Abstract
Taraxacum officinale Weber (dandelion) is a very ubiquitous species, and it can grow in urban environments on metal-polluted sediments deposited in the gutters. This study represents a preliminary step to verify the presence of metals in sediments collected in urban streets in Pisa and to assess the alteration in dandelion metabolites in order to understand its adaptation to polluted environments. The soil and sediments were collected at three urban streets and analyzed for total and extractable Cr, Pb, Cu, Ni, and Zn. The total values of Pb and Zn in street sediments exceeded the limits for residential areas of soils. Zn was the most mobile of the metals analyzed. Floating cultivations trials were set up with dandelion seedlings and street sediments. The metals were analyzed in roots and leaves. Antioxidant power, anthocyanins, polyphenols, non-protein thiols (NP-TH) and chlorophylls were measured in dandelion leaves. The first two parameters (anthocyanins and antioxidant power) were higher in the polluted samples compared to the control; chlorophyll content was lower in the treated samples, whereas NP-TH showed no differences. NP-TH groups determined in roots were associated with the root content of Zn and Pb. These results indicate that dandelion can tolerate plant stress by altering its metabolite content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eroded urban soil settles in the street gutters, and the sediment is composed of soil, dust and can contain metals. Many studies have reported that urban sediments collected in streets are contaminated by trace metals. Ewen et al. (2009) found zinc (Zn), copper (Cu), and lead (Pb) in the sediments in inner city roads; Kremova et al. (2009) found cadmium (Cd), Cu, nickel (Ni), Fe, and Zn in street sediments, which were higher than in the nearby soils; and Irvine et al. (2009) found Cu, Zn, and Pb associated with urban and traffic areas. Nevertheless, in some cases, gutters can represent a niche for the development of ruderal plants, such as dandelion. Taraxacum officinale Weber (dandelion) is a common herbaceous perennial plant of the Asteraceae family with a wide geographic distribution, and it is associated with disturbance in anthropic ecosystems and ruderal sites (Pignatti 1982); this wide distribution reflects its broad ecological amplitude. Dandelion is often found in degraded soils and in urban environment in Italy (Benvenuti 2004), growing in very shallow spaces between the cracks in the pavements.
Some authors have reported that dandelion is able to take up a variety of metals into its tissues, suggesting that the plant could be used worldwide as a trace metal indicator (Kuleff and Djingova 1984; Kabata-Pendias and Dudka 1991; Simon et al. 1996; Normandin et al. 1999). In particular, dandelion has been proven to react quantitatively to air and soil pollution with As, Br, Cd, Cu, Fe, Hg, Mn, Ni, Pb, Pt, Pd, Rh, and Zn (Kuleff and Djingova 1984; Kabata-Pendias and Dudka 1991; Djingova and Kuleff 1993; Djingova et al. 2003; Maric et al. 2013), although the correspondence between the air–soil pollution and the concentration in the plant is usually not simply linear. Keane et al. (2001) also indicated that dandelion leaves strongly differ in metal content according to the season, and highlighted a lack of correlation between leaf and soil concentrations of some metals (Pb, Zn, and chromium (Cr)). The roots of the dandelion act as a storage organ (Cyr and Bewley 1990), and for this reason, it is likely to be the place where the plant keeps all its excess elements, such as pollutants, but only few studies reported the content of metals in roots (Kabata-Pendias and Dudka 1991; Krolak 2003).
Plants growing in contaminated heavy metal environments avoid detrimental effects in different ways, which involve a disorder of cell metabolism leading to a growth reduction and lower biomass production (Hall 2002). Tolerance is achieved through the activation of various defense mechanisms including the different localization of metal ions in roots and shoots, with an accumulation and storage in nontoxic forms (Dickinson et al. 2009) or active transport of ions into the vacuole, and the formation of complexes with organic acids or peptides (Memon and Schröder 2009). Metal contamination induces oxidative stress producing reactive oxygen species. Therefore, to counteract this damage, plant cells deactivate this metal stress inducing highly efficient antioxidant defense mechanisms (Clijsters et al. 1999; Singh et al. 2004). Antioxidant substances and antioxidant enzymes play a vital role in providing cellular defense against the free radicals produced by oxidative stress (Sinha et al. 2007; Sinha and Saxena 2006). Antioxidants are often reducing agents that can remove free radical intermediates mainly due to the availability of their hydroxyl groups in the molecule, and inhibit other oxidation reactions by being oxidized themselves (Rice-Evans et al. 1996).
