Abstract
Polychlorinated biphenyls (PCBs) pose a threat to the environment due to their high adsorption capacity to soil organic matter, stability and low reactivity, low water solubility, toxicity and ability to bioaccumulate. With Icelandic soils, research on contamination issues has been very limited and no data has been reported either on PCB degradation potential or rate. The goals of this research were to assess the bioavailability of aged PCBs in the soils of the old North Atlantic Treaty Organization facility in Keflavík, Iceland and to find the best biostimulation method to decrease the pollution. The effectiveness of different biostimulation additives (N fertiliser, white clover and pine needles) at different temperatures (10 and 30 °C) and oxygen levels (aerobic and anaerobic) were tested. PCB bioavailability to soil fauna was assessed with earthworms (Eisenia foetida). PCBs were bioavailable to earthworms (bioaccumulation factor 0.89 and 0.82 for earthworms in 12.5 ppm PCB soil and in 25 ppm PCB soil, respectively), with less chlorinated congeners showing higher bioaccumulation factors than highly chlorinated congeners. Biostimulation with pine needles at 10 °C under aerobic conditions resulted in nearly 38 % degradation of total PCBs after 2 months of incubation. Detection of the aerobic PCB degrading bphA gene supports the indigenous capability of the soils to aerobically degrade PCBs. Further research on field scale biostimulation trials with pine needles in cold environments is recommended in order to optimise the method for onsite remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the key functions of soils is to filter, absorb and transform various substances (Jones et al. 2010), e.g. soil pollutants such as polychlorinated biphenyls (PCBs). PCBs are organic hydrocarbons that have 1–10 chlorine atoms attached to biphenyl and 209 different congeners exist theoretically, of which 20–60 are most widely used (Safe 1994; Erickson 1997; BEST 2001; Abraham et al. 2002; Ohtsubo et al. 2004; Vasilyeva and Strijakova 2007). PCBs were produced between 1929 and late 1970s and used in industrial applications such as transformers, capacitors, hydraulic liquids, lubricants, flame retardants and plastics due to their thermal stability and low reactivity, low water solubility and high vaporisation temperature. They pose a serious risk to the environment with their bioaccumulation and biomagnification potential (Erickson 1997; Fagervold et al. 2007; Jörundsdóttir 2009) and toxicity (Ross 2004; Ulbrich and Stahlmann 2004) and high adsorption capacity to the soil organic matter and clay particles.
Up to 85 % of the global biosphere is permanently exposed to temperatures below 5 °C (Margesin 2007). Soils of cold regions and environments are key players in pollution research due to currently experiencing faster and larger-scale environmental changes than any other areas on earth (UNEP/AMAP 2011). They accumulate pollutants from lower latitudes, which has a great impact on their people’s traditional use of natural resources and food systems (Jones et al. 2010; UNEP/AMAP 2011). PCB biodegradation can take place through mineralization and cometabolism (Gomes et al. 2013): under anaerobic conditions, biodegradation occurs through dehalorespiration when bacteria capable of anaerobic respiration use PCBs as electron acceptors (Vasilyeva and Strijakova 2007), while aerobic degradation relies on oxidative destruction of PCBs with the help of various genes, e.g. on bphA and dehydrogenase (Wiegel and Wu 2000; Ohtsubo et al. 2004). In the biphenyl pathway, PCBs are first transformed to chlorobenzoic acid (CBA) by bacteria that uses biphenyl as a carbon and energy source and followed by CBA-degrading bacteria that transform the pollutant to less toxic forms (Ohtsubo et al. 2004). Research of cold soils has found that temperature is not the only governing factor of pollutant degradation and functioning of the microbial communities (Mohn et al. 1997; Master and Mohn 1998; Kuipers et al. 2003; Welander 2005; Aislabie et al. 2006; Lambo and Patel 2007; Zharikov et al. 2007), but knowledge of the dynamic soil environment, contaminants and contaminant degraders are of pivotal importance. Soil pH, close to neutral being optimal for degradation, has an effect on the adsorption of PCBs into organic matter and therefore bioavailability and biodegradation as well (Jota and Hassett 1991; Borja et al. 2005; Aislabie et al. 2006). Sufficient amount of carbon in easily consumable form, such as terpenes (Hernandez et al. 1997), is one of the necessities for improving the living conditions for dechlorinating microorganisms (Wiegel and Wu 2000; Ohtsubo et al. 2004; Aislabie et al. 2006). Furthermore, adequate amount of electron donors, such as nitrate (NO3 −), is crucial to the rate, extent and route of any anaerobic reductive dehalogenation process (Tiedje et al. 1993; Wiegel and Wu 2000; Abraham et al. 2002; Borja et al. 2005).
