Abstract
Pot culture experiments were conducted in a glasshouse to evaluate the effects of four efficient Cr(VI)-reducing bacterial strains (SUCR44, SUCR140, SUCR186, and SUCR188) isolated from rhizospheric soil, and four arbuscular mycorrhizal fungi (AMF—Glomus mosseae, G. aggregatum, G. fasciculatum, and G. intraradices) alone or in combination, on Zea mays in artificially Cr(VI)-amended soil. Presence of a strain of Microbacterium sp. SUCR140 reduced the chromate toxicity resulting in improved growth and yields of plants compared to control. The bioavailability of Cr(VI) in soil and its uptake by the plant reduced significantly in SUCR140-treated plants; the effects of AMF, however, either alone or in presence of SUCR140 were not significant. On the other hand, presence of AMF significantly restricted the transport of chromium from root to the aerial parts of plants. The populations of AMF chlamydospores in soil and its root colonization improved in presence of SUCR140. This study demonstrates the usefulness of an efficient Cr(VI)-reducing bacterial strain SUCR140 in improving yields probably through reducing toxicity to plants by lowering bioavailability and uptake of Cr(VI) and improving nutrient availability through increased mycorrhizal colonization which also restricted the transport of chromium to the aerial parts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr), used in several industrial processes, has attained wide public and regulatory attention because of its toxicity to environmental ecosystems in certain oxidation states. Cr oxidation states vary between −2 and +6, but only the +3 and +6 states are stable under commonly observed environmental conditions (Mishra et al. 1995). Cr (VI) exists in solution as Cr2O4 2−, and due to structural similarity with SO4 2−, it enters into the living organisms via sulfate transport pathways (Cervantes et al. 2001). Inside the cells, reaction of Cr(VI) with biological reductants produces short- or long-lived Cr intermediates of different valency states that in turn react with hydrogen peroxide to generate free radical (Mabbett et al. 2002). The toxic properties of Cr (VI) originate from the action of this form itself as an oxidizing agent as well as from the formation of reactive oxygen species (Pandey et al. 2005; Shanker et al. 2005). Due to generation of free radicals, it is toxic (Wise et al. 2004) to all forms of living systems including microorganisms by causing oxidative stress (Ackerley et al. 2006) beside causing DNA damage (Mabbett et al. 2002) and altered gene expression (Bagchi et al. 2002). Moreover, Cr(VI) is also mutagenic (Puzon et al. 2002), carcinogenic (Codd et al. 2003), and teratogenic (Asmatullah et al. 1998), and has been recognized as a priority pollutant (Cheung and Gu 2007). Although hexavalent chromium is highly toxic, its trivalent form is relatively inert and much less toxic than the hexavalent form (Krishna and Philip 2005). Excessive Cr causes toxicity to plants, as exhibited by altered metabolic processes including impaired photosynthesis, uptake of nutrients, chlorosis, and membrane damage resulting in reduced root growth, stunting, and finally plant death (Shanker et al. 2005).
Many heavy-metal-resistant bacteria have been reported bearing exceptional ability to promote the growth of the host plant by various mechanisms such as atmospheric nitrogen fixation, solubilization of phosphorus and minerals in soil, production of plant growth regulators (hormones) as well as siderophores etc. (Glick et al. 1999). Moreover, microbes possessing chromate-reducing activity can detoxify Cr(VI) either enzymatically or through the production of metabolites (Losi et al. 1994). The rhizosphere offers a complex and dynamic microenvironment where microbes develop unique communities interacting with root systems that have potential application to detoxify hazardous compounds including toxic metals (Burd et al. 2000; Rajkumar and Freitas 2008). Cr(VI)-resistant bacteria possessing such reducing ability as well as plant-growth-promoting features have raised high hopes for cost-effective and eco-friendly measures for sustainable agriculture in soil contaminated with chromium (Rajkumar et al. 2005, 2006). Furthermore, mycorrhizal fungi are recognized as biological agents that potentially increase the tolerance of plants to heavy metal toxicity (Vivas et al. 2003, 2005, 2006). Moreover, mycorrhizal performance, particularly that of the autochthonous strain, was improved by the bacterium and both contributed to better plant growth and establishment in metal-contaminated soils like Zn and Cd (Vivas et al. 2003, 2005, 2006). The reduction of growth due to Cr interference with nutritional elements uptake can be improved through mycorrhizal inoculation. Karagiannidis and Hadjisavva Zinoviadi (1998) showed that arbuscular mycorrhizal fungi (AMF) can enhance yield simultaneously reducing the chromium content in crop plants. Previous reports suggest that enhanced levels of Cr(VI) are also toxic to mycorrhizal fungi and reduce their colonization in plants (Davies et al. 2001; Citterio et al. 2005). There is very little information available about the synergistic effect of chromate-reducing rhizobacteria with AMF on reducing chromium toxicity in crop plants and improving crop yields. Presuming efficient Cr(VI)-reducing bacterial strains in soil lowering down the bioavailability of Cr(VI) may help in improving colonization and consequently population of AMF beside reducing plant toxicity, the present studies were carried out to explore the possibility of some efficient Cr(VI)-reducing rhizobacteria and AMF on growth and yields of Zea mays in artificially Cr(VI)-amended soil with an assumption that higher yields of plants could be achieved through reduced Cr(VI) toxicity and improved mycorrhization.