The uptake of some soil metal pollutants has been determined in many studies, but few data are available concerning the change in metabolites content that are modified in the presence of metal stress or tolerance. Therefore, we analyzed the presence of some metabolites involved in stress adaptation in the leaves to verify the influence of metal contamination on the physiology of dandelion.
Nonenzymatic cellular antioxidant metabolites include phenolic compounds, flavonoid anthocyanins, and also non-protein thiols (NP-TH, e.g., cysteine, glutathione). In chamomile, brassica and other species, different forms of phenolic metabolites contribute to a tolerance to metal excess and participate in active antioxidative protection (Kováčik and Klejdus 2008; Mobin and Khan 2007). One of the most important mechanisms for metal detoxification in plants is the chelation of metals by low molecular weight proteins such as metallothioneins and peptide ligands, the phytochelatins (PCs) (Hall 2002; Gupta et al. 2013). The functional significance of PCs is attributed to the presence of NP-TH groups, responsible for the coordination of metals (Hall 2002; Maestri et al. 2010). Thus, the presence of NP-TH groups is very important for understanding the overall mechanism of the tolerance to heavy metals.
Previous studies indicate that dandelion may accumulate metals from the atmosphere as well as the soil (Kabata-Pendias and Dudka 1991; Djingova and Kuleff 1993); for this reason, it has been proposed as a biomonitor of environmental metal pollution (Kuleff and Djingova 1984; Simon et al. 1996). Collier et al. (2010) found that dandelion clones grown in metal-polluted sites showed morphological adaptation (reduced total biomass), while no studies determined the primary and secondary metabolites in dandelion to correlate the adaptation to metals with physiological responses.
The aims of this study were (1) to quantify the metal content in street sediments and evaluate the uptake in dandelion and (2) to assess the adaptation mechanisms of dandelion grown on metal-polluted street sediment, particularly the physiological response, through the evaluation of its secondary metabolites. For this purpose, we assessed the presence of metals (Cr, Cu, Ni, Zn, and Pb) in the urban street sediments and in the soils nearby, and their bioavailability; metals and physiological parameters as antioxidant power, anthocyanins, polyphenols, NP-TH, and chlorophylls, were also measured in dandelion.
This paper will contribute to scientific knowledge about the physiological and biochemical responses of dandelion to environmental metal present in street sediment, its defense mechanisms, and the potential of this species to grow in polluted environments.
Material and methods
Study area, sampling, and experimental trials
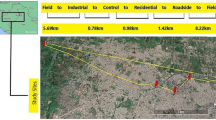
Pisa is a medium-sized town located on the very highly populated coast of Central Tuscany (Italy) with a population of around 90,000. Three sampling sites were chosen to collect soil and street sediment in the main roads of the inner city area as follows: Via Bonanno, Via Conte Fazio, and Piazza Guerrazzi (Fig. 1). The soil samples were taken at 0–20-cm depth, and the street sediment samples, consisting in the solid runoff, were collected from the nearby gutters. The collected sediments were used for growing dandelion in the experimental trial. For this purpose, the sediments were mixed with vermiculite (ratio 1:1), added in order to create a more suitable texture and allow the best cultivation conditions for the plants. The control plants were grown on potting soil + vermiculite (1:1). To exclude any possible risk of additional metal contamination, we analyzed metals in the potting soil and vermiculite, and they resulted, respectively, as follows (values are in milligram per kilogram): Cu 4.9 and 0.3; Ni 12.0 and 10.0; Pb 24.2 and 13.4; Zn 18.1 and 5.3. The seeds of dandelion were collected in the urban area of Pisa, in the summer 2008, sown in the substrate (sediment + vermiculite) in the autumn, and grown for 96 days in greenhouse at the Department of Agriculture, Food and Environment of Pisa University (latitude 43°42′15′′N; longitude 10°25′38′′E). The growing conditions were average temperature 20 °C, humidity 70–80 %, and 14/10 h photoperiod at approximately light intensity of 300 μmol m–2 s–1. The plants were grown individually in a pot of 0.5 L, and fertilized with a Hoagland nutrient solution. The culture was set in a floating system prepared as in Rideout and Gooden (1998). At the end of the growing period, the plant materials (leaves and roots) were washed, weighed, and stored at −80 °C throughout the analysis, with the exception of the sampling for chlorophyll determination and dry weight.