Studies on PCB remediation of northern circumpolar soils are scarce (Lambo and Patel 2007; Kalinovich et al. 2012) and in Iceland, in particular, very limited research exists on soil pollution and bioremediation (Meyles and Schmidt 2005). The goal of the study was to test biostimulation methods for reduction of soil PCB concentrations. The specific aims were to investigate whether (1) PCBs adsorbed to these soils are bioavailable, whether (2) biological degradation activity in Icelandic sub-arctic soils could be increased at ambient temperature with both conventional (fertiliser N) and unconventional (white clover and pine needle) amendments and whether (3) increased temperature would augment bioremediation potential of the soils in question. Considering that climate is already getting wetter and warmer in Iceland (Björnsson et al. 2008) with increasing soil temperatures, it is imperative to study how such changes affect the soils of northern latitudes and hence the fate of pollutants within soils.
Materials and methods
Scene setting
The former North Atlantic Treaty Organization facility and United States Naval Air Station Keflavík (NASKEF) was situated at Keflavík International Airport in Iceland from the WWII until the autumn of 2006 (Almenna Consulting Engineers 2008 and 2010, personal communication). PCBs, mainly Aroclor 1260, were used at NASKEF in transformer oils in great quantities and were stored in old machinery at the army sales facilities but were mainly phased out of use during early 1990s (Almenna Consulting Engineers 2010, personal communication). The soil from the research area (63°57′29″N, 22°34′59″W) was divided into three pollution categories: more than 50 ppm (in microgram per gram dry weight) PCB, 1–50 ppm PCB and less than 1 ppm PCB; bioremediation of this study was applied to soils containing 1–50 ppm PCBs. The groundwater around the study site has been confirmed to contain no detectable PCBs (ÍSOR 2008). The climate in the study area is relatively mild, cold temperate oceanic. The mean annual precipitation is 1,100 mm and temperature 4.4 °C (Icelandic Meteorological Office database 2010, personal communication).
Soil samples
Soils from the area have been previously described by Arnalds (2004) and Arnalds et al. (2009) and classified as Brown Andosols. Soils were sampled according to NORDTEST Technical Report No. 329 (Karstensen et al. 1997). At the laboratory, soils were sieved (2 mm), stored in glass jars at 4 °C in the dark and allowed to equilibrate for 2 weeks prior to the experiment. A control soil was sampled from the same area and confirmed not to contain any detectable PCBs. Soils used in this research are characterised in Table 1. Soil moisture content was calculated from oven-dried samples (105 °C) and water-holding capacity (WHC) was determined according to Smith and Mullins (2001). Soil pH (H2O) was measured in 1:5 soil/water suspension with a glass calomel electrode (Oakton pH/mV/°C Meter pH 1000 Series, Chicago, IL, USA). C tot and N tot were measured by thermal combustion (Elemental Analyzer Vario MAX CN, Analysensysteme GmbH Germany) and since the studied soils contained no carbonates, C tot was taken as C org. Cation exchange capacity (CEC) was determined according to Blakemore et al. (1987) using Sampletek Vacuum Extractor (Macro Industries, INC). The exchangeable bases (Na+, Mg+2, K+ and Ca+2) were determined by gas diffusion with FIALAB 3500B (Fialab Instruments, USA) and NH4-H in a flow injection FIAstar 5010 analyzer (Tecator, Hoganas, Sweden). The content (in percent) of ammonium oxalate extractable Al, Si, Fe and Mn were analysed by inductively coupled plasma optical emission spectrometry (Spectro, Germany). Allophane content was estimated by multiplying Si % by 6 (Parfitt 1990) and ferrihydrite by multiplying Fe % by 1.7 (Parfitt and Childs 1988).