Material and methods
Preparation of soil samples with artificial Cr(VI) contamination
Artificial contamination of soil with Cr(VI) was carried out by a method described earlier (Papassiopi et al. 2009). Potting mixture containing soil and vermicompost (both autoclaved, 1:10 v/v) were mixed with an aqueous solution containing the appropriate concentration of potassium chromate in order to obtain the respective concentration (100 mg kg−1) of Cr(VI) per kilogram of soil. Wet soils were periodically stirred for 3 days to obtain homogenous distribution of Cr(VI) and left at room temperature for air drying. The air-dried soil was used for pot experiments.
Plant material and growth conditions
The experiments were performed under glasshouse conditions with minimum and maximum temperature of 25 and 34 °C, respectively, a relative humidity of 60–70 %, and an approximate 16:8 (day/night) photoperiod. The soil used in this experiment was a sandy loam (Ustifluvent) with pH 7.35, EC 0.38 dSm−1, 3.35 g kg−1 organic carbon, 182 kg ha−1 available N (alkaline permanganate extractable), 15.9 kg ha−1 available P (0.50 M NaHCO3 extractable), and 92 kg ha−1 available K (1 N NH4OAc extractable). The vermicompost mixed in soil was produced from mixture of distillation waste (plant-spent, de-oiled herb) of aromatic grasses (Cymbopogon winterianus and Cymbopogon flexuosus) in a vermicomposting unit for 90 days using adult clitellate Eudrilius eugineae, an epigeic species of earthworm (Singh et al. 2012c, 2013a). The vermicompost contained 1.05 % N, 0.65 % P, and 0.71 % K.
Preparation of bio-inoculums
Cr(VI)-reducing bacterial inoculums
Four efficient Cr(VI)-reducing bacteria [Bacillus cereus SUCR44 (JN674188), Microbacterium sp. SUCR140 (JN674183), Bacillus thuringiensis SUCR186 (JN674184), and B. subtilis SUCR188 (JN674195)] used in this study were earlier isolated from rhizospheric soil irrigated with tannery effluent (Soni et al. 2013) and maintained at Microbial Technology Department of CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP) by sub-culturing on nutrient agar (sodium chloride, 5.0 g L−1; beef extract, 1.5 g L−1; yeast extract, 1.5 g L−1; peptic digest of animal tissue, 5.0 g L−1; agar 12.0 g L−1; pH 7.0 ± 0.2; Himedia, India) plates amended with 100 mg L−1 of Cr(VI) as potassium chromate. Cells grown for 18 h in 1,000 mL nutrient broth (sodium chloride, 5.0 g L−1; beef extract, 1.5 g L−1; yeast extract, 1.5 g L−1; peptic digest of animal tissue, 5.0 g L−1; pH 7.0 ± 0.2; Himedia, India) were harvested (OD at 600 nm were 1.2 ± 0.1) by centrifugation at 6,000×g for 10 min at 4 °C, washed, and resuspended in 250 mL of saline water (0.85 % NaCl) of pH 7.0. Vermicompost-based inoculum (Kalra et al. 2010; Singh et al. 2012a, 2013c) was prepared by mixing the resuspended cells in 2 kg sterilized vermicompost which was incubated for 7 days at 28 °C. At the time of application, the population of SUCR strains (SUCR44, SUCR140, SUCR186, and SUCR188) was 2.1 to 2.5 × 109 CFU g−1 of vermicompost and 5 g of such vermicompost-based inoculum was used for each pot placed near the seeds.
