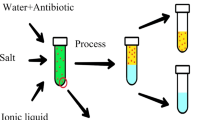
Abstract
Nonsteroidal anti-inflammatory drugs and antibiotics are classes of active pharmaceutical ingredients (APIs), which are continuously contaminating the ecosystem through various anthropogenic activities. Because of their pseudo-persistence in the aquatic environment and their potentially chronic effects on aquatic life, it is important to closely monitor their concentrations in the aquatic environment using a sensitive analytical method. Sustainable aqueous biphasic systems (ABSs) composed of ionic liquids and biodegradable organic salt (sodium malate) were proposed. The phase diagrams of the systems were firstly determined, and [N4444]Cl-based ABS was selected for the simultaneous extraction and preconcentration of seven APIs. With the developed ABS, extraction efficiencies of APIs close to 100% were obtained. For the developed method, limits of detection (LODs) of 45, 65, 76, 14, 60, 48, and 51 ng L–1 were obtained for indomethacin, ibuprofen, diclofenac, naproxen, ketoprofen, flurbiprofen, and chloramphenicol, respectively, providing from 1216- to 1238-fold improvement as compared with the analysis without preconcentration. From an economic and environmental point of view, we can predict the prospects and competitive position of the method developed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Active pharmaceutical ingredients (APIs) are a group of persistent and bioactive contaminants, including antibiotics, hormones, nonsteroidal anti-inflammatory drugs (NSAIDs), and so on. They are frequently used for medication of infectious diseases, inflammation, osteoporosis, bronchial asthma, periodontal disease, and rheumatic disorders (Davies and Anderson 1997; Launer 2003). In particular, NSAIDs and chloramphenicol are widely used because of their low price and wide applicability. Approximately, 70 million NSAIDs are prescribed each year in the USA, 10 million in Canada, and 20 million in the UK. Wang et al. reported that the annual consumption of NSAIDs in China is 5 billion dollars. Due to the large annual consumption, these drugs are continuously discharged into the wastewater via municipal, medical, and industrial wastewater, and disposal of un-consumed or expired products (Kummerer 2009; Rehman et al. 2015). According to Barbara and Sun, APIs undergo several processes during the wastewater treatment of which they are not completely eliminated from wastewater (Kasprzyk-Hordern et al. 2009; Sun et al. 2014). Finally, the APIs in wastewater treatment plant effluents are continuously released into the aquatic environment including surface water, groundwater, tap water, river water, and even drinking water.
Literature survey exhibits that the concentrations of APIs in aquatic environment is generally between ug L−1 and ng L−1 (Fernandez et al. 2010; Lolic et al. 2015). Among all detected NSAIDs in the environment, diclofenac, ibuprofen, and naproxen, three NSAIDs with huge global consumption by the population often showed the highest concentration and detection rates (He et al. 2017). Hence, diclofenac, ibuprofen, and naproxen have been included in the list of top 10 priority pharmaceuticals that should be monitored in aquatic ecosystems by European Union (Barbosa et al. 2016; Tiedeken et al. 2017). Although APIs are present in trace concentration level in aquatic environment, the pseudo-persistence due to continuous discharge could result in health-related problems of aquatic animals. It was reported that prolonged exposure to some APIs in environmentally relevant concentrations will damage the general health of fish and otter (Cuklev et al. 2011; Richards 2014; Stancova et al. 2015). Accordingly, it is necessary to develop improved analytical methods regarding detecting and quantifying APIs in aquatic environment for environmental monitoring (Almeida et al. 2017b; He et al. 2017).
With regard to the trace amount of APIs in aquatic environment, extraction and preconcentration steps are highly demanded prior to instrumental analysis, both to increase their concentrations up to values that can be quantified by analytical equipment and to remove major interferences. So far, various sample preparation methods have been developed for the extraction of APIs, such as salting-out liquid phase extraction, liquid-liquid microextraction (LLME), solid phase extraction (SPE), dispersive solid phase extraction (d-SPE), and magnetic solid phase extraction (MSPE) (Amiri et al. 2016; Grueiro Noche et al. 2011; Larsson et al. 2009; Trinanes et al. 2015; Yahaya et al. 2013). These methods have their own advantages, but the enrichment effect of them is generally poor, which limits the sensitivity of the methods. In comparison with traditional methods, aqueous biphasic system (ABS) is a very promising sample pretreatment method due to its advantages of simplicity, high enrichment factor, reproducibility, and so on.
Typically, ABSs are formed by two aqueous-rich phases based on polymer-polymer, polymer-salt, salt-salt, or salt-ionic liquid (IL) combination (Gutowski et al. 2003; Li et al. 2010). The system undergoes a two-phase separation when the phase-forming components above are given concentrations. In addition to the large water content, the non-volatile nature of polymers and salts allows the phase-forming components to be recovered and recycled, and hence, ABSs can be seen as an environmentally friendly approach. The distribution of the target compound between the two phases depends on the nature of the compound and the composition of the two-phase system.