Soil PCB analyses
Analysis of PCB in soil samples was undertaken according to NORDTEST Technical Report No. 329 guidelines (Karstensen et al. 1997, 1998). Following extraction and clean-up, the determination of total PCBs and individual PCB congeners were carried out with Agilent 6890 N GC with DB1701 column (60 m, 0.25 mm i.d., 0.25 μm film) equipped with an electron capture detector. A mixture of Aroclor 1260, 50, and 500 ppm in transformer oil (Accustandard, USA) were used to determine the total amount of PCBs by comparing and quantifying 20 different peaks in the mixture with a five-point standard curve (2, 10, 50, 100 and 500 ppm Aroclor 1260). The individual congeners were determined using nine individual PCB standards (#28, 52, 101, 118, 138, 153, 170, 180 and 187) from Accustandard, USA, using a six-point standard curve of 0.5, 2, 8, 25, 100 and 200 pg/μl of each congener. The method was originally developed by this laboratory, which also coordinated a ring test involving 21 laboratories (Karstensen et al. 1998). The method was tested using a PCB-contaminated certified reference soil (CRM481) from IRMM (BCR). The laboratory participates in two different quality assurance schemes every year, Quasimeme BT2 (PCBs and pesticides in marine biota) and AMAP (PCBs, pesticides, PBDEs in human serum), thus testing standard solutions.
Bioavailability of PCBs in soil to earthworms
The uptake of PCBs by earthworms (Eisenia foetida) was determined according to Hallgren et al. (2006). E. foetida is a widely used earthworm in bioavailability studies and results obtained may be considered as worst case scenarios (Hallgren et al. 2006). Earthworms and compost soil were confirmed to contain no PCBs. The earthworms were 3.0–9.5 cm long and weighed 0.11–0.67 g.
Microcosms were prepared by carefully mixing 20 g field moist polluted soil and 20 g of fresh compost soil in a glass jar. Deionised water (5 g) was added to achieve a suitably moist environment. Nine identical microcosms were prepared; three with unpolluted control soil, three with soil containing 25 ppm PCBs (final soil mixture, 12.5 ppm) and three with soil containing 50 ppm PCBs (final soil mixture, 25 ppm). Ten earthworms were weighed and added to each jar. The jars were covered with parafilm and placed in desiccators where the drying stones had been replaced by water to create stable humidity and no evaporation. The desiccators were stored in darkness at room temperature for a period of 10 days. After incubation, the earthworms were removed, rinsed and stored at −20 °C until analysis (Hallgren et al. 2006).
Extraction of PCBs from the earthworms was carried out by the Jensen extraction method (Jensen et al. 1983) as described in Ólafsdóttir et al. (1995), Jensen et al. (2003) and Ólafsdóttir et al. (2005). The total PCBs and individual PCB congeners were determined by gas chromatography as described above.
Presence of PCB-degrading bacterial genes and groups
Soil DNA was extracted in four replicates from 0.25 g of unpolluted control soil, soil containing 25 ppm of PCBs and soil containing 50 ppm of PCBs with PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA). Manufacturer’s instructions were followed except for disrupting the cells by beadbeating with FastPrep instrument for 30 s at speed 5.5 m s−1. Extracted DNA was quantified with Qubit Quant-iT dsDNA HS Assay kit by Qubit 1.0 fluorometer (Invitrogen, USA) and the quality was checked by agarose gel electrophoresis with ethidium bromide staining and NanoDrop ND1000 UV–vis spectrophotometer (NanoDrop Technologies, USA).
The presence of bacterial genes and taxa related to PCB degradation was tested by PCR with seven different previously published primer sets. Gene bphA has been associated with aerobic PCB degradation (Witzig et al. 2006), genes fcbA, fcbB and ohb with degradation of PCB dechlorination products (Rodrigues et al. 2001, 2006) and gene cbrA with anaerobic dechlorination by Dehalococcoides (Watts et al. 2005). Taxon-specific 16S ribosomal RNA gene primers were used to target the dechlorinating members of Chloroflexi (Wagner et al. 2009). The primer sequences and annealing temperatures are presented in Table 2. One microliter of the DNA extract (0.02–0.86 ng of DNA) was used as template with duplicate PCR reactions for each DNA extract. The reaction mixtures with final volume of 50 μl contained 0.2 mM of each dNTP (Finnzymes, Finland), 0.5 mM of both primers (Oligomer, Finland), 0.05 % of bovine serum albumin (BSA acetylated, Promega), 1 × Biotools reaction buffer with 2 mM MgCl2 and 1 U of DNA polymerase (Biotools, Spain). Peltier Thermal Cycler DNA Engine (MJ Research) was used for the amplification with the following programme: initial denaturation at 95 °C for 5 min, followed by 35 cycles (40 cycles for cbrA) of denaturation at 94 °C for 45 s, annealing at variable temperature for 1 min and elongation at 72 °C for 2 min. The presence and size of PCR product was checked on a 1.5 % agarose gel.