Soil, sediment, and plant analysis
Texture, pH, and cation exchange capacity (CEC) were determined from the soil samples by standard methods (ASA-SSSA 1996). The organic matter (OM) content was determined using a CHNS analyzer Carlo Erba NA 1500. The total concentrations of Pb, Cu, Ni, Cr, Cd, and Zn were measured in soil and sediments after nitric–perchloric microwave digestion (HNO3/HClO4 mixture 2.5:1 ratio) by atomic absorption spectroscopy (Wright and Stuczynski 1996). Sediment samples were also subject to chemical extraction with CaCl2 according to Basta and Gradwohl (2000) and Takáč et al. (2009). The fractions extracted with CaCl2 represent the amount of metal immediately available that is the easily exchangeable fraction linked with weak electrostatic forces. The plant organs were harvested and stored as reported above. Roots, before drying, were washed in an ultrasound bath to eliminate any soil particles that might have remained on the surfaces, using a Branson Sonifier 250 ultrasonic processor in an ice bath for 10 min. To determine the heavy metal content, the samples of dried vegetable tissues were ground and then digested using acid–oxidant digestion (HNO3/HClO4 mixture 2.5:1 ratio), with a microwave system “ETHOS-900” (MILESTONE S.r.l., Bergamo, Italy), equipped with a pulsed-mode emission in Teflon vials (Tassi et al. 2008). The following microwave program was used for plant samples: 250 W for 10 min with 5 min of ventilation. The same procedure was used for soil and sediment using a different microwave program: 200 W for 10 min and then, after a pause of 1 min, 250 W for 10 min followed by 5 min of ventilation. After digestion, all the samples were made up to 25 mL with Milli-Q water and then analyzed by flame AAS (Varian AA 240FS).
Metabolites analysis
Chlorophylls were determined spectrophotometrically according to Moran (1982); five-leaf discs 10-mm diameter per plant were punched, and their photosynthetic pigments were extracted by direct immersion into 5 ml of solvent N,N-dimethylformamide. After storing the samples in dark at 4 °C for 24 h, the absorbance of the pigment extract was then quantified by spectrophotometer with absorbance reading at wavelengths 664, 647, and 625 nm. The chlorophyll a (Chla), chlorophyll b (Chlb), and total chlorophyll contents of the leaves were calculated on fresh weight basis. Chlorophyll determinations were repeated three times per treatment.
For anthocyanins determination, leaves were ground in one volume methanol/HCl (v/v 99/1 %) with the addition of two-thirds volume of distilled water. Extracts were recovered, and one volume of chloroform was added to remove chlorophylls through mixing and centrifugation (1 min at 14,000 g). Anthocyanins containing in the aqueous phase were recovered and absorption was determined spectrophotometrically at a wavelength 535 nm (Cheng and Breen 1991). Calculation of anthocyanins was based on the standard curve prepared using cyanidin chloride. The contents were expressed as milligrams per gram dry weight. Mean values were obtained from three independent replicates.
For the measurements of total antioxidant power and total soluble phenolic compounds, plant leaves were extracted by homogenization in 70 % ethanol (w/v, 1/10). After incubation for 30 min at 4 °C, the samples were centrifuged at 15,000 g for 10 min, then the supernatants were utilized for the further analyses. The antioxidant power was determined by using the ferric reducing antioxidant power (FRAP) method with minor modification (Szőllősi and Szőllősi Varga 2002). The working solution always freshly prepared contained 7.5 mM acetate buffer, pH 3.6, 0.1 mM tripyridyltriazine (TPTZ), and 0.05 mM FeCl3 6H2O. At low pH, when the tripyridyltriazine (FeIII-TPTZ) complex is reduced to the ferrous form (FeII), an intensive blue color of (FeII-TPTZ) can be monitored spectrophotometrically at 593 nm. Aqueous solution of known Fe(II) concentration was used for calibration (in a range of 100–1,000 μmol L−1) was used as external standard reference.
Total soluble phenolic compound content was assayed with the method based on Folin–Ciocalteu’s phenol reagent and spectrophotometric determination (Singleton and Rossi 1965). Sample extract (0.05 mL) was introduced in a vial, then 0.5 mL of Folin–Ciocalteu’s reagent and 0.45 mL of saturated sodium carbonate solution (75 g L−1) were added and mixed. After incubation at room temperature for 2 h, absorption at 765 nm was measured. Results were expressed as milligram of chlorogenic acid per gram of fresh weight. Quantification data were the mean of four results.