Among the various ABSs, the one composed of salt and IL was frequently used for the preconcentration of trace organic compounds in aquatic environment (Almeida et al. 2017a; Zawadzki et al. 2016). ILs are green organic salts, non-flammable, with high thermal and chemical stabilities and strong solubilization power. Moreover, researchers are able to tailor their polarities and affinities by manipulating the chemical structures of their ions, which greatly improves the practical capacity of ILs (Li et al. 2010). Therefore, we have a reason to believe that there is an ABS composed of IL and salt suitable for the extraction of APIs. Most of the previously reported ABSs are composed of ILs and high-charge-density inorganic salts which were based on phosphate, sulfates, and carbonate anions (Zawadzki et al. 2016; Almeida et al. 2017a). However, high-concentration discharges of these inorganic salts in the aquatic environment raise some environmental issues. Aiming at overcoming the environmental impacts of these high-charge-density salts, biodegradable and more biocompatible organic salts were introduced in the composition of IL-based ABSs.
In this work, sustainable ABSs composed of green solvent (ILs) and biodegradable organic salt (sodium malate) were proposed for the extraction and preconcentration of APIs. The phase diagrams of ILs and sodium malate–based aqueous biphasic systems were firstly determined according to the previous work and used to infer their formation ability (Malekghasemi et al. 2016; Merchuk et al. 1998). The developed ABS pretreatment procedure was applied to the simultaneous extraction and preconcentration of APIs in the aquatic environment. To achieve the highest extraction efficiency and enrichment factors, the main parameters were studied such as the composition of the two-phase system and the proportion of each composition. Meanwhile, the concentrations of APIs in IL-rich phase were determined by HPLC-UV. In general, the benefit of our work includes (i) a sustainable ABS composed of green solvent [N4444]Cl and biodegradable organic salt; (ii) one-step ABS pretreatment procedure that could simultaneously extract and preconcentrate APIs in aquatic environment; and (iii) higher recoveries, relatively lower LODs, and better precision.
Experiment section
Materials and samples
The ILs, 1-butyl-3-methylimidazolium chloride ([C4C1im]Cl), 1-pentyl-3-methylimidazolium chloride ([C5C1im]Cl), 1-hexyl-3-methylimidazolium chloride ([C6C1im]Cl), and 1-octyl-3-methylimidazolium chloride ([C8C1im]Cl), were purchased from the Center for Green Chemistry and Catalysis (Lanzhou, China). Tetrabutylphosphonium chloride and tetrabutylammonium bromide were supplied by Sigma-Aldrich. Tetrabutylammonium chloride was obtained from Adamas Reagent Co., Ltd.-beta. All ILs were dried at 70 °C for 72 h to reduce the water contents. The chemical structures of ILs were shown in Fig. 1A.
One wide-spectrum antibiotic, chloramphenicol, and six NSAIDs, ibuprofen, indomethacin, flurbiprofen, diclofenac, naproxen, and ketoprofen, were used in this work. These drugs were acquired from Sigma-Aldrich chemicals (Shanghai, China). The structures of the studied APIs are shown in Fig. 1B. The sodium malate monohydrated, C4H2Na2O4·H2O, > 99.0 wt% pure, was acquired from Alfa Aesar (Tianjin, China). The water used throughout this work was prepared by a Millipore Milli-Q Plus pure water system (MA, USA). The methanol used was HPLC grade (Tedia Co, Fairfield, OH).
Six river water samples were collected from Yuhangtang River, Shangtang River, Yueya River, Xixi Wetland, Beijing-Hangzhou Canal, and Qiantang River. The sample of running water was taken from the tap in the laboratory (tap water). Three hospital wastewater samples were collected from the hospital wastewater treatment plants of local hospitals in Hangzhou. The collected volume for each sample was about 500 mL. After filtration with 0.22-μm nylon membrane, the water samples were taken to the laboratory in clean glass bottles at 4 °C. Within 10 h of sampling, all samples were double distilled and treated with a Milli-Q system. These samples can effectively test the applicability of the method, as the literature shows that the matrix and the concentrations of APIs in these samples vary greatly.