AMF inoculums
Inoculums of four species of Glomus, i.e., Glomus mosseae (Gm), G. aggregatum (Ga), G. fasciculatum (Gf), and G. intraradices (Gi) were obtained from Microbial Culture Collection of CSIR-CIMAP, Lucknow, India. These AMF were propagated with host maize plants (Z. mays L.) for 10 weeks in a vermicompost as a potting medium and subsequently left to shade dry for 2 weeks. Maize roots containing AMF mycelium were cut into 1-cm segments, and thoroughly mixed into potting medium (vermicompost) acted as potential inoculum consisted of chlamydospores and colonized root of AMF (Singh et al. 2012b, 2013b) . The composite inoculum was stored at 5 °C until use. Five grams of such inoculum was used for each pot at a time of sowing. The inoculum potential of composite samples was 7.3 ± 0.6 spores g−1.
Experimental design
This experiment was conducted as a completely randomized design with three replications. Three seeds were sown in each plastic pot (15 cm height and 10 cm internal diameter). Various treatments include:
Control: without any inoculum
Bacterial inoculum only: SUCR44, SUCR140, SUCR186, and SUCR188
AMF inoculum only: Ga, Gi, Gf, and Gm
Bacteria + AMF inoculums: SUCR44 + Ga, SUCR44 + Gi, SUCR44 + Gf, SUCR44 + Gm, SUCR140 + Ga, SUCR140 + Gi, SUCR140 + Gf, SUCR140 + Gm, SUCR186 + Ga, SUCR186 + Gi, SUCR186 + Gf, SUCR186 + Gm, SUCR188 + Ga, SUCR188 + Gi, SUCR188 + Gf, and SUCR188 + Gm
Seedlings were thinned to one plant per pot, 5 days after germination. The plants were watered regularly to maintain the optimum moisture level (water holding capacity 0.44 mL g−1). The experiments were repeated twice.
Harvesting and biomass measurements
Harvesting was done after 60 days of sowing. The whole plants were uprooted from the pots and washed repeatedly with de-ionized water, blotted dry then roots and shoots were separated manually. Root and shoot length (control and treated) were measured with the help of a meter scale. Biomass was estimated on dry weight basis (g) after oven drying at 70 °C till a constant weight was obtained. At a time of harvesting, rhizospheric soil samples were also collected for determining the microbial population.
Determination of bioavailable Cr(VI) [soluble Cr(VI)] in soil
Bioavailable Cr(VI) in the soil was measured by a method described by Rtidel and Terytze (1999). Ten grams of soil was shaken with 48 mL 0.1 M phosphate buffer (pH 8.0) containing 1 mL (0.4 M) aluminum sulfate and 1 mL (1 M) sodium sulfite for 30 min at 250 rpm, followed by membrane-filtration (0.45 μm). Ten milliliters soil extract was taken and mixed with 20 mL of distilled water and 1 mL sodium hypochlorite. Afterwards, 5 g sodium chloride and 1 mL (7 M) phosphoric acid were added. The solution was then transferred to a 50-mL volumetric flask. One milliliter of diphenylcarbazide (DPCZ) solution was then added, and the flask was filled to the mark with water. After 10 min the absorbance was measured at 540 nm. A separate 10 mL of soil filtrate treated in the same manner with only 1 mL of acetone instead of DPCZ solution was used as blank.
Estimation of chromium in plants
Root and shoot samples were vigorously shaken with 0.01 M EDTA solution and water to exclude contaminant Cr on the surface. The washed root or shoot samples were then dried at 70 °C till the constant weight was obtained. Dried root and shoot tissues were grinded into fine powder using a porcelain mortar. About 200 mg of powdered plant tissue was taken in Teflon container with 10 mL of digestion mixture [concentrated HNO3 and HF (2:1, v/v)] and digested in microdigester (Analytik, Jena, Germany) for 75 min at 200 °C and 200 bar pressure. After digestion, the samples were allowed to cool and then filtered through Whatmann (no. 1) filter in a 25 mL of measuring flask and the volume of filtrate was made to 25 mL using deionized water. Total Cr content in the digest was determined by atomic absorption spectroscopy (PerkinElmer).
