32 bat species belonging to four families and six feeding habits. Only nine species were represented by more than three specimens (SI-Table 1 in supplementary information). When combining all samples, the median fur Hg concentration in Santa Rita (SAN), the site closest to gold mining activities, was 0.66 µg g−1 (range from <LOQ to 8.67 µg g−1, Kaplan-Meier estimated mean 1.18 ± 1.49 µg g−1, n = 75). In Malinowski (MAL), located at an intermediate distance from gold mining activities, the median fur Hg concentration was 0.23 µg g−1 (range from <LOQ to 4.86 µg g−1, Kaplan-Meier estimated mean 0.45 ± 0.71 µg g−1, n = 95), whereas in Tahuamanu (TAH), the most distant site from gold mining activities, the median fur Hg concentration was 0.22 µg g−1 (range from <LOQ to 3.66 µg g−1, Kaplan-Meier estimated mean 0.42 ± 0.62 µg g−1, n = 34). The highest Hg concentration (8.67 µg g−1) was found in an adult male Phyllostomus elongatus (omnivorous/insectivorous; (Bernard 2002, 2001)) sampled in SAN.
From all fur samples, 79 (~40%) showed Hg concentrations below the LOQ. Almost all <LOQ-values (97%) corresponded to frugivorous species, mostly from the genus Artibeus (SI-Table 1). Fur Hg concentrations in frugivorous species (median 0.26 µg g−1, Kaplan-Meier estimated mean 0.48 ± 0.64 µg g−1, n = 154) were significantly lower (p < 0.001, Mann–Whitney test) than in non-frugivorous species (median 1.16 µg g−1, Kaplan-Meier estimated mean 1.80 ± 1.83 µg g−1, n = 29) (singleton species omitted). Among the frugivorous species, Carollia perspicillata showed the highest median Hg concentration (median 0.66 µg g−1), whereas P. elongatus showed the highest median Hg concentration (median 1.90 µg g−1) among the non-frugivorous species. No sample surpassed the threshold of >10 µg g−1 at which adverse neurochemical effects are reported in wild mice (Burton et al. 1977) and captive mink (Wobeser et al. 1976).
To determine the effects of ASGM-related Hg releases on regional bat populations, we further examined the genera and species captured in the three study locations, i.e., Artibeus (A. planirostris (frugivorous, n = 35)), Carollia (C. brevicauda (frugivorous, n = 13) and C. perspicillata (frugivorous, n = 65)), and Phyllostomus (P. elongatus (omnivorous/insectivorous, n = 16)). At the genus level, Hg concentrations in fur differed significantly between Artibeus, Carollia, and Phyllostomus in all three study locations (p < 0.007, KW-ANOVA; SI-Table 2). Artibeus showed in all locations the lowest Hg concentrations, followed by Carollia and Phyllostomus (p ≤ 0.03, Mann-Whitney test). At a species level, A. planirostris showed lower Hg concentrations than the other three bat species in ASGM-affected locations SAN and MAL (Fig. 2a–b). In all three locations, Hg concentrations in P. elongatus were higher than in A. planirostris and C. perspicillata (Fig. 2a–c). In locations SAN and TAH, Hg concentrations observed in P. elongatus did not differ from those in C. brevicauda. We found no significant difference between Hg concentrations in C. brevicauda and in C. perspicillata in any of the study locations (Fig. 2a–c).