Phase diagrams and tie lines
The ternary phase diagrams corresponding to the ABSs composed of ionic liquids and sodium malate were determined through the cloud point titration method at ambient temperature and atmospheric pressure. The sodium malate solution was added dropwise to the ionic liquid solution until a cloudy solution was formed, which was considered to be a cloud point and the concentration of each component in the solution at that time was recorded. Thereafter, deionized water was added dropwise to the cloudy solution to make the solution clarify, and then the sodium malate solution was added again to reach a new cloud point. This action is repeated until enough two nodes were measured. The experimental binodal curves were correlated by using the Merchuk equation (Merchuk et al. 1998):
where A, B, and C are the constants obtained through the data regression, while IL and salt are the weight percentage of IL and salt, respectively.
The tie line lengths (TLLs) corresponding to the mixture compositions used in this work were measured through a well-established gravimetric method initially introduced by Rogers et al. A ternary mixture of IL + salt + H2O at the biphasic region was prepared and stirred vigorously for at least 24 h. Then, the two coexisting phases were separated carefully and weighed with a precision of ± 10−4 g. The TLs were determined by the application of the lever-arm rule through the relationship between the weight of the top (IL-rich) phase and that of the overall system. For the calculation of each TL, a system of four equations and four unknown variables was solved:
where α is known to be the ratio between the mass of the IL-rich phase (top) and the overall system, and subscripts “IL”, “salt,” and “M” refer to the top phase, bottom phase, and mixture, respectively. The tie line length (TLL) was calculated by Eq. (6):
Screening of IL-based ABS for the complete extraction of APIs
For the selection of improved IL-based ABS for the completely extraction of APIs, several ternary systems (IL + C4H2Na2O4 + H2O) were prepared within the biphasic region at constant weight fraction percentages of each component: 30 wt% of IL + 19 wt% of C4H2Na2O4 + 51wt% of aqueous solutions (Table S1). The concentration of APIs used in the initial aqueous solutions was 5.0 mg L−1. These mixtures were vigorously stirred and left to thermodynamic equilibrate for 24 h at 25 (± 1) °C. Subsequently, the IL-rich phase and salt-rich phases were carefully separated and weighed. The amount of each API in each phase was quantified through an Ultimate 3000 high-performance liquid chromatography (Thermo Scientific, USA). The extraction experiments for each system were carried out in triplicate, and three injections per sample were performed. The extraction efficiency of each system for the APIs, EE%, were determined according to
where CIL and Csalt are the concentrations of APIs in the IL-rich and salt-rich aqueous phases and WIL and Wsalt are the weights of IL-rich and salt-rich phases, respectively.
Concentration of APIs in the [N4444]Cl-based aqueous biphasic systems
After the screening of ionic liquids, the system composed of [N4444]Cl + C4H2Na2O4 + H2O with a TLL of 71.0 was selected to develop the extraction and concentration platforms for APIs. As previous studies concluded, the concentration of APIs in the wastewater real sample and other matrix were in the order of μg L−1 (Gavrilescu et al. 2015); thus APIs (0.5 μg L−1) were used for the investigation of the enrichment factor in different compositions of the phase-forming components. The ABS with different composition was magnetically stirred at ambient condition for about 1 h and then left for 24 h to separate the two phases (Table S2). The IL-rich phase was carefully collected and weighted. The concentration of APIs in the IL-rich phase was evaluated by high-performance liquid chromatography with an ultraviolet detector (HPLC-UV). The extraction efficiency and enrichment factors (EF) of APIs along the characterized TL were evaluated by Eq. (7) and Eq. (8), respectively:
where CIL is the concentration of APIs in IL-rich phase and CIN is the initial API concentration in aqueous solution.
Extraction and determination of APIs
The ABS formed by 0.05 g [N4444]Cl, 3.8 g C4H2Na2O4, and 6.15 g water sample was prepared, stirred at ambient condition for about 1 h, and then left to equilibrate for 24 h. The phases were separated and the concentrations of APIs were quantified by the HPLC-UV method.
The determination of APIs was carried out by an Ultimate 3000 UHPLC system (Thermo Scientific, USA) with a variable wavelength UV detector. The separation of target compounds was performed on an Accucore C18 column (150 × 4.6 mm, 2.6 μm) at 40 °C, and a mobile phase consisted of water containing 0.1% acetic acid (A) and methanol (B) at a flow rate of 0.4 mL min−1. Gradient elution was set as follows: 0–4 min, 50% A; 4–20 min, 40% A. The temperature of autosampler was maintained at 10 °C, and the injection volume was 10 μL. Based on the full scan results in Fig. S1, the detector wavelengths for the quantification were set at 225 nm, 255 nm, and 277 nm.