Bioremediation amendments
Soils used for bioremediation treatments were carefully mixed from polluted soil and control soil with a final concentration of 27 ppm PCBs (analysed in three technical replicates). Soils were incubated (aerobically and anaerobically) at 10 and 30 °C in heat-controlled incubators for 2 months in order to see if higher temperature increases the degradation of PCBs. Treatments included autoclaved sterile control, active control without amendments, 50 kg N ha−1, 100 kg N ha−1, pulverised white clover (Trifolium repens; 0.5 g dry mass in 10 g field moist soil) and pulverised pine needles (Pinus contorta; 0.5 g dry mass in 10 g field moist soil). After the additions, 10 g of field moist soil mixture was transferred to five replicate 20-ml amber vials (Agilent Technologies, Germany) that served as microcosms. Microcosms were covered with ultraclean screw cap with septa (Agilent technologies, Germany). The aerobic samples were adjusted to 60 % WHC for optimal microbial activity (Alexander 1999) and only closed loosely in order to let air in continuously to the samples. Anaerobic conditions were obtained by addition of 10 ml deionized water into the microcosms.
Soil dehydrogenase activity
Dehydrogenase enzymes are involved in the aerobic degradation of PCBs (Ohtsubo et al. 2004) through the biphenyl pathway. Dehydrogenase activity was determined according to the modified method of Trevors (1984). One gram of soil was placed into sterilised and foil-covered plastic 50-ml centrifuge bottles (Sarstedt, Germany). Ten milliliter of sterile substrate solution (0.1 % p-iodonitrotetrazolium chloride) and 0.5 M TES buffer (adjusted to pH 7.8 with 0.5 M NaOH) was added and samples were placed on an end-over shaker for 18 h at room temperature. After shaking, 10 ml of ethanol was added and samples were centrifuged (Universal 320R, Hettich, Germany) for 20 min at 4 °C and 2,700×g. Absorbance of the supernatant at 490 nm was measured with a linear spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences, Sweden). Standard curves were determined using 1, 2, 3 and 5 ppm iodonitetrazolium formazan.
Data analyses
Basic statistical analyses of the results were performed with SAS 9.1 for Windows and Excel Analysis ToolPak. In addition, PCB data was analysed with a non-parametric multivariate test based on Gower dissimilarity that gives equal weights to the different variables (congeners). The significance of the difference in congener abundance was evaluated by permutation test in the CAP programme (Canonical Analysis of Principal Coordinates, mode: Generalised Discriminant Analysis) available at http://www.stat.auckland.ac.nz/~mja/Programs.htm (Anderson and Robinson 2003). Statistical testing was based on 9,999 permutations.
Results
Bioavailability of PCBs in soil to earthworms
The earthworms in the control soil appeared to be in good condition throughout the study whereas the earthworms in the polluted soils faced higher mortality. Of the 30 earthworms added in the beginning of the experiment, only five remained alive in the 12.5 ppm PCB soil and nine in the 25 ppm PCB soil, compared to all remaining alive in the control soil. The PCB accumulation in earthworms, in 12.5 ppm PCB soil and 25 ppm PCB soil, respectively, is presented on the x-axis of Fig. 1a. Bioaccumulation factor (BAF, PCB concentration in earthworms; in milligram per gram of fresh weight) divided by PCB concentration in the soil (in milligram per gram of dry weight) for earthworms was 0.89 and 0.82 for earthworms in 12.5 ppm PCB soil and in 25 ppm PCB soil, respectively. The earthworms accumulated both higher chlorinated and less chlorinated congeners (Table 3). Congener abundance and proportions, as evaluated by generalised multivariate discriminant analysis without and with data standardisation, were significantly different in the two PCB levels (P = 0.027 and P = 0.016, respectively; Fig. 1). Earthworms accumulated PCB 153 in greatest quantity, as it was the congener of the highest concentration in both soil mixtures. The highly chlorinated PCB 153, 180 and 187 were accumulated in great quantity, as well as PCB 138 and 101. However, BAFs were highest for the least chlorinated congeners (Table 3).