Median mercury (Hg) concentrations in the fur of the bat species A. planirostris, C. brevicauda, C. perspicillata, and P. elongatus in the southeastern Amazon of Peru. Bats were sampled at three locations varying in distance to the major regional ASGM operations. a–c Hg concentrations in bat species grouped by genus at each study location. d Hg concentration in bat populations grouped by species. Locations: SAN and MAL are ASGM-affected, whereas TAH is distant from ASGM activities and considered unaffected (see sampling locations in Fig. 1). Data are presented as raw data points (diamonds) and grouped box plots. For each population, only the data above the maximum limit of quantification (LOQ, dashed line) are shown. Populations of A. planirostris at the different study locations were not compared due to the high number of values below the LOQ. Letters represent results of pairwise comparisons using Mann-Whitney tests, indicating significant differences in fur Hg concentrations among species or sampling locations. Populations with different letters are significantly different at the 0.05 significance level
When comparing populations of the same bat species at the different study locations (Fig. 1d), only the populations of C. perspicillata differed significantly among the three study locations (p < 0.001, KW-ANOVA; p ≤ 0.006, Mann-Whitney tests; Fig. 2d). The median Hg concentration in C. perspicillata in SAN (1.19 µg g−1), the closest location to gold mining activities, was about two and a half times higher than that in conspecifics in MAL, and about five times higher than the median Hg concentration in conspecifics in TAH, the location furthest away from the ASGM. Populations of P. elongatus differed significantly only between locations SAN and TAH (p = 0.028, Mann-Whitney test). No significant differences were found among populations of C. brevicauda (p = 0.077, KW-ANOVA). Populations of A. planirostris at the different study locations were not compared due to the high percentage (83%) of <LOQ-values.
We found no significant differences in Hg concentrations among specimens of different sex for C. perspicillata (median female 1.05 µg g−1, median male 0.55 µg g−1, n = 28 and 37, respectively) or for P. elongatus (median female 1.68 µg g−1, median male 1.83 µg g−1, n = 6 and 13, respectively) when pooling all sites (SI-Fig. 1). Only at SAN, female C. perspicillata (median 1.43 µg g−1 Hg, n = 15) showed significantly higher (p < 0.004, Mann-Whitney test) Hg concentrations than their male conspecifics (median 0.74 µg g−1 Hg, n = 17). We also did not detect any significant difference in Hg concentrations among individuals of different age (i.e., juveniles, sub-adults, adults) neither for C. perspicillata nor for P. elongatus (SI-Fig. 2).
Discussion
Our results suggest that fur Hg concentrations in bats depend on differences in feeding habits and that bats of higher trophic levels accumulate higher Hg concentrations than bats of lower trophic levels. This supports the findings on Hg concentrations in frugivorous and insectivorous bats in Malaysia (Syaripuddin et al. 2014). The low Hg concentrations found in most Artibeus specimens (83% of all data <LOQ, see Fig. 2 and SI-Table 1) suggest that the detected Hg concentrations reflect dietary Hg exposure rather than exposure to Hg in dust particles (i.e., exogenous Hg).
Differences in Hg concentrations between Carollia and Artibeus species in ASGM-affected locations are likely due to dietary differences. Whereas A. planirostris is a canopy specialist that feeds mainly on fruits from several species of figs (Ficus), the short-tailed frugivorous bats, C. brevicauda and C. perspicillata, are understory specialists feeding primary on fruits from understory shrubs, e.g., Piper (Giannini and Kalko 2004; Thies et al. 2006). Many Piper species are early succession plants that grow near disturbed areas caused by human activities, including gold mining activities (Thies and Kalko 2004). A higher consumption of Piper may explain the higher Hg concentrations observed in Carollia, as these plants are suitable for phytoremediation of Hg-contaminated soils (Marrugo-Negrete et al. 2016). Further, higher Hg concentrations in Carollia may reflect a higher consumption of non-fruit items. Although carolliine and stenodermatine bats are generally known as fruit-eating phyllostomid subfamilies (Fleming 1988; Ribeiro Mello et al. 2004; Thies et al. 1998; Thies and Kalko 2004), both of them have been reported to occasionally supplement their fruit diet (probably opportunistically) with nectar, pollen, and insects, especially during low fruit availability (Arata et al. 1967; Fleming et al. 1972; Heithaus et al. 1975; Maguiña et al. 2012). According to York and Billings (2009), insectivory in Carollia may be more extensive than previously suggested, and the genus may, therefore, be trophically positioned between obligate herbivores and obligate insectivores. Particularly in the southeastern Peruvian Amazon, carolliine bats are reported to have a more diverse diet than stenodermatine species and to complement their primary Piper-based diet with other fruits and insects (Bravo et al. 2012). Because fruits in the southeastern Peruvian Amazon are lower in some nutrients (e.g., sodium) than fruits in other tropical areas, and carolliine bats in this region rarely lick soils that constitute natural sources of minerals for diet supplementation or fruit detoxification, the insects consumed by Carollia bats may serve as a supplementary source for the limited nutrients in Piper fruits (Bravo et al. 2012, 2008). Higher Hg concentrations in Carollia than in Artibeus may, thus, be partially explained by the more generalist diet of Carollia and by a higher consumption of fruits with the capacity of accumulating Hg.