Validation of the method
To evaluate the performance of the proposed method, a series of Shangtang river water spiked with known amounts of standard solutions were prepared. To guarantee that this river water did not contain the APIs under study, it was extracted by the selected ABS, and the chromatogram was shown in Fig. S2. The calibration curves were established by plotting the peak areas against the API concentrations in the range of 0.03–20 μg L−1. The limit of detection (LOD) and limit of quantification (LOQ) were defined as signal-to-noise ratio (S/N) of 3 and 10, respectively. And they were detected by continuously decreasing the spiked concentration. The repeatability and reproducibility of the method were determined by analyzing five concentrations (1.0 μg L−1, 5.0 μg L−1, 10.0 μg L−1, 15.0 μg L−1, and 20.0 μg L−1) of APIs within 1 day and 6 days continuously. The results obtained were calculated for % RSD. For the determination of API recoveries in real samples, hospital wastewater sample, river water, and tap water were spiked at five concentration levels (1.0 μg L−1, 5.0 μg L−1, 10.0 μg L−1, 15.0 μg L−1, and 20.0 μg L−1) and been analyzed by the developed method. As real sample may contain target analytes, non-spiked samples were also analyzed. API recoveries from different sample were calculated using the following equation:
where Cs is the concentration of APIs found in spiked samples, Cns is the concentration of APIs found in non-spiked samples, and C0 is the concentration spiked in the samples.
Result and discussion
Phase diagrams and tie lines
The parameters of Eq. (1) derived from the nonlinear regression of different ABSs, and their corresponding determination coefficients, were shown in Table 1. According to the r2, the correctness of the nonlinear regression was confirmed. The ternary phase diagrams of novel ABSs composed of ILs and sodium malate were depicted in Fig. S3, and the details of weight fraction data were given in the supplementary materials. As shown in Fig. S3A, the phase-forming ability followed the order: [C4C1im]Cl ≪ [C5C1im]Cl ≪ [C6C1im]Cl≪ [C8C1im]Cl. This rank was in close agreement with previous studies: an increase in the cation alkyl-chain length led to an increase in the fluid hydrophobicity and poorer solubility of the ionic liquid in water (Huddleston et al. 2001). Figure S3B presented the influence of the cation core; [N4444]Cl and [P4444]Cl have an identical ability for the phase split, whereas [C4C1im]Cl was weaker than them. The two quaternary salts ([N4444]Cl and [P4444]Cl) have highly shielded charges, located mostly on the heteroatom surrounded by four alkyl chains, thus leading to a higher tendency toward salting out from aqueous media (Bridges et al. 2007). The imidazolium-based ionic liquid ([C4C1im]Cl) has a cation with a charge more evenly dispersed along the entire heterocycle and a greater ability to interact with water via hydrogen-bonding. The inspection of Fig. S3C indicated the bromide-based ionic liquids presented higher aptitude to form ABS than chloride-based counterpart. Anion with lower electron pair donation ability presents lower ability to create hydration complexes, and therefore more easily salted-out by conventional salts. Taking this idea into account, the ability of an ionic liquid anion to produce ABS depends on their hydrogen bond accepting strength (Ventura et al. 2009).
Screening of IL-based ABS for the complete extraction of APIs
For an adequate extraction and preconcentration, the appropriate selection of the phases was always required. ABS consisting of approximately 30 wt% of IL + 19 wt% of C4H2Na2O4 + 51 wt% of aqueous solution containing APIs was initially used to assess the ability of ILs of different chemical structures to extract APIs (Table S1). The extraction efficiency of the investigated ABSs for APIs were depicted in Fig. 2 and Table S3 and follows the rank: [N4444]Cl ≈ [N4444]Br > [P4444]Cl > [C8C1im]Cl > [C6C1im]Cl ≈ [C5C1im]Cl ≈ [C4C1im]Cl (Bridges et al. 2007). In general, all the APIs preferentially partition to the IL-rich phase, with extraction efficiency higher than 92% in all systems evaluated. The higher affinity of APIs toward the IL-rich phase correlates well with their octanol–water partition coefficient (Kow) value (Table 2). Furthermore, the extraction efficiency of APIs was closely related to the hydrophobicity of the IL that forms aqueous biphasic system. Due to the high hydrophobicity, [N4444]Cl, [N4444]Br, and [P4444]Cl showed higher extraction ability for APIs. Moreover, among the ILs studied, [N4444]Cl has the characteristics of lower toxicity (Egorova and Ananikov 2014; Zhao et al. 2007), higher thermal stability, lower price, and commercially available (Golding et al. 2002; Ropponen et al. 2004). In consideration of the extraction efficiency and the advantages associated with [N4444]Cl discussed above, [N4444]Cl-based ABS was selected for the determination of APIs in environmental aqueous samples.
Concentration of APIs using [N4444]Cl-based ABS
According to the previous studies, one primary requisite of using ABS as sample pretreatment method was the presence of long tie lines. As shown in Table 3 and Fig. S4, longer TLL not only cut down the cross contamination of each phase by enriching the component in the opposite layer but also can afford higher enrichment factors without observable sacrifice of extraction efficiency. Therefore, the longest TLL (circa 77.1) was further studied for concentration purposes.