The non-protein thiols (NP-SH groups) content in tissues were extracted and determined using the method of Ellman et al. (1961). Plant material (0.5 g) was homogenized in 7 % sulfosalicylic acid. After centrifugation at 10,000 g for 20 min at 4 °C, NP-SH content was measured in the supernatant by reaction with Ellman’s reagent and absorbance was recorded at 412 nm.
Reagents, materials, and chemical instrumentation
All the reagent and chemicals are purchased by Sigma-Aldrich (St Louis, Mo, USA). Organic solvents were purchased from J.T. Baker (Mullinckrodt, Buker, Inc., Phillipipsburg, NI 088659). The spectrophotometric measurements were carried out using a UV1204 UV/VIS spectrophotometer (Shimadzu).
Statistical analysis
Statistical analysis was carried out using specialized software (Statgraphics 5.1, Statistical Graphics Corp., USA). One-way ANOVA with a Student–Neumann–Keuls test was used to test differences in metabolite accumulation. Means were separated by the least significance difference test at p < 0.05.
Results and discussion
Soils and street sediments analyses
The chemical and physical characteristics of the soils near the street sediment collecting points are shown in Table 1. Soil texture was between loamy-sand (LS) and sandy loam (SL); the content of sand was very high, as it is often the case in urban soils where the content of coarse material greatly affects the texture. Also, the other soil parameters were consistent with similar studies on urban soils (Biasioli et al. 2006; Bretzel and Calderisi 2006); pH was sub-alkaline (8), estimating a very low mobility for the metals analyzed, especially Pb and Zn (Businelli et al. 2009); CEC was around 25 cmol kg−1, and this value was consistent with the content of OM (6–9 %). The total metal content of the soils showed that Cu, Cr, and Ni were within the average value for Italian non-contaminated soils (Bretzel and Calderisi 2011; Biasioli et al. 2006), whereas Pb and Zn values in Via Bonanno and Via Conte Fazio were >100 mg kg−1, but in Piazza Guerrazzi, those metals showed lower values, data are reported in Table 2.
Street sediment analysis showed that Cu, Cr, Ni, Pb, and Zn were present in all the samples. Total Zn reached the highest value in Via Bonanno (>800 mg kg–1) as reported in Table 2; this was the only case of extra content, in the other cases, all the metals were within the average values for urban sediments (Kremova et al. 2009; Irvine et al. 2009). Between the CaCl2 extractable values, only Zn was very high in the sample Via Bonanno. All the other values were low (<0.5), as reported in Table 3. The pH of street sediments was 7, revealing a very low mobility for Cu, Pb, and Zn (Businelli et al. 2009).
Some samples of street sediment showed higher metal values compared to those of the nearby soils; this may be due to the close deposition of metals from vehicles and to runoff, but also to the affinity of the contaminants to the smallest particles because of their high exchangeable surface. In fact, the finest particles are the most subject to erosion and though represent the major percentage of the sediment. Cu and Ni were higher in Via Conte Fazio and Piazza Guerrazzi street sediments than in the respective soils (Table 2). Zn and Pb were higher only in Piazza Guerrazzi. It has been reported that the highest concentration of metals in street sediments is due to the percentage of the finest fraction compared to the soils (Kremova et al. 2009). In any case, all these metals showed a very low mobility; the values were very low in the CaCl2 extraction (Table 3). Only Zn showed a noticeable amount of CaCl2 extracted (19 mg kg−1), which was consistent with the high total content in the same samples.
Metals in plants
Only sediment, and not soil, was employed for the experiments as dandelion easily grows in the cracks of the gutters, where only sediment is present, deposited by erosion. The plants grown in pots containing street sediments were stunted, they had smaller leaves than the control plants. This is a common response of dandelion grown in polluted soil (Collier et al. 2010). The analyses of metal content in roots revealed that they were richer in Pb and Zn than in Cu, Cr, and Ni (Fig. 2). This was especially true for the plants grown on Via Bonanno sediment which had the following values: Pb >100 mg kg–1 and Zn >90 mg kg–1. Pb was present in Via Conte Fazio and in the control, even though the content of Pb was the lowest in the sediment.
Previous data obtained with dandelion grown in polluted soil of Poland showed that Pb accumulation is related to scarce Zn absorption in particular Zn concentration in soil above 200 mg kg–1 inhibited Zn uptake (Krolak 2003).
The values of cumulation factor (FC) for Pb in the configuration root/sediment were different along the trials, showing a value of 1 for Via Bonanno, 0.24 for Piazza Guerrazzi, and 0.55 for Via Conte Fazio, whereas FC values for Zn were approximately 0.11–0.12.