Chromium uptake in different plant parts was calculated using the bioaccumulation factor (BAF). The BAF presents an index of a plant’s ability to accumulate a particular metal relative to its concentration in medium (Ghosh and Singh 2005). For chromium metal, BAF was calculated as:
The translocation factor (TF), which represents the translocation efficiency of plants, is expressed as the ratio of chromium concentration in shoot tissue and chromium concentration in root tissue (Tappero et al. 2007)
Microbial population estimation
For determining the AM fungi colonization, fine root from plants were cut into 5 mm long pieces, washed with 10 % trypan blue, and percentage root calculated as described by McGonigle et al. (1990). Positive counts for mycorrhizal colonization included the presence of aseptate hyphae/vesicles/arbuscules. The wet sieving and decanting method was used to isolate AM fungal spores and estimate abundance (Gerdemann and Nicolson 1963). Population of SUCR strains were determined by serial dilution technique with 0.85 % saline solution using nutrient agar medium supplemented with 100 mg L−1 of Cr(VI), supplied in nutrient agar medium as potassium chromate.
Statistical analysis
The collected data of two trials were subjected to statistical analysis for analysis of variance method (ANOVA), suitable to completely randomized design (CRD), with the help of software ASSISTAT Version 7.6 beta (2012). The data on percentage root colonization by AM fungi was analyzed using arcsine square transformed values. The experimental data from the two trials had a similar variance value; hence, the data were combined for further analysis. Significant differences among treatments were based on the F test in ANOVA and means were calculated using Duncan’s multiple range test under a significance level of P ≤ 0.05 and P ≤ 0.01. The standard error (SE) of the mean in vertical bar charts was computed with Sigma Plot 10. The results and discussion are based on the mean data of two trials.
Results and discussion
Effects of bioinoculants on growth characteristic of Z. mays in artificially Cr(VI)-amended soil
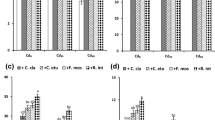
Rhizobacterial strains showed differential effectiveness on growth parameters as indicated by root length, plant height, and total dry mass production when inoculated singly (Singh et al. 2009) or co-inoculated with AM fungi (Singh et al. 2013c). From Figs. 1, 2, 3a and b, it can be inferred that root length, plant height, and dry biomass of Z. mays varies significantly (at P ≤ 0.05) among the different treatments. As compared to control, treatment with SUCR140 resulted in maximum increase in growth of Z. mays in terms of root length (96.43 %), plant height (153.18 %), dry root biomass (88.52 %), and dry shoot biomass (66.43 %). Role of Cr(VI)-reducing bacteria in improving plant growth has been earlier reported by several other workers (Zayed and Terry 2003; Mohanty and Patra 2011). The increase in growth of Z. mays by application of rhizobacterial strains could be due to reduction of toxic Cr(VI) to relatively nontoxic Cr(III) (Salunkhe et al. 1998). Co-inoculation of bacterial strains with AMF further improved the plant growth. SUCR140 showed a remarkable synergy with Gf in term of improving growth and yields. As compared to control, co-inoculation of SUCR140 + Gf showed 135.72, 191.48, 156.40, and 108.65 % increase in root length, plant height, dry root biomass, and dry shoot biomass, respectively. This increase in growth of plant by application of AMF in presence of SUCR 140 could be because of lower toxicity of reduced chromate to plants as well as improved mycorrhiza colonization of roots further improving growth of plants through increased nutrient acquisition. Our observations are in agreement with the results of Khan (2001), who reported that mycorrhizae are known to produce growth-stimulating substances for plants, improving mineral nutrition and increased growth and biomass under heavy-metal-contaminated soil necessary for effective phytoremediation to become a commercially viable strategy for decontamination of polluted soils.
a Effect of bioinoculants on dry shoot weight of Z. mays in soil inoculated with 100 mg kg−1 of Cr(VI) in soil. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05. b Effect of bioinoculants on dry root weight of Z. mays in soil inoculated with 100 mg kg−1 of Cr(VI) in soil. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05
The effect of Cr(VI) (100 mg kg−1 of soil) on plant was found to be highly toxic as the length of the roots, plant height, dry root, and shoot biomass were significantly reduced (64.35, 65.95, 40.18, and 85.44 %) as compared to the plants not treated with chromium (data not provided). Cr(VI) generally accumulates in roots because it binds with cell wall of root and retards cell division and cell elongation (Woolhouse 1983). The cell divisions are arrested by changing mitotic index reflected by frequency of cell division phases, an important parameter when determining the rate of root growth (Liu et al. 1993; Castro et al. 2007; Chidambaram et al. 2009). Under Cr(VI) stress, the mitotic index may change resulting in the decline of root growth (Hayat et al. 2012). It was also assumed that percentage of all mitotic phases decreases and interphase increases, indicating that fewer or no cells enter the division cycle, while those found in mitotic phases are arrested. Another possibility of decrease in root growth, on exposure of Cr(VI), may be damaging of plasma membrane of root, causing leakage of cell content and collapse of epidermal cells of root hairs (Castro et al. 2007).