Different initial compositions along the same TL, and with a TLL value circa 77.1, lead to different weight ratios of the coexisting phases (as shown in Fig. 3). The extraction efficiency values, respective standard deviations, and enrichment factors for different initial compositions were depicted in Table 3. The inspection of Table 3 indicates that complete extractions of APIs were always obtained for all the mixture compositions evaluated. The experimental enrichment factors were ranged from 0.88 to 1238, meaning that it is possible to preconcentrate the APIs by cutting down the volume of the IL-rich phase without losing extraction efficiency. The results indicated that IL-based ABS can simultaneously extract and concentrate different classes of APIs by three orders of magnitude (form μg L−1 to mg L−1). Hence, ABS composed of 0.5 wt% [N4444]Cl + 38.0 wt% sodium malate + 61.5 wt% aqueous solution was selected for the determination of APIs.
Schematic illustration behind the use of IL-based ABS as concentration platforms. Phase diagram of the system composed of [N4444]Cl + C4H2Na2O4 + H2O, at (25 ± 1) °C: binodal data ( ); TL data (
); TL data ( ); initial compositions (
); initial compositions ( ). In the inset, the enrichment factors of corresponding initial compositions are shown
). In the inset, the enrichment factors of corresponding initial compositions are shown
Performance of the method
Under the optimized conditions, the proposed method was coupled with HPLC and applied to the determination of APIs in aqueous samples. The method validation parameters, such as linearity, LOD, LOQ, recoveries, and precision for APIs, were summarized in Table 4. The response function was found to be linear in the range of 0.2–20 μg L−1 with determination coefficient (r2) higher than 0.998. The LOD and LOQ were ranging from 0.01 to 0.08 μg L−1 and 0.03–0.24 μg L−1, respectively. In the hospital wastewater samples, the recoveries of APIs at concentration level of 1.0 μg L−1, 5.0 μg L−1, 10.0 μg L−1, 15.0 μg L−1, and 20.0 μg L−1 were in the ranges 91.8–99.2%, 93.3–97.5%, 93.5–98.9%, 95.9–99.4%, and 97.4–99.3%, respectively (Table 5). In the river water and tap water, the recoveries of APIs ranged from 92.5 to 99.3% (Table S4 and Table S5). The results show that the matrix does not react with the APIs and has no effect on the recoveries. The RSD representing for repeatability and reproducibility of the method were between 0.5–9.6% and 1.4–8.5%, respectively (Table 5).
Real sample analysis
The optimized ABS system coupled with HPLC-UV was applied for the determination of APIs in real water samples: tap water, six river water, and three hospital wastewater. Among the samples, only naproxen was detected in one hospital wastewater. The chromatograms of the normal hospital wastewater and hospital wastewater spiked with 5.0 μg L−1 APIs after extraction were shown in Fig. 4. No obvious interference was observed in Fig. 4, which indicates the good selectivity of the proposed method.
Chromatograms of the determination of APIs in real water samples. (A) blank control sample, (B) normal hospital wastewater sample spiked 5.0 μg L−1 APIs, and (C) normal hospital wastewater sample. Peaks: (1) chloramphenicol; (2) ketoprofen; (3) naproxen; (4) flurbiprofen; (5) indomethacin; (6) diclofenac; (7) ibuprofen
Figure S5 shows the HPLC-UV chromatograms of the non-spiked and spiked river water samples with no ABS pretreatment and the spiked water after ABS pretreated. APIs were not identified in the non-spiked river water, meaning that they are present in concentrations below the LOD. As expected, APIs in spiked river water were disappeared after preconcentrated by the ABS system, in agreement with the high recoveries of the method.
Furthermore, the proposed method was compared with some previously methods for the determination of APIs in water samples (Table 6) (Arghavani-Beydokhti et al. 2018; Aydin et al. 2018; Bazregar et al. 2016; Cruz-Vera et al. 2009; Lee et al. 2014; Liang et al. 2016; Park and Myung 2015; Parrilla Vazquez et al. 2013; Toledo-Neira and Alvarez-Lueje 2015; Topac et al. 2018). The extraction solvent used in previous works were chloroform, 1-undecanol, 1,2-dichloroethane, dichloromethane, and 1-octyl-3-methylimidazolium. In contrast, the developed methods use small amounts of green solvent [N4444]Cl as extraction solvent, which is more environmentally friendly. Compared with other methods in Table 6, the developed method has higher extraction recoveries (98.1–99.6%) and narrower recovery range, indicating that this method can extract all the APIs effectively and has been applied in a wide range. The enrichment factors of most methods in Table 6 are below 200, while the enrichment factors of the developed method are 1216–1238. The results show that the method can extract and preconcentrate APIs effectively and, correspondingly, improve the sensitivity of the method. Moreover, the LODs of the developed method are lowest of all the methods’ list in Table 6, which reveals the applicability of the developed method for the determination of trace APIs in environmental samples.