We found no influence of sex or age on Hg concentrations in the fur of C. perspicillata and P. elongatus (SI-Fig. 1–2). The non-significant difference between Hg concentrations in fur of juveniles, sub-adults, and adults of both species also indicates that these bats do not accumulate Hg over time. These results suggest that Hg releases from gold mining activities in the Madre de Dios region enter the regional terrestrial trophic web and that wildlife species living closer to gold mining communities or amalgam roasting sites are more intensely exposed to Hg than those living in areas distant from anthropogenic Hg sources. The lack of statistical significance between Hg concentrations and sex or age may also be due to a low power of the statistical tests, i.e., the tests had a low probability of detecting an existing difference (Steidl et al. 1997). The misuse of power analysis for interpreting non-significant study results, such as retrospective power analysis, has been highlighted by various authors (Gerard et al. 1998; Steidl et al. 1997; Thomas 1997). These authors note, however, that retrospective power analysis may be effectively used to estimate the sample size that would have resulted in a high probability of detecting biologically significant effects (Steidl et al. 1997). Sample sizes in our study may not have been large enough to achieve an acceptable power of 0.8 to detect a biologically significant effect (Cohen 1988). For example, it would have been necessary to test 90 P. elongatus of each sex to achieve an 80% probability of detecting the true effect of sex in Hg concentrations of the species, whereas 149 P. elongatus of each sex would have been required to achieve a 95% probability of observing differences among the populations (SI-Table 3). Larger sample sizes may have led to somewhat different outcomes, because larger samples are more likely than smaller ones to result in rejection of a false null hypothesis (Cohen 1992), although according to Steidl et al. (1997), a low statistical power analysis does not indicate whether or not an effect actually exists.
The scenario is similar for the non-significant differences in Hg concentrations between some bat populations of the same species (Fig. 1d). The different outcomes obtained for P. elongatus and C. perspicillata at the different study locations possibly indicate that P. elongatus roams over larger distances than C. perspicillata. Carollia species are generally smaller than Phyllostomus species, feeding on more localized food sources than do Phyllostomus bats, which sometimes need to fly great distances to obtain their food. Nonetheless, the lack of a significant difference among populations of P. elongatus and C. brevicauda may also be attributed to the small sample size and to the large variability within the data because in both cases the maximum Hg concentrations increased from TAH to MAL and to SAN (Fig. 2d). A retrospective power analysis demonstrates, for example, that at least 26 P. elongatus from both SAN and MAL would have been required to achieve an adequate power of 0.8 (SI-Table 4).
Our study demonstrates that local gold mining activities are Hg sources that increase the exposure of local wildlife to Hg. The actually encountered Hg concentrations are, however, relatively low. The levels we detected do not surpass the toxicological threshold at which adverse neurological effects have been reported in small mammals (>10 µg g−1). They are in the range of Hg concentrations found in the fur of frugivorous and insectivorous bat species from nonpoint source affected sites in Malaysia (0.012–9.5 µg g−1) (Syaripuddin et al. 2014), and in the range of the fur of insectivorous bat species of nonpoint source affected sites in the U.S. (1.5–3.3 µg g−1) (Karouna-Renier et al. 2014; Nam et al. 2012).