Leaves collected from dandelion grown in street sediment contained metals, particularly Pb, Zn, and Cu (Fig. 3). The levels of detected Zn and Pb were higher than that detected in roots, as already reported in previous papers (Krolak 2003). Davies (1992) reported that Zn in radish plants correlated better to exchangeable Zn in soil, while Pb correlated better to its total in soil. This was confirmed by our study; Pb concentrated in the roots of the dandelion, Via Conte Fazio, Piazza Guerrazzi, and controls, showed 40–60 mg kg–1 in the roots (Fig. 2). On the other hand, Pb content in Via Bonanno roots showed a particular behavior, in fact it was very high (>100 mg kg–1), approximately twice the content compared to the other samples, thus suggesting a better uptake of Pb in the presence of high levels of Zn.
Synergistic actions have been reported between the metals (Bjerre and Schierup 1985). On the other hand, Wei et al. (2005) reports that Taraxacum mongolicum is a Zn excluder. If this is the case with Taraxacum officinalis, it could explain the relatively low content of Zn in the roots. The presence of Zn in the street sediments seems to improve the uptake of Pb, although Pb in the roots correlated with the total Pb in all the other samples (i.e., Via Conte Fazio, Piazza Guerrazzi, and control). The results of the metal uptake in the roots suggest an affinity of dandelion to Pb. In fact, the values in the roots were higher than the Cu, Cr, Ni, and Zn values, confirming the uptake and indicating the presence of Pb in street sediments. The translocation factor (leaves/roots) for Zn was 1.48, Pb 1.53, and Cu 0.68. These values showed that Zn and Pb were present in highest concentration in leaves, while Cu was present in highest concentration in roots, as in Krolak (2003).
The correspondence between the air–soil pollution and the concentration in plants is not usually simply linear (Keane et al. 2001), in fact it has been already described that dandelion metal accumulation follows the soil metal levels, particularly for Cr, Mn, Pb, and Zn (Keane et al. 2001); although for other metals (Cd, Cu, Fe, and Ni), there is a lack of a significant relationship between leaf and soil concentrations. In this study, the values of leaves/sediment ratio for Pb (FC) were 1.6 for Via Bonanno, 0.37 for Piazza Guerrazzi, and 0.86 for Via Conte Fazio, whereas FC values for Zn were in the range of 0.16–0.18. However, the Via Bonanno street sediment had the highest level of Pb and Zn, and the correspondence of the highest level of Pb and Zn in root and leaves confirms the uptake and migration of metal from sediment to the aerial part of plants.
Metabolites in plants
Plants defenses towards metal toxicity implement different strategies. Pb induces various antioxidants (enzymatic and nonenzymatic) in many plants, as horsegram, bengalgram (Reddy et al. 2005), privet Ligustrum ovalifolium (Gajic’ et al. 2009), and the submerged macrophyte Vallisneria (Wang et al. 2011).
Antioxidant power and metabolites involved in stress adaptation were determined in the leaves to verify the influence of contamination on the physiology of dandelion grown on street sediments; the results are shown in Table 4.
The photosynthetic pigments Chla, Chlb, and total chlorophyll were determined in leaves collected from 96-day-old T. officinale plants. Control plants showed an usually higher content of Chla and Chlb than the treated plants, with a loss of about 20 % total Chl in stressed plants. The concentrations of both Chla and Chlb decreased with the same ratio as the total chlorophyll; the Chla to Chlb ratio calculated for control plants had a rate of approximately 3.19, whereas the ratios calculated with the values of contaminated plants ranged between 3.16 and 3.28, with a slight increase for the Via Conte Fazio site. The fact that this ratio was maintained means that a hydrolysis of Chla can be ruled out.
The importance of monitoring the total Chl concentration and Chla to Chlb ratio as an early warning system for the toxic effect of metal accumulation in plants is well documented (Li et al. 2009). Heavy metals can affect each component at different levels, contributing to changes in plant physiology. The chlorophyll values detected in our data confirm the involvement of metals in the decrease of Chla and Chlb. Moreover, heavy metal accumulation had a negative effect on the ratio of Chla/Chlb, due in particular to a faster hydrolysis ratio of Chla compared with Chlb when plants are under stress (Abdel-Basset et al. 1995). However, in dandelion, the ratio of Chla to Chlb was constant in the different samples, suggesting that dandelion activated some other physiological mechanism for the growth in metal-contaminated street sediments.