Conclusion
In this work, a simple, green, rapid, and sensitive method was developed for the monitoring of seven APIs in aquatic environment utilizing HPLC-UV coupled with ABS pretreatment procedure. Aiming at overcoming environmental impacts of these high-charge-density salts used in traditional ABS, sustainable ABS composed of green solvent (ILs) and biodegradable organic salt (sodium malate) was proposed. Extraction efficiencies ranging between 97% and 100% were obtained for the IL-rich phase revealing the high affinity of APIs to the hydrophobic phase. In particular, excellent extraction efficiencies of APIs were attained with [N4444]Cl-based ABS. By tuning the mixture point composition for a minimum IL-rich phase volume, the enrichment factor of APIs can reach 1238 in a single step. The proposed methodology allows the increase of the APIs concentrations by three orders of magnitude (from ng L−1 to μg L−1), thus overcoming the LODs of conventional analytical equipment commonly used in the detection and monitoring of aquatic environment. The performance of the ABS-HPLC-UV method (linearity, LODs, recoveries, and RSDs) was evaluated. Adequate repeatability, good linearity, and the low quantification limit demonstrated that the proposed method can serve as a powerful alternative for the determination of APIs in aquatic environments.
Data availability
All data generated or analyzed during this work are included in this published article and supplementary information files.
References
Almeida HFD, Marrucho IM, Freire MG (2017a) Removal of nonsteroidal anti-inflammatory drugs from aqueous environments with reusable ionic-liquid-based systems. ACS Sustain Chem Eng 5:2428–2436. https://doi.org/10.1021/acssuschemeng.6b02771
Almeida HFD, Freire MG, Marrucho IM (2017b) Improved monitoring of aqueous samples by the preconcentration of active pharmaceutical ingredients using ionic-liquid-based systems. Green Chem 19:4651–4659. https://doi.org/10.1039/C7GC01954H
Amiri M, Yadollah Y, Safari M, Asiabi H (2016) Magnetite nanoparticles coated with covalently immobilized ionic liquids as a sorbent for extraction of non-steroidal anti-inflammatory drugs from biological fluids. Microchim Acta 183:2297–2305. https://doi.org/10.1007/s00604-016-1869-5
Arghavani-Beydokhti S, Rajabi M, Asghari A (2018) Coupling of two centrifugeless ultrasound-assisted dispersive solid/liquid phase microextractions as a highly selective, clean, and efficient method for determination of ultra-trace amounts of non-steroidal anti-inflammatory drugs in complicated matrices. Anal Chim Acta 997:67–79. https://doi.org/10.1016/j.aca.2017.10.005
Aydin S, Aydin ME, Beduk F, Tekinay A, Kilic H (2018) Analysis of diclofenac in water samples using in situ derivatization-vortex-assisted liquid-liquid microextraction with gas chromatography-mass spectrometry. Acta Pharma 68:313–324. https://doi.org/10.2478/acph-2018-0024
Barbosa MO, Moreira NF, Ribeiro AR, Pereira MF, Silva AM (2016) Occurrence and removal of organic micropollutants: an overview of the watch list of EU decision 2015/495. Water Res 94:257–279. https://doi.org/10.1016/j.watres.2016.02.047
Bazregar M, Rajabi M, Yamini Y, Asghari A, Hemmati M (2016) Tandem air-agitated liquid-liquid microextraction as an efficient method for determination of acidic drugs in complicated matrices. Anal Chim Acta 917:44–52. https://doi.org/10.1016/j.aca.2016.03.005
Bridges NJ, Gutowski KE, Rogers RD (2007) Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS). Green Chem 9:177–183. https://doi.org/10.1039/b611628k
Cruz-Vera M, Lucena R, Cárdenas S, Valcárcel M (2009) One-step in-syringe ionic liquid-based dispersive liquid–liquid microextraction. J Chromatogr A 1216:6459–6465. https://doi.org/10.1016/j.chroma.2009.07.040
Cuklev F, Kristiansson E, Fick J, Asker N, Forlin L, Larsson DG (2011) Diclofenac in fish: blood plasma levels similar to human therapeutic levels affect global hepatic gene expression. Environ Toxicol Chem 30:2126–2134. https://doi.org/10.1002/etc.599
Davies NM, Anderson KE (1997) Clinical pharmacokinetics of diclofenac-therapeutic insights and pitfalls. Clin Pharmacokinet 33:184–213. https://doi.org/10.2165/00003088-199733030-00003
Egorova KS, Ananikov VP (2014) Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 7:336–360. https://doi.org/10.1002/cssc.201300459