Mercury concentrations in non-frugivorous bats in the study site closest to gold mining activities (SAN median 3.23 ± 2.33 µg g−1; ~ 15 km distance to major ASGM areas) are much lower than Hg concentrations found in the fur of insectivorous bats in North America (up to 132 µg g−1) sampled in areas identified as biological Hg hotspots or near (~ 25 km distance) anthropogenic Hg point sources (Karouna-Renier et al. 2014; Little et al. 2015b; Nam et al. 2012; Wada et al. 2010; Yates et al. 2014). The large difference in Hg concentrations may be explained by trophic dietary differences, but also by differences in Hg bioavailability and assimilation in bats. On one side, in contrast to most insectivorous bats from North America, insectivorous phyllostomid bat species are mostly gleaners and do not frequently prey on emerging aquatic insects that are reported to be important links in the transfer of Hg from aquatic to terrestrial food webs (Chaves-Ulloa et al. 2016; Speir et al. 2014). On the other side, fresh fruit consumption in the Amazon region has been suggested to reduce Hg levels in blood and hair and to have a protective role against Hg dietary exposure (Passos et al. 2003, 2008, 2007). Although this is not yet fully understood, this effect may be linked to the content of selenium in certain tropical fruits. Selenium is known to antagonize the toxicity of both Hg and methyl-Hg, due to its capacity to form an inert complex (Khan and Wang 2009). A high-fiber diet has also been reported to promote a faster elimination of Hg from the body (Rowland et al. 1986). Finally, while the Hg released from former chlor-alkali and textile industries in the U.S. (mercuric sulfate and mercuric chloride) is highly reactive and bioavailable for uptake by organisms, the Hg released by ASGM is in the form of metallic Hg, which is relative unreactive and not bioavailable. Thus, the availability of Hg for biota uptake strongly depends on the physicochemical form in which Hg exists in a system.
Conclusions
Findings from our study demonstrate that Hg concentrations in bat fur respond to differences in feeding habits, and hence, bat fur is a good medium for monitoring the exposure of Hg through the food web in bat populations. Any assessment of the effects of Hg releases from ASGM must be done on a species or genus level and consider species-specific characteristics. At least for two species, populations in ASGM-affected sites harbored higher Hg concentrations than populations at more distant sites. As Hg concentrations reflect Hg dietary exposure, Hg emissions from local amalgam roasting sites, indeed, seem to deposit locally and enter the terrestrial food web. This study emphasizes the importance to consider the feeding habits when assessing Hg concentrations in bats and other wildlife species. As other authors, we encourage future studies on wildlife conservation to estimate a priori minimum sample sizes required to achieve high probabilities of detecting biologically significant effects.