The reduction in the plant height in chromate-affected plants might be mainly due to the reduced root growth and consequent lesser nutrient and water transport to the above ground parts of the plant. In addition to this, chromium transport to the aerial part of the plant can have a direct impact on cellular metabolism of shoots contributing to the reduction of plant height (Shanker et al. 2005). There is also a good possibility of Cr(VI) interaction with endogenous phytohormones that control plant growth processes (Moya et al. 1995). The negative effect on dry matter production could be essentially an indirect effect of chromate on plants resulting from oxidative damage to the photosynthetic and mitochondrial apparatus/processes (Dixit et al. 2002)
Effect of bioinoculants in reducing chromium bioavailability in artificially Cr(VI)-contaminated soil
The data related to bioavailability of Cr(VI) [soluble fraction of Cr(VI)] in soil after harvesting (after 60 days) has been shown in Fig. 4. The bioavailable Cr(VI) was present to a tune of 35 mg kg−1 in soil at the time of harvesting in control pots. These results are consistent with other studies where almost similar amount of chromium was found bioavailable as Cr(VI) (Mandiwana et al. 2007; Polti et al. 2011). As compared to control, a significant reduction of bioavailable Cr(VI) was recorded on SUCR bacterial inoculations alone or in combination with AMF. However, no such significant changes were observed in pots inoculated with AMF singly. It is clear from Fig. 4 that the maximum reduction in bioavailable chromium occurs in soil inoculated with SUCR 140 + Gi or SUCR140 alone. The reduced bioavailability might be because of the higher amount of chromium-reducing metabolites produced by SUCR140 (Soni et al. 2013) although the possibility of adsorption of Cr(VI) by these strains must also be looked into (Kanga et al. 2007). Considering the control value as 100 %, the bioavailability of chromium reduced by 91.75 % and 89.69 % by aforesaid bioinoculant treatments respectively; though statistically at par with each other. This shows that AMF do not play a significant role in reducing the bioavailability of Cr(VI) probably it lacks any mechanism available with them to reduce Cr(VI).
Effect of bioinoculants on Cr uptake in Z. mays
The uptake of chromium (Fig. 5a and b) was significantly higher in control plants as compared to the plants treated with SUCR bacterial inoculums. Generally, the concentrations of metals are higher in root rather than aerial part of plants. The higher Cr(VI) concentration in root is due to its immobilization in vacuole of root cells (Shanker et al. 2005). It was noticed that, inoculation of SUCR140 and SUCR 44 either singly or in co-inoculated forms, significantly reduce the uptake of chromium to plants (Fig. 5a and b). However, maximum reduction in uptake of chromium was observed in plants inoculated with SUCR140 which reduced further when co-inoculated with AMF; no significant differences in uptake of Cr(VI) in root and shoot were, however, observed among the different species of Glomus co-inoculated with SUCR140 indicating that AMF may not play any significant role in reducing the uptake of Cr(VI) from soil. Considering the uptake of chromium in control as 100 %, the uptake of chromium by SUCR 140 was reduced by 45.56 and 36.82 % in root and aerial part of plants, respectively (Fig. 5a and b). The reduction in uptake of chromium therefore could be purely due to SUCR strains reducing Cr(VI) into Cr(III), i.e., high available and soluble form to less available and insoluble form in soil.
a Effect of bioinoculants on uptake of chromium by root. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05. b Effect of bioinoculants on uptake of chromium by shoot and leave. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05
The bioaccumulation factor and translocation factor are presented in Fig. 6a, and b, respectively. From Fig. 6a, it can be inferred that SUCR140 + AMF inoculated plants showed minimum accumulation of chromium; no significant differences were observed among the single and combined treatments of SUCR 140 with various species of Glomus. Considering the control value as 100 %, the chromium accumulation by SUCR 140 + AMF was reduced to 42–52 %.