The antioxidant power, measured with the concentration-dependent FRAP, indicated that the stressed plants had statistically higher values than the control plants. The highest value was found in the Via Conte Fazio sample, which exhibited an antioxidant power (ferric equivalent 49.98 mg g–1 FW) twice the control value.
Total polyphenol content displayed (Table 4) a slight increase in levels in two samples (Via Bonanno and Piazza Guerrazzi), although there were no statistical differences with the control value (0.421 mg g–1 FW). Only the Via Conte Fazio sample exhibited the highest level (0.663 mg g–1 FW). The polyphenol content seems to follow the same increase already observed for the antioxidant power.
Anthocyanins detected in plants grown on street sediments indicated that stress conditions increased the anthocyanin content. A slight increase in anthocyanins was observed in Via Conte Fazio, and a strong increase in the other two samples. In the Via Bonanno samples, the value was five times higher than the control plants (Table 4).
Further analyses were conducted to determine NP-TH in leaves and roots (Table 5). The leaves of three samples (Via Bonanno, Via Conte Fazio, and Piazza Guerrazzi) showed a lower NP-TH content than the control, but in Via Conte Fazio, the difference was not statistically significant. NP-TH in polluted roots was very similar to the control. Only Via Bonanno content (0.268 μmol g–1 FW) was statistically different.
Our results highlight that dandelion tolerates metal stress, adopting particular strategies with an increase of the antioxidant system.
The results indicated a relationship between antioxidant compounds (antioxidant power, anthocyanins, and NP-TH) and metal stress in via Bonanno. Anthocyanin pigments are involved in many physiological phenomena; the induction of anthocyanin accumulation might help in the protection of the photosynthetic apparatus, screening it from the deleterious effects of stress generated by superoxide radicals (Mobin and Khan 2007). This phenomenon happens without limiting the photosynthesis, in fact in our results, Chla/Chlb ratio is not affected by metal stress.
Besides the general response of pigments to metal contamination, dandelion seems to react in different ways to the uptake of metals. Our results indicated that the plants that accumulated Pb and Zn in the roots (Via Bonanno) improved the content of NP-TH groups in roots, and anthocyanins in leaves. These data are in accordance with Mishra et al. (2006), who indicated a correlation between metals and the partitioning of NP-TH in leaves and roots of Ceratophyllum.
The plants that showed a lower content of metals in roots (Piazza Guerrazzi and Via Conte Fazio) produced a very high content of antioxidant power and polyphenols in leaves. This is in accordance with the activation of antioxidants as a general response to stress. The stressed leaves of the plants showed increases in the levels of antioxidants in varying degrees and provided endogenous protection effects. The use of chlorophyll content and total phenolics as physiological and biochemical markers for the tolerance to some Pb is recently been accepted (Gajic’ et al. 2009; Wang et al. 2011), and the results obtained with dandelion confirm the involvement of a general oxidative stress in plants due to metal toxicity.
Conclusion
Our results indicate that dandelion can grow on metal-contaminated sediments by simply altering its physiological parameters. Photosynthetic pigments, anthocyanins, and secondary metabolites such as polyphenols, NP-TH, and the antioxidant power play a role in the defense mechanisms. In addition, dandelion leaves appear to be the main organ for metal accumulation, especially Pb. Consequently, this study confirmed the possibility of using dandelion as an indicator of Pb and Zn in the soil.
Dandelion’s ability to grow in degraded urban environment led us to indicate dandelion as a species suitable to revegetate urban areas. We believe this is a very interesting perspective, since few studies have been carried out on the ability of plants, potentially interesting for ornamental purposes, to tolerate a polluted environment and make it look beautiful at the same time.
Further investigation is necessary to better correlate the metabolites with metal pollution in soil.