References
Arata AA, Vaughn JB, Thomas ME (1967) Food habits of certain colombian bats. J Mammal 48:653–655
Asner GP, Llactayo W, Tupayachi R, Luna ER (2013) Elevated rates of gold mining in the Amazon revealed through high-resolution monitoring. Proc Natl Acad Sci U S A. 110:18454–18459
Basu N, Scheuhammer A, Grochowina N, Klenavic K, Evans D, O’Brien M, Chan HM (2005) Effects of mercury on neurochemical receptors in wild river otters (Lontra canadensis). Environ Sci Technol 39:3585–3591
Beal SA, Jackson BP, Kelly MA, Stroup JS, Landis JD (2013) Effects of historical and modern mining on mercury deposition in southeastern Peru. Environ Sci Technol 47:12715–12720
Bernard E (2001) Vertical stratification of bat communities in primary forests of Central Amazon, Brazil. J. Trop. Ecol. 17:2001
Bernard E (2002) Diet, activity and reproduction of bat species (Mammalia Chiroptera) in Central Amazonia, Brazil. Rev. Bras. Zool. 19:173–188
Bose-O’Reilly S, Schierl R, Nowak D, Siebert U, William JF, Owi FT, Ir YI (2016) A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environ Res 149:274–281
Bravo A, Harms KE, Emmons LH (2012) Keystone resource (Ficus) chemistry explains lick visitation by frugivorous bats. J Mammal 93:1099–1109
Bravo A, Harms KE, Stevens RD, Emmons LH (2008) Collpas: Activity hotspots for frugivorous bats (Phyllostomidae) in the Peruvian Amazon. Biotropica 40:203–210
Brooks WE, Sandoval E, Yepez MA, Howell H (2007) Peru mercury inventory 2006. U.S. Geological Survey Open-File Report 2007-1252. http://pubs.usgs.gov/of/2007/1252/. Accessed 19 May 2017
Burton GV, Alley RJ, Rasmussen GL, Orton P, Cox V, Jones P, Graff D (1977) Mercury and behavior in wild mouse populations. Environ Res 14:30–34
CAMEP (Carnegie Amazon Mercury Ecosystem Project) (2013) Mercury in Madre de Dios - Mercury concentrations in fish and humans in Puerto Maldonado. Research Brief #1 [Online]. Standford: CAMEP. https://dge.carnegiescience.edu/research/CAMEP/CAMEP%20Research%20Brief%20-%20Puerto%20Maldonado%20English%20-%20FINAL.pdf. Accessed 19 May 2017
Carrera JP (2003) Distribución de murciélagos (Chiroptera) a través de un gradiente altitudinal en las estribaciones orientales de los Andes Ecuatorianos. (Licentiate thesis). Pontificia Universidad Católica del Ecuador, Quito, Ecuador
Chaves-Ulloa R, Taylor BW, Broadley HJ, Cottingham KL, Baer NA, Weathers KC, Ewing HA, Chen CY (2016) Dissolved organic carbon modulates mercury concentrations in insect subsidies from streams to terrestrial consumers. Ecol Appl 26:1771–1784
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, NJ
Cohen J (1992) Statistical power analysis. Curr Dir Psychol Sci 1:98–101
Deza Arroyo NE (1996) Mercury Accumulation in Fish from Madre de Dios, a Goldmining Area in the Amazon Basin, Perú (Master thesis). Oregon State University, Corvallis, USA
Díaz MM, Aguirre LF, Barquez RM (2011) Clave de identificación de los murciélagos del cono sur de Sudamérica (Key to the bats of the Southern Cone of South America). Centro de Estudios en Biología Teórica y Aplicada, Cochabamba
Diringer SE, Feingold BJ, Ortiz EJ, Gallis JA, Araújo-Flores JM, Berky A, Pan WKY, Hsu-Kim H (2015) River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru. Environ. Sci. Process. Impact 17:478–487
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Eckley CS, Hintelmann H (2006) Determination of mercury methylation potentials in the water column of lakes across Canada. Sci Total Environ 368:111–125