a Effect of bioinoculants on bioaccumulation factor. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05. b Effect of bioinoculants on translocation factor. Error bars shown as standard error of mean, different letters above the error bars show significant difference at P ≤ 0.05
The TF showed the translocation efficiency of plants (Fig. 6b), i.e., ratio of particular metal in shoot tissue and root tissue. The TF values <1, suggest restricted transport of chromium from root to shoot (Gheju et al. 2009) who also reported that Cr(VI) is slowly translocated from root to the aerial part of plants. These results are also consistent with our studies. We observed that SUCR strains alone are not effective in reducing the translocation, but the plants treated with AMF alone or co-inoculated with SUCR inoculums showed relatively lower transport of chromium to the aerial parts. Among different species of Glomus, minimal translocation was noticed in plants inoculated with Ga, and combined inoculations of Ga with SUCR 44 or SUCR 140 resulted in the least translocation of chromium. As compared to control, the reduction in translocation of chromium from root to aerial parts was to a tune of 38.20 and 30.41 %, on aforesaid treatments, respectively. Our results suggest that, although AMF did not reduce or improve the uptake of chromium in root but it restricted the transportation of chromium to the aerial parts. The reduction in translocation of chromium from root to aerial parts could be due to immobilization of chromium by mycorrhizal fungi. This capability was particularly substantial in case of Ga. The fungi may immobilize metals in several ways including the binding of heavy metals to chitin in the fungal cell walls causing a reduction in the translocation of heavy metals to the shoots of the plants. Also fungal vesicles may be involved in storing toxic metals and thereby avoiding their translocation to upper parts of the plants (Gother and Paszkowski 2006). Several studies have indicated an increased retention of Zn in the roots of AMF inoculated plants such as clover and maize (Zhu et al. 2001; Chen et al. 2001, 2003).
Microbial population estimation
In general, inoculation with SUCR strains significantly improved AMF colonization as well as its population in soil as measured by the number of chlamydospores per gram of soil (Table 1). The roots of the plants in presence of SUCR strains showed considerably higher colonization with AMF, maximum being with SUCR 140 compared to the treatments in which AMF were inoculated alone. Maximum increase in number of AMF spores were noticed in the treatments containing SUCR140; an increase of 81–122 % to over single AMF inoculation. Likewise, colonization of roots increased by 75–100 % in plants inoculated with SUCR140. However, the population of SUCR strains was not affected significantly when inoculated singly or in combination with AMF. Improved AMF colonization as well as its population in soil could be due to reduction of Cr(VI) in soil to relatively non-toxic forms by SUCR strains providing a favorable micro-rhizo environment for better root growth and colonization of AMF.
Conclusion
The present study establishes that application of efficient strain of Cr(VI)-reducing bacteria like SUCR140 (Microbacterium sp.) can lower the chromium toxicity to the plant by reducing the bioavailability of toxic Cr(VI). The reduced Cr(VI) toxicity levels in soil can help in promoting the growth, proliferation, and colonization of mycorrhizal fungi, resulting in improved growth and yield of crop plants. To our knowledge, this study is first of its kind demonstrating the usefulness of Cr(VI)-reducing plant-growth-promoting rhizobacteria in improving yields via improved symbiotic relationship of the plants with AMF. The reduction in uptake of Cr(VI) in presence of efficient chromium-reducing bacterial strains and further translocation of Cr(VI) through improved colonization of AMF would prevent higher accumulation of chromium in aerial parts of edible use.