References
Abdel-Basset R, Issa AA, Adam MS (1995) Chlorophyllase activity: effect of heavy metals and calcium. Photosynthetica 31:421–425
ASA-SSSA (1996) Methods of Soil Analysis, Part 1 and 3 Physical and Chemical Methods, 2nd edn. ASA-SSSA, Madison, Wisconsin, USA
Basta NT, Gradwohl R (2000) Estimation of cadmium, Pb, and zinc bioavailability in smelter-contaminated soils by a sequential extraction procedure. J Soil Conta 9:149–164
Benvenuti S (2004) Weed dynamics in the Mediterranean urban ecosystem: ecology, biodiversity, and management. Weed Res 44:341–354. doi:10.1111/j.1365-3180.2004.00410.x
Biasioli M, Barberis R, Ajmon-Marsan F (2006) The influence of a large city on some soil properties and metals content. Sci Total Environ 356:154–164. doi:10.1016/j.scitotenv.2005.04.033
Bjerre GK, Schierup HH (1985) Uptake of six heavy metals by oat as influenced by soil type and additions of cadmium, lead zinc, and copper. Plant Soil 88:57–69
Bretzel F, Calderisi M (2006) Metal contamination in urban soils of coastal Tuscany (Italy). Environ Monit Assess 118:319–335. doi:10.1007/s10661-006-1495-5
Bretzel F, Calderisi M (2011) Contribution of a municipal solid waste incinerator to the trace metals in the surrounding soil. Environ Monit Asses 182:523–533. doi:10.1007/s10661-011-1894-0
Businelli D, Massaccesi L, Onofri A (2009) Evaluation of Pb and Ni mobility to groundwater in calcareous urban soil of Ancona, Italy. Water Air Soil Pollut 201:185–193. doi:10.1007/s11270-008-9936-0
Cheng GW, Breen PJ (1991) Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hort Sci 116:865–869
Clijsters H, Cuypers A, Vangronsveld J (1999) Physiological responses to heavy metals in higher plants: defence against oxidative stress. Z Naturforsch 54c:730–734
Collier MH, Keane B, Rogstad SH (2010) Productivity differences between dandelion (Taraxacum officinale; Asteraceae) clones from pollution impacted versus non-impacted soils. Plant Soil 329:173–183
Cyr DR, Bewley JD (1990) Proteins in the roots of the perennial weeds chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Weber) are associated with overwintering. Planta 182:370–374. doi:10.1007/BF02411387
Davies BE (1992) Interrelationships between soil properties and the uptake of cadmium, copper, lead, and zinc from contaminated soil by radish (Raphanus sativus L.). Water Air Soil Pollut 63:331–342
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytoremediat 11:97–114
Djingova R, Kuleff I (1993) Monitoring of heavy metal pollution by Taraxacum officinale. In: Markert B (ed) Plants as Bio-Monitors. VCH Verlagsgesellschaft, Weinheim, pp 433–460
Djingova R, Kovacheva P, Wagner G, Markert B (2003) Distribution of platinum group elements and other traffic-related elements among different plants along some highways in Germany. Sci Total Environ 308:235–246
Ellman GL, Courtney DD, Andres V, Festherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ewen C, Anagnostopoulou MA, Ward NI (2009) Monitoring of heavy metal leveles in roadsite dusts of Thessaloniki, Greece in relation to motor vehicle traffic density and flow. Environ Monit Assess 157:483–498. doi:10.1007/s10661-008-0550-9
Gajic’ G, Mitrovic’ M, Pavlovic’ P, Stevanovic’ B, Djurdjevic’ L, Kostic’ O (2009) An assessment of the tolerance of Ligustrum ovalifolium Hassk to traffic-generated Pb using physiological and biochemical markers. Ecotoxicol Environ Saf 72:1090–1101
Gupta DK, Huang HG, Corpas FJ (2013) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161. doi:10.1007/s11356-013-1485-4
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Irvine KN, Perrelli MF, Ngoen-klan R, Droppo IG (2009) Metal levels in street sediments from an industrial city: spatial trends, chemical fractionation, and management implications. J Soil Sediments 9:328–341. doi:10.1007/s11368-009-0098-5
Kabata-Pendias A, Dudka S (1991) Trace metal contents of Taraxacum officinale (dandelion) as a convenient environmental indicator. Environ Geochem Health 13:108–113. doi:10.1007/BF01734301
Keane B, Collier MH, Shann JR, Rogstad SH (2001) Trace metal content of dandelion (Taraxacum officinale) leaves in relation to soil contamination and airborne particulate matter. Sci Total Environ 281:63–78. doi:10.1016/S0048-9697(01)00836-1
Kováčik J, Klejdus B (2008) Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep 27:605–615. doi:10.1007/s00299-007-0490-9