Engstrom DR, Fitzgerald WF, Cooke CA, Lamborg CH, Drevnick PE, Swain EB, Balogh SJ, Balcom PH (2014) Atmospheric Hg emissions from preindustrial gold and silver extraction in the Americas: a reevaluation from lake-sediment archives. Environ Sci Technol 48:6533–6543
Fleming TH (1988) The short-tailed fruit bat: a study in plant-animal interactions. University of Chicago Press, Chicago
Fleming TH, Hooper ET, Wilson DE (1972) Three Central American bat communities: structure, reproductive cycles, and movement patterns. Ecology 53:556–569
Gerard PD, Smith DR, Weerakkody G (1998) Limits of retrospective power analysis limits. J. Wildl. Manage. 62:801–807
Giannini NP, Kalko EKV (2004) Trophic structure in a large assemblage of phyllostomid bats in Panama. Oikos 105:209–220
Gutleb AC, Helsberg A, Mitchell C (2002) Heavy metal concentrations in fish from a pristine rainforest valley in Peru: a baseline study before the start of oil-drilling activities. Bull Environ Contam Toxicol 69:523–529
Gutleb AC, Schenck C, Staib E (1997) Giant otter (Pteronura brasiliensis) at risk? Total mercury and methylmercury levels in fish and otter scats, Peru. Ambio 26:511–514
Heithaus ER, Fleming TH, Opler PA (1975) Foraging petterns and resource utilization in 7 species of bats in a seasonal tropical forest. Ecology 56:841–854
Helsel DR (2002) Statistics for censored environmental data using Minitab® and R, 2nd edn. Wiley, Hoboken, NJ, p 324
Hickey MBC, Fenton MB (1996) Behavioural and thermoregulatory responses of female hoary bats, Lasiurus cinereus (Chiroptera: Vespertilionidae), to variations in prey availability. Ecoscience 3:414–422
Hickey MBC, Fenton MB, MacDonald KC, Soulliere C (2001) Trace elements in the fur of bats (Chiroptera: Vespertilionidae) from Ontario and Quebec, Canada. Bull Environ Contam Toxicol 66:699–706
Jones G, Jacobs DS, Kunz TH, Wilig MR, Racey Pa (2009) Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 8:93–115
Kalko EKV, Handley CO, Handley D (1996) Organization, diversity, and long-term dynamics of a Neotropical bat community. In: Cody M, Smallwood J (eds) Longterm Studies in Vertebrate Communities. Academic Press, Los Angeles, p 503–553
Karouna-Renier NK, White C, Perkins CR, Schmerfeld JJ, Yates D (2014) Assessment of mitochondrial DNA damage in little brown bats (Myotis lucifugus) collected near a mercury-contaminated river. Ecotoxicology 23:1419–1429
Khan MA, Wang F (2009) Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Env Toxicol Chem 28:1567–1577
Kunz TH, Anthony ELP (1982) Age estimation and post-natal growth in the bat Myotis lucifugusitle. J Mammal 63:23–32
Lamborg CH, Fitzgerald WF, Damman AWH, Benoit JM, Balcom PH, Engstrom DR (2002) Modern and historic atmospheric mercury fluxes in both hemispheres: Global and regional mercury cycling implications. Global Biogeochem Cycles 16:51
Laperche V, Hellal J, Maury-Brachet R, Joseph B, Laporte P, Breeze D, Blanchard F (2014) Regional distribution of mercury in sediments of the main rivers of French Guiana (Amazonian basin). Springerplus 3:519–521
Little ME, Burgess NM, Broders HG, Campbell LM (2015a) Distribution of mercury in archived fur from little brown bats across Atlantic Canada. Environ Pollut 207:52–58
Little ME, Burgess NM, Broders HG, Campbell LM (2015b) Mercury in little brown bat (Myotis lucifugus) maternity colonies and its correlation with freshwater acidity in Nova Scotia, Canada. Environ Sci Technol 49:2059–2065
Maguiña R, Amanzo J, Huamán L (2012) Dieta de murciélagos filostómidos del valle de Kosñipata, San Pedro, Cusco -Perú. Rev Peru Biol 19:159–166
Marrugo-Negrete J, Marrugo-Madrid S, Pinedo-Hernández J, Durango-Hernández J, Díez S (2016) Screening of native plant species for phytoremediation potential at a Hg-contaminated mining site. Sci Total Environ 542:809–816