References
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381
Asmatullah Qureshi SN, Shakoori AR (1998) Hexavalent chromium induced congenital abnormalities in chick embryos. J Appl Toxicol 18(3):167–171
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity and oxidative mechanism of different forms of chromium. Toxicology 180:5–22
Burd GI, Dixon DG, Glick BR (2000) Plant growth promoting bacteria that decreases heavy metal toxicity in plants. Can J Microbiol 46:237–245
Castro RO, Trujillo MM, Bucio JL, Cervantes C, Dubrovsky J (2007) Effects of dichromate on growth and root system architecture of Arabidopsis thaliana seedlings. Plant Sci 172:684–691
Cervantes C, Campos-Garcia J, Gutierrez-Corona F, Loza-Tavera H, Torres-Guzman JC, Moreno-Sanchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Chen B, Christie P, Li X (2001) A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza. Chemosphere 42:158–192
Chen BD, Li XL, Tao HQ, Christie P, Wong MH (2003) The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 50:839–846
Cheung KH, Gu JD (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad 59:8–15
Chidambaram A, Sundaramoorthy P, Murugan A, Sankar Ganesh K, Baskaran L (2009) Chromium induced cytotoxicity in blackgram (Vigna mungo L.). Iran J Environ Health Sci Eng 6:17–22
Citterio S, Prato N, Fumagalli P, Aina R, Massa N, Santagostinoa A, Sgorbati S, Berta G (2005) The arbuscular mycorrhizal fungus Glomus mosseae induces growth and metal accumulation changes in Cannabis sativa L. Chemosphere 59:21–29
Codd R, Irwin JA, Lay PA (2003) Sialoglycoprotein and carbohydrate complexes in chromium toxicity. Curr Opi Chem Biol 17(2):213–219
Davies FT Jr, Puryear JD, Newton RJ, Egilla JN, Grossi JAS (2001) Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J Plant Physiol 158:777–786
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ 25:687–690
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Brit Mycol Soc 46:235–246
Gheju M, Balcu I, Ciopec M (2009) Analysis of hexavalent chromium uptake by plants in polluted soils. Ovidius Univ Ann Chem 20:12–131
Ghosh M, Singh SPA (2005) A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut 133:365–371
Glick BR, Patten CL, Holguin G, Penrose GM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London
Gother V, Paszkowski U (2006) Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 223:1115–1122
Hayat S, Gulshan Khalique G, Irfan M, Wani AS, Tripathi BN, Ahmad A (2012) Physiological changes induced by chromium stress in plants: an overview. Protoplasma. doi:10.1007/s00709-011-0331-0
Kalra A, Chandra M, Awasthi A, Singh AK, Khanuja SPS (2010) Natural compound enhancing growth and survival of rhizobial inoculants in vermicompost based formulation. Biol Fertil Soil 46:521–524
Kanga SY, Lee JU, Kim KW (2007) Biosorption of Cr(III) and Cr(VI) onto the cell surface of Pseudomonas aeruginosa. Biochem Engin J 36:54–58
Karagiannidis N, Hadjisavva Zinoviadi S (1998) The mycorrhizal fungus Glomus mosseae enhances growth, yield and chemical composition of a durum wheat variety in 10 different soils. Nutr Cycl Agroecos 52:1–7
Khan AG (2001) Relationship between chromium biomagnification accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environ Int 26:417–423
Krishna RK, Philip L (2005) Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater 121:109–117
Liu DH, Jaing WS, Li MX (1993) Effect of chromium on root growth and cell division of Allium cepa. Isr J Plant Sci 42:235–243
Losi ME, Frankenberger WT (1994) Chromium-resistant microorganisms isolated from evaporation ponds of a metal processing plant. Water Air Soil Pollut 74:405–413
Mabbett AN, Lloyd JR, Macaskie LE (2002) Effect of complexing agents on reduction of Cr(VI) by Desulfovibrio vulgaris ATCC29579. Biotechnol Bioeng 79(4):389–397
Mandiwana KL, Panichev N, Kataeva M, Siebert S (2007) The solubility of Cr(III) and Cr(VI) compounds in soil and their availability to plants. J Hazard Mat 147:540–545
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 11:495–501
Mishra S, Singh V, Srivastava S, Srivastava R, Srivastava MM, Dass S, Satsangi GP, Prakash S (1995) Studies on uptake of trivalent and hexavalent chromium by maize (Zea mays). Food Chem Toxicol 33:393–397
Mohanty M, Patra HK (2011) Attenuation of chromium toxicity by bioremediation technology. Env Contam Toxicol 210:1–34
Moya JL, Ros R, Picazo I (1995) Heavy-metal hormone interactions in rice plants: effects on growth, net photosynthesis, and carbohydrate distribution. J Plan Gro Regulat 14:61–67
Pandey V, Dixit V, Shyam R (2005) Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) exposed to hexavalent chromium. Chemosphere 61:40–47
Papassiopi N, Kontoyianni A, Vaxevanidou K, Xenidis A (2009) Assessment of chromium biostabilization in contaminated soils using standard leaching and sequential extraction techniques. Sci Tot Environ 407:925–936