Fernandez C, Gonzalez-Doncel M, Pro J, Carbonell G, Tarazona JV (2010) Occurrence of pharmaceutically active compounds in surface waters of the Henares-Jarama-Tajo River system (Madrid, Spain) and a potential risk characterization. Sci Total Environ 408:543–551. https://doi.org/10.1016/j.scitotenv.2009.10.009
Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F (2015) Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol 32:147–156. https://doi.org/10.1016/j.nbt.2014.01.001
Golding J, Forsyth S, MacFarlane DR, Forsyth M, Deacon GB (2002) Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: novel low cost ionic liquids. Green Chem 4:223–229. https://doi.org/10.1039/B201063A
Grueiro Noche G, Fernandez Laespada ME, Perez Pavon JL, Moreno Cordero B, Muniategui Lorenzo S (2011) In situ aqueous derivatization and determination of non-steroidal anti-inflammatory drugs by salting-out-assisted liquid-liquid extraction and gas chromatography-mass spectrometry. J Chromatogr A 1218:6240–6247. https://doi.org/10.1016/j.chroma.2011.06.112
Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD (2003) Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc 125:6632–6633. https://doi.org/10.1021/ja0351802
He BS, Wang J, Liu J, Hu XM (2017) Eco-pharmacovigilance of non-steroidal anti-inflammatory drugs: necessity and opportunities. Chemosphere 181:178–189. https://doi.org/10.1016/j.chemosphere.2017.04.084
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD (2001) Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem 3:156–164. https://doi.org/10.1039/B103275P
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2009) The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res 43:363–380. https://doi.org/10.1016/j.watres.2008.10.047
Kummerer K (2009) The presence of pharmaceuticals in the environment due to human use--present knowledge and future challenges. J Environ Manag 90:2354–2366. https://doi.org/10.1016/j.jenvman.2009.01.023
Larsson N, Petersson E, Rylander M, Jonsson JA (2009) Continuous flow hollow fiber liquid-phase microextraction and monitoring of NSAID pharmaceuticals in a sewage treatment plant effluent. Anal Methods 1:59–67. https://doi.org/10.1039/b9ay00015a
Launer LJ (2003) Nonsteroidal anti-inflammatory drug use and the risk for Alzheimer's disease-dissecting the epidemiological evidence. Drugs 63:731–739. https://doi.org/10.2165/00003495-200363080-00001
Lee CH, Shin Y, Nam MW, Jeong KM, Lee J (2014) A new analytical method to determine non-steroidal anti-inflammatory drugs in surface water using in situ derivatization combined with ultrasound-assisted emulsification microextraction followed by gas chromatography mass spectrometry. Talanta 129:552–559. https://doi.org/10.1016/j.talanta.2014.06.027
Li Z, Pei Y, Wang H, Fan J, Wang J (2010) Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. TrAC Trends Anal Chem 29:1336–1346. https://doi.org/10.1016/j.trac.2010.07.014
Liang N, Huang P, Hou X, Li Z, Tao L, Zhao L (2016) Solid-phase extraction in combination with dispersive liquid-liquid microextraction and ultra-high performance liquid chromatography-tandem mass spectrometry analysis: the ultra-trace determination of 10 antibiotics in water samples. Anal Bioanal Chem 408:1701–1713. https://doi.org/10.1007/s00216-015-9284-z
Lolic A, Paiga P, Santos LH, Ramos S, Correia M, Delerue-Matos C (2015) Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: occurrence and environmental risk. Sci Total Environ 508:240–250. https://doi.org/10.1016/j.scitotenv.2014.11.097
Malekghasemi S, Mokhtarani B, Hamzehzadeh S, Hajfarajollah H, Sharifi A, Mirzaei M (2016) Phase diagrams of aqueous biphasic systems composed of ionic liquids and dipotassium carbonate at different temperatures. Fluid Phase Equilib 415:193–202. https://doi.org/10.1016/j.fluid.2016.02.015
Merchuk JC, Andrews BA, Asenjo JA (1998) Aqueous two-phase systems for protein separation studies on phase inversion. J Chromatogr B 711:285–293. https://doi.org/10.1016/S0378-4347(97)00594-X
Park SY, Myung SW (2015) Simultaneous determination of nonsteroidal anti-Inflammatory drugs in aqueous samples using dispersive liquid-liquid microextraction and HPLC analysis. Bull Kor Chem Soc 36:2901–2906. https://doi.org/10.1002/bkcs.10601