Meech JA, Veiga MM, Tromans D (1998) Reactivity of mercury from gold mining activities in darkwater ecosystems. Ambio 27:92–98
Melamed R, Trigueiro FE, Villas Bôas RC (2000) The effect of humic acid on mercury solubility and complexation. Appl Organomet Chem 14:473–476
Miller CL, Watson DB, Lester BP, Howe JY, Phillips DH, He F, Liang L, Pierce EM (2015) Formation of soluble mercury oxide coatings: Transformation of elemental mercury in soils. Environ Sci Technol 49:120105–12111
Miura T, Koyama T, Nakamura I (1978) Mercury content in museum and recent specimens of Chiroptera in Japan. Bull Environ Contam Toxicol 20:696–701
Moreno-Brush M, Rydberg J, Gamboa N, Storch I, Biester H (2016) Is mercury from small-scale gold mining prevalent in the southeastern Peruvian Amazon? Environ Pollut 218:150–159
Morrison DW (1978) Lunar phobia in a neotropical fruit bat, Artibeus jamaicensis (Chiroptera: Phyllostomidae). Anim Behav 26:852–855
Mosquera C, Chávez ML, Pachas VH, Moschella P (2009) Estudio diagnóstico de la actividad minera artesanal en Madre de Dios (Diagnostic Study of artisanal mining activities in Madre de Dios), Fundación Conservación Internacional, Lima
Moya MI, Montaño-Centellas F, Aguirre LF, Tordoya J, Martínez J, Galarza MI (2008) Variación temporal de la quiropterofauna en un bosque de yungas en Bolivia. Mastozoología Neotrop. 15:349–357
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nam DH, Yates D, Ardapple P, Evers DC, Schmerfeld J, Basu N (2012) Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology 21:1094–1101
Neitzel V (2002) Die Kalibration von Analysenverfahren (Teil 2) Nicht lineare Kalibrationsfunktionen (Calibration of Analytical Methods (part 2) non linear calibration functions). Chem Labor Biotech 53
O’Shea TJ, Everette aL, Ellison LE (2001) Cyclodiene insecticide, DDE, DDT, arsenic, and mercury contamination of big brown bats (Eptesicus fuscus) foraging at a Colorado Superfund site. Arch Environ Contam Toxicol 40:112–120
Passos CJ, Mergler D, Gaspar E, Morais S, Lucotte M, Larribe F, Davidson R, De Grosbois S (2003) Eating tropical fruit reduces mercury exposure from fish consumption in the Brazilian Amazon. Environ Res 93:123–130
Passos CJS, Da Silva DS, Lemire M, Fillion M, Guimarães JRD, Lucotte M, Mergler D (2008) Daily mercury intake in fish-eating populations in the Brazilian Amazon. J Expo Sci Environ Epidemiol 18:76–87
Passos CJS, Mergler D, Fillion M, Lemire M, Mertens F, Guimarães JRD, Philibert A (2007) Epidemiologic confirmation that fruit consumption influences mercury exposure in riparian communities in the Brazilian Amazon. Environ Res 105:183–193
Ribeiro Mello MA, Menezes Schittini G, Selig P, Godoy Bergallo H (2004) A test of the effects of climate and fruiting of Piper species (Piperaceae) on reproductive patterns of the bat Carollia perspicillata (Phyllostomidae). Acta Chiropterologica 6:309–318
Roach RR, Busch S (2004) Mercury exposure aboard an ore boat. Environ Health Perspect 112:910–913
Roulet M, Lucotte M, Canuel R, Rheault I, Tran S, De Freitos Gog YG, Farella N, Souza do Vale R, Sousa Passos CJ, De Jesus da Silva E, Mergler D, Amorim M (1998) Distribution and partition of total mercury in waters of the Tapajós River Basin, Brazilian Amazon. Sci Total Environ 213:203–211
Rowland IR, Mallett AK, Flynn J, Hargreaves RJ (1986) The effect of various dietary fibres on tissue concentration and chemical form of mercury after methylmercury exposure in mice. Arch Toxicol 59:94–98