a Distribution of the earthworm samples on the single canonical axis formed with discriminant analysis, testing the hypotheses that PCB congener uptake differs in E. foetida incubated in soil with different PCB contamination level (permutated P value 0.027). x-Axis is the total PCB uptake, which was below detection limit in worms incubated in clean control soil. b Correlations of the individual PCB congeners with the same canonical axis
Presence of PCB degrading bacterial genes and groups
Soil DNA concentration (average ± standard deviation) varied from 2.14 ± 1.57 μg g−1 soil fresh weight in unpolluted control soil to 0.13 ± 0.04 in soil containing 25 ppm of PCBs to 0.36 ± 0.06 in soil containing 50 ppm of PCBs. A single strong PCR product of the expected size (approximately 500 bp) was systematically produced with bphA primers from all the PCB-contaminated soil extracts with no or faint products from the unpolluted control soil. No PCR products were amplified from either contaminated or control soil DNA extracts with any of the primers related to dechlorination or degradation of dechlorination products.
PCB degradation and biological activity
PCB bioremediation trial
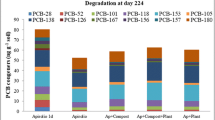
Large variation in total PCB concentration was observed after the bioremediation trial, possibly due to treatment effects on the extractability of the aged PCBs. Best reduction in total PCBs was obtained in the aerobic pine needle treatment at 10 °C, which yielded a 38 % reduction in the total PCBs after 2 months, final concentration being 16.9 ppm (95 % confidence interval, 16.1–17.6 ppm) compared to 27.0 ppm at the beginning of the experiment. For the pine needle treatment, a reduction compared to sterile and non-sterile controls was observed also at aerobic 30 °C, whereas the other additives did not seem to improve PCB degradation in any incubation condition (Table 4).
The abundance of the different PCB congeners was significantly different in the untreated, anaerobically treated and aerobically treated samples (permutated P = 0.001). In the constrained ordination, the aerobically treated samples received generally higher scores than the anaerobically treated ones on the canonical axis 1, with which congeners 28, 52 and 118 showed negative correlation (Fig. 2). Best reduction in total PCBs was obtained in the aerobic pine needle treatment at 10 °C, which yielded a 38 % reduction in the total PCBs after 2 months, final concentration being 16.9 ppm compared to 27.0 ppm at the beginning of the experiment (Table 4).
Canonical plots showing the results of generalised discriminant analysis for PCB congener abundance in bioremediation experiment. a Visualises the distribution (scores) of the original and bioremediated-contaminated soil samples in constrained ordination, testing the hypothesis that PCB composition differs in untreated, anaerobically treated and aerobically treated samples (permutated P value 0.001). Dash line circles the aerobic pine needle treatments. b The correlation of the individual PCB congeners with the same canonical axes, illustrating their association to the separation of the differently treated samples
Soil dehydrogenase activity
The highest values for dehydrogenase activity were measured for white clover treatments and the second highest for the pine needles treatment (Fig. 3). All treatments with fertiliser addition (50 and 100 kg N ha−1) resulted in decreased dehydrogenase activity. Activity was typically higher at 10 °C than at 30 °C. In general, PCB concentration had a significant negative correlation with dehydrogenase activity (Pearson r = −0.67, p < 0.001).