Parrilla Vazquez MM, Parrilla Vazquez P, Martinez Galera M, Gil Garcia MD, Ucles A (2013) Ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction coupled with liquid chromatography-quadrupole-linear ion trap-mass spectrometry for simultaneous analysis of pharmaceuticals in wastewaters. J Chromatogr A 1291:19–26. https://doi.org/10.1016/j.chroma.2013.03.066
Rehman MS, Rashid N, Ashfaq M, Saif A, Ahmad N, Han JI (2015) Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 138:1045–1055. https://doi.org/10.1016/j.chemosphere.2013.02.036
Richards NL (2014) Merging wildlife and environmental monitoring approaches with forensic principles: application of unconventional and non-invasive sampling in eco-pharmacovigilance. J Forensic Res 5:228–244. https://doi.org/10.4172/2157-7145.1000228
Ropponen J, Lahtinen M, Busi S, Nissinen M, Kolehmainen E, Rissanen K (2004) Novel one-pot synthesis of quaternary ammonium halides: new route to ionic liquids. New J Chem 28:1426–1430. https://doi.org/10.1039/b409062b
Stancova V, Zikova A, Svobodova Z, Kloas W (2015) Effects of the non-steroidal anti-inflammatory drug (NSAID) naproxen on gene expression of antioxidant enzymes in zebrafish (Danio rerio). Environ Toxicol Pharmacol 40:343–348. https://doi.org/10.1016/j.etap.2015.07.009
Sun Q, Lv M, Hu A, Yang X, Yu CP (2014) Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J Hazard Mater 277:69–75. https://doi.org/10.1016/j.jhazmat.2013.11.056
Tiedeken EJ, Tahar A, McHugh B, Rowan NJ (2017) Monitoring, sources, receptors, and control measures for three European union watch list substances of emerging concern in receiving waters-a 20 year systematic review. Sci Total Environ 574:1140–1163. https://doi.org/10.1016/j.scitotenv.2016.09.084
Toledo-Neira C, Alvarez-Lueje A (2015) Ionic liquids for improving the extraction of NSAIDs in water samples using dispersive liquid-liquid microextraction by high performance liquid chromatography-diode array-fluorescence detection. Talanta 134:619–626. https://doi.org/10.1016/j.talanta.2014.11.067
Topac N, Karadas C, Kara D (2018) Development of a preconcentration method for indomethacin in natural waters using dispersive liquid-liquid microextraction based on solidification of floating organic drop technique. Water Qual Res J Can 53:41–50. https://doi.org/10.2166/wqrj.2018.036
Trinanes S, Carmen Casais M, Carmen Mejuto M, Cela R (2015) Selective determination of COXIBs in environmental water samples by mixed-mode solid phase extraction and liquid chromatography quadrupole time-of-flight mass spectrometry. J Chromatogr A 1420:35–45. https://doi.org/10.1016/j.chroma.2015.10.004
Ventura SPM, Neves CMSS, Freire MG, Marrucho IM, Oliveira J, Coutinho JAP (2009) Evaluation of anion influence on the formation and extraction capacity of ionic-liquid-based aqueous biphasic systems. J Phys Chem B 113:9304–9310. https://doi.org/10.1021/jp903286d
Yahaya N, Mitome T, Nishiyama N, Sanagi MM, Ibrahim WAW, Nur H (2013) Rapid dispersive micro-solid phase extraction using mesoporous carbon COU-2 in the analysis of cloxacillin in water. J Pharm Innov 8:240–246. https://doi.org/10.1007/s12247-013-9164-z
Zawadzki M, e Silva FA, Domańska U, Coutinho JAP, Ventura SPM (2016) Recovery of an antidepressant from pharmaceutical wastes using ionic liquid-based aqueous biphasic systems. Green Chem 18:3527–3536. https://doi.org/10.1039/C5GC03052H
Zhao D, Liao Y, Zhang Z (2007) Toxicity of ionic liquids. Clean (Weinh) 35:42–48. https://doi.org/10.1002/clen.200600015
Author information
Authors and Affiliations
Contributions
Weixia Li performed all experiments and was a major contributor in writing the manuscript. Xiaomei Zheng analyzed and interpreted the data regarding the phase diagrams. Pengyue Zhang analyzed and interpreted the data regarding the tie lines. Guoping Tu analyzed and interpreted the data regarding the determination of APIs. Suyin Zhang compared the proposed method with some previously methods. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1026 kb).
Rights and permissions
About this article
Cite this article
Li, W., Zheng, X., Tu, G. et al. Novel aqueous biphasic system based on ionic liquid for the simultaneous extraction of seven active pharmaceutical ingredients in aquatic environment. Environ Sci Pollut Res 28, 17853–17864 (2021). https://doi.org/10.1007/s11356-020-11751-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11751-7