Speir SL, Chumchal MM, Drenner RW, Cocke WG, Lewis ME, Whitt HJ (2014) Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ Toxicol Chem 33:2506–2509
Steidl RJ, Hayes JP, Schauber E (1997) Statistical power analysis in wildlife research. J. Wildl. Manage. 61:270–279
Syaripuddin K, Kumar A, Sing KW, Halim MRA, Nursyereen MN, Wilson JJ (2014) Mercury accumulation in bats near hydroelectric reservoirs in Peninsular Malaysia. Ecotoxicology 23:1164–1171
Thies W, Kalko EKV (2004) Phenology of neotropical pepper plants (Piperaceae) and their association with their main dispersers, two short-tailed fruit bats, Carollia perspicillata and C. castanea (Phyllostomidae). Oikos 104:362–376
Thies W, Kalko EKV, Schnitzler H-U (2006) Influence of environment and resource availability on activity patterns of Carollia castanea (Phyllostomidae) in Panama. J Mammal 87:331–338
Thies W, Kalko EKV, Schnitzler H-U (1998) The roles of echolocation and olfaction in two Neotropical fruit-eating bats, Carollia perspicillata and C. castanea, feeding on Piper. Behav Ecol Sociobiol 42:397–409
Thomas L (1997) Retrospective power analysis. Conserv Biol 11:276–280
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
UNEP (United Nations Environmental Programme) (2013) Global MercuryAssessment 2013: Sources, Emissions, Releases, and Environmental Transport. UNEP, Geneva
Usman K, Habersetzer J, Subbaraj R, Gopalkrishnaswamy G, Paramanandam K (1980) Behaviour of bats during a lunar eclipse. Behav Ecol Sociobiol 7:79–81
Wada H, Yates DE, Evers DC, Taylor RJ, Hopkins WA (2010) Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology 19:1277–1284
Wobeser G, Nielsen NO, Schiefer B (1976) Mercury and Mink II. Experimental methyl mercury intoxication. Can J Comp Med 40:34–45
Yates DE, Adams EM, Angelo SE, Evers DC, Schmerfeld J, Moore MS, Kunz TH, Divoll T, Edmonds ST, Perkins C, Taylor R, O’Driscoll NJ (2014) Mercury in bats from the northeastern United States. Ecotoxicology 23:45–55
York HA, Billings SA (2009) Stable-isotope analysis of diets of short-tailed fruit bats (Chiroptera: Phyllostomidae: Carollia). J Mammal 90:1469–1477
Zukal J, Pikula J, Bandouchova H (2015) Bats as bioindicators of heavy metal pollution: history and prospect. Mamm. Biol.-Z Saugertierkd 80:220–227
Acknowledgements
This research was partially funded by Tambopata Reserve Society (TReeS), World Wildlife Foundation (WWF-Peru), and AG Umweltgeochemie—TU Braunschweig. We are greatful for the logistic support of Servicio Nacional de Areas Protegidas por el Estado (Sernanp), Servicio Nacional Forestal y de Fauna Silvestre (Serfor), Asociación para la Investigación y Desarrollo Integral (Aider), as well as of Jose Luis Mena and Nadesna Cortes. Thanks to Katherin Bernabé, Emilio Bonifaz, Deyber Gil, Werner Pinedo, and Fredy Mollehuanca for their assistance in the field. We appreciate the guidance on the data treatment and the constructive comments of Dagmar Söndgerath, Johan Rydberg, Dania Richter, and Marta Pérez-Rodríguez that greatly helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Ethical approval
This article does not contain any studies with human participants. Capture and handling of bats were conducted under the research permit (N° 006-2015-SERNANP-JEF) obtained from the Peruvian authorities of the national protected areas (Sernanp).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moreno-Brush, M., Portillo, A., Brändel, S.D. et al. Mercury concentrations in bats (Chiroptera) from a gold mining area in the Peruvian Amazon. Ecotoxicology 27, 45–54 (2018). https://doi.org/10.1007/s10646-017-1869-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-017-1869-1