Dehydrogenase activity before and after different bioremediation treatments at 10 and 30 °C. Line mean value of three replicate and columns represent a bulked sample from three samples. A aerobic, AN anaerobic, 1 sterile control, 2 active control, 3 50 kg N ha−1, 4 100 kg N ha−1, 5 white clover and 6 pine needles
Discussion and conclusions
Bioavailability of PCBs in soil to earthworms
PCBs in the studied soils were bioavailable to earthworms (E. foetida) increasing the risk of the mobility of pollutants to the surrounding environment. Earthworms process a large quantity of soil daily and in addition they may absorb pollutants through their thin external barrier, which may increase the bioaccumulation of PCBs in the food web when higher organisms, such as birds, consume earthworms (Ville et al. 1995). Explanations for the observed high bioavailability include the low organic matter content of the soils (e.g. Wågman et al. 2001), the low CEC of the studied soils compared to Icelandic soils in general (Sigurgeirsson et al. 2005) as well as lower allophane and ferrihydrite contents than reported by Guicharnaud and Paton (2006). Ideally, earthworms will be able to release some of the most recalcitrant parts of the pollutants and at the same time enhance the soil properties including porosity and aeration of the soil. In this study, the earthworms were able to absorb and accumulate both highly and less chlorinated PCB congeners (Fig. 1, Table 3). This is in accordance with other studies done on Aroclor mixtures (e.g. Tharakan et al. 2006). Interestingly, we found bioaccumulation factors to vary for the different congeners. BAF correlated negatively (r = −0.766, P = 0.01) with the number of chlorines, suggesting that the test in fact indicated not higher accumulation but higher bioavailability of the less chlorinated congeners. To our knowledge, this is the first report of systematic patterns observed in BAFs for different PCB congeners.
PCB degradation and biological activity
Aroclor 1260 has previously been shown not to be susceptible to aerobic biodegradation (Crawford and Crawford 2005). In contrast, our study found aerobic biostimulation at 10 °C with pine needles result in 38 % reduction of total PCBs in 2 months. The success with pine needles could be explained by the increase in dehydrogenase activity and the terpenes in the pine needles acting like a natural substrate for biphenyl-degrading bacteria (Herdandez et al. 1997; Park et al. 1999). Lack of degradation due to white clover amendment may be related to flavonoids not being able to function as growth substrates for PCB-degrading bacteria as described in previous studies (Donnelly et al. 1994; Pieper 2005). Preliminary experiment treatments with plant detritus and roots did not result in degradation of the total PCBs in the samples, which could indicate lack of easily available nitrogen for the microorganisms (Michel et al. 2001). Fertiliser treatments did not decrease total PCBs, possibly due to fertiliser adsorption to the allophane and ferrihydrite surfaces, which in turn would make the nutrients less available for active soil microorganisms (Shoji et al. 1993).
PCB degradation by cold-adapted bacteria has been reported previously (e.g. Welander 2005; Lambo and Patel 2007) and biodegradation in cold environments, including frozen soils (Aislabie et al. 2006), is far from impossible. Factors enhancing biodegradation are 60 % WHC (Aislabie et al. 2006), close to neutral soil pH (Wiegel and Wu 2000; Fava et al. 2003) and temperature (Wu et al. 1997; Guicharnaud et al. 2010). In this study, increase in temperature benefitted neither PCB degradation nor microbial dehydrogenase activity. The increase in dehydrogenase activity obtained after addition of white clover and pine needles is in agreement with Wilke and Bräutingam (1992). The observed strong amplification of the aerobic bphA gene furthermore indicates the indigenous capability of the soils to aerobically degrade PCBs. In aerobic oxidative PCB degradation, bphA encodes the first fundamental step of the biphenyl upper pathway, in which biphenyl is converted to dihydrodiol and further to CBA. Our inability to detect any genes related to anaerobic PCB degradation potential corroborates the negative results received with the anaerobic treatments. Together, these observations suggest that reductive dehalogenation might require bioaugmentation of effective anaerobic degrader bacteria into the studied soils.
Conclusions
This study demonstrated a 38 % aerobic biodegradation of PCBs with pine needle biostimulation at 10 °C in 2 months. These results give an indication that bioremediation at average Icelandic summer field temperatures, with appropriate biostimulation, could be feasible. This is supported by reported findings of the study by Guicharnaud et al. (2010), which showed that the biological properties of the Icelandic soils are adapted to work at low temperatures and governed by substrate availability to microorganisms rather than temperature. Earthworms accumulated both less and higher chlorinated PCBs effectively, which indicates their bioavailability in the studied Icelandic soils as well as risk of biomagnification of PCBs in the food chain.
The