Kremova K, Robertson D, Cveckova V, Rapant S (2009) Road-deposited sediment, soil, and precipitation (RDS) in Bratislava, Slovakia: compositional and spatial assessment of contamination. J Soil Sediments 9:304–316. doi:10.1007/s11368-009-0097-6
Krolak E (2003) Accumulation of Zn, Cu, Pb, and Cd by dandelion (Taraxacum officinale Web) in environments with various degrees of metallic contamination. Pol J Environ Stud 12:713–721
Kuleff I, Djingova R (1984) The dandelion Taraxacum officinale a monitor for environmental pollution? Water Air Soil Pollut 21:77–85
Li Y, Liu J, Liu Y, Li X (2009) Effects of EDTA on mechanism of lead accumulation in Typha orientalis Presl. Bull. Environ Contam Toxicol 83:553–557. doi:10.1007/s00128-009-9787-4
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13
Maric M, Antonijevic M, Alagic S (2013) The investigation of the possibility for using some wild and cultivated plants as hyperaccumulators of heavy metals from contaminated soil. Environ Sci Pollut Res 20:1181–1188. doi:10.1007/s11356-012-1007-9
Memon AR, Schröder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res 16:162–175. doi:10.1007/s11356-008-0079-z
Mishra S, Srivastava S, Tripathia RD, Govindarajan R, Kuriakose SV, Prasad MNV (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition, and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–611. doi:10.1016/j.jplph.2006.03.003
Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol 9:1376–1381
Normandin L, Kennedy G, Zayed J (1999) Potential of dandelion (Taraxacum officinale) as a bioindicator of manganese arising from the use of methylcyclopentadienyl manganese tricarbonyl in unleaded gasoline. Sci Total Environ 239:165–171
Pignatti S (1982) Flora d’Italia. Edagricole, Bologna
Reddy AM, Kumar SG, Jyonthsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead-induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rideout JW, Gooden DT (1998) Phosphorus nutrition of tobacco seedlings grown in greenhouse float culture. J Plant Nutr 21:307–319
Simon L, Martin HW, Adriano DC (1996) Chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Web) as phytoindicators of cadmium contamination. Water Air Soil Pollut 91:351–362. doi:10.1007/BF00666269
Singh S, Saxena R, Pandey K, Bhatt K, Sinha S (2004) Response of antioxidants in sunflower (Helianthus annuus L.) grown on different amendments of tannery sludge: its metal accumulation potential. Chemosphere 57:1663–1673. doi:10.1016/j.chemosphere.2004.07.049
Singleton VL, Rossi JAJ (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagent. Amer J Enol Viticol 16:144–158
Sinha S, Saxena R (2006) Effect of iron on lipid peroxidation, and enzymatic and nonenzymatic antioxidants and bacoside—a content in medicinal plant Bacopa monnieri L. Chemosphere 62:1340–1350. doi:10.1016/j.chemosphere.2005.07.030
Sinha S, Mallick S, Misra RK, Singh S, Basant A, Gupta AK (2007) Uptake and translocation of metals in Spinacia oleracea L. grown on tannery sludge-amended and contaminated soils: effect on lipid peroxidation, morpho-anatomical changes, and antioxidants. Chemosphere 67:176–187. doi:10.1016/j.chemosphere.2006.08.026
Szőllősi R, Szőllősi Varga I (2002) Total antioxidant power in some species of Labiatae (adaptation of FRAP method). Acta Biol Szeged 46:125–127
Takáč P, Szabová T, Kozáková Ľ, Benková M (2009) Heavy metals and their bioavailability from soils in the long-term polluted Central Spiš region of SR. Plant Soil Environ 55:167–172
Tassi E, Pouget J, Petruzzelli G, Barbafieri M (2008) The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 71:66–73
Wang C, Lu J, Zhang S, Wang PF, Hou J, Qian J (2011) Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natane. Ecotox Environ Saf 74:1297–1303
Wei S, Zhou Q, Wang X (2005) Identification of weed plants excluding the uptake of heavy metals. Environ Intern 31:829–834. doi:10.1016 j envint.2005.05.045
Wright RJ, Stuczynski T (1996) Atomic absorption and flame emission spectrometry. In: Sparks DL (ed) Methods of Soil Analysis: Part 3. Chemical Methods and Processes. Soil Sci Soc Am Book Series 5, SSSA, Madison, pp 65–90
Acknowledgments
This study was supported by funds from the University of Pisa. The authors would like to thank Irene Rosellini for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vera Slaveykova
Rights and permissions
About this article
Cite this article
Bretzel, F., Benvenuti, S. & Pistelli, L. Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ Sci Pollut Res 21, 2325–2333 (2014). https://doi.org/10.1007/s11356-013-2147-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2147-2