Polti MA, Atjiána MC, Amorosoa MJ, Abatea CM (2011) Soil chromium bioremediation: synergic activity of actinobacteria and plants. Int Biodet Biodeg 65:1175–1181
Puzon GJ, Petersen JN, Roberts AG, Kramer DM, Xun L (2002) A bacterial flavin reductase system reduces chromates (III)–NAD+ complex. Biochem Biophy Res 294(1):76–81
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834–842
Rajkumar M, Nagendran R, Lee KJ, Lee WH (2005) Characterization of a novel Cr6+ reducing Pseudomonas sp. with plant growth-promoting potential. Curr Microbiol 50:266–271
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62:741–748
Rtidel H, Terytze K (1999) Determination of retractable chromium(VI) in soil using a photometric method. Chemosphere 39(4):697–708
Salunkhe PB, Dhakephalkar PK, Paknikar KM (1998) Bioremediation of hexavalent chromium in soil microcosms. Biotechnol Lett 20:749–751
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:735–753
Singh R, Paramaeswarn TN, Prakasa Rao EVS, Puttanna K, Kalra A, Srinivas KVNS, Bagyaraj DJ, Divya S (2009) Effect of arbuscular mycorrhizal fungi and Pseudomonas fluorescens on root-rot/wilt, growth and yield of Coleus forskohlii. Biocontrol Sci Technol 19:835–841
Singh R, Divya S, Awasthi A, Kalra A (2012a) Technology for efficient and successful delivery of vermicompost colonized bioinoculants in Pogostemon cablin (patchouli) Benth. World J Microbiol Biotechnol 28:323–333
Singh R, Kalra A, Ravish BS, Divya S, Paramaeswarn TN, Srinivas KVNS, Bagyaraj DJ (2012b) Effect of potential bioinoculants and organic manures on root-rot and wilt, growth, yield and quality of organically grown Coleus forskohlii in semiarid tropical region of Bangalore (India). Plant Pathol 61:700–708
Singh R, Singh R, Soni SK, Patel RP, Kalra A (2013a) Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crops Prod 45:335–342
Singh R, Singh R, Soni SK, Singh SP, Chauhan UK, Kalra A (2013b) Vermicompost from biodegraded distillation waste improves soil properties and essential oil yield of Pogostemon cablin (patchouli) Benth. Appl Soil Ecol 70:48–56
Singh R, Soni SK, Awasthi A, Kalra A (2012c) Evaluation of vermicompost doses for management of root-rot disease complex in Coleus forskohlii under organic field conditions. Aust Plant Pathol 41(4):397–403
Singh R, Soni SK, Kalra A (2013c) Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza 23:35–44
Soni SK, Singh R, Awasthi A, Singh M, Kalra A (2013) In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environ Sci Pollut Res 20(3):1661–1674
Tappero R, Peltier E, Gra¨fe M, Heidel K, Ginder-Vogel M, Livi KJT, Rivers ML, Marcus MA, Chaney RL, Sparks DL (2007) Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol 175:641–654
Vivas A, Barea JM, Biró B, Azcón R (2006) Effectiveness of autochthonous bacterium and mycorrhizal fungus on Trifolium growth, symbiotic development and soil enzymatic activities in Zn contaminated soil. J Appl Microbiol 100:587–598
Vivas A, Vörös I, Biró B, Campos E, Barea JM, Azcón R (2003) Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environ Pollut 126:179–189
Vivas A, Barea JM, Azcón R (2005) Interactive effect of Brevibacillus brevis and Glomus mosseae, both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soil. Environ Pollut 134:257–266
Wise SS, Elmore LW, Holt SE, Little JE, PG A n, Bryant BH, Pierce WSJ (2004) Telomerase mediated lifespan extension of human bronchial cells does not affect hexavalent chromium induced cytotoxicity or genotoxicity. Mol Cell Biochem 255(1–2):103–112
Woolhouse HW (1983) Toxicity and tolerance in the responses of plant metals. In: Lange A et al (eds) Encyclop plant physiol. Vol.12 C. Springer, Berlin, pp 245–300
Zayed AM, Terry N (2003) Chromium in the environment: factors affecting biological remediation. Plant Soil 249:139–156
Zhu YG, Christie P, Scott Laidlaw A (2001) Uptake of Zn by arbuscular mycorrhizal white clover from Zn-contaminated soil. Chemosphere 42:193–199
Acknowledgments
The authors wish to thank the Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing necessary facilities and encouragement during the course of investigation and the Indian Council of Medical Research (ICMR), New Delhi, India, for providing financial support to SKS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Soni, S.K., Singh, R., Awasthi, A. et al. A Cr(VI)-reducing Microbacterium sp. strain SUCR140 enhances growth and yield of Zea mays in Cr(VI) amended soil through reduced chromium toxicity and improves colonization of arbuscular mycorrhizal fungi. Environ Sci Pollut Res 21, 1971–1979 (2014). https://doi.org/10.1007/s11356-013-2098-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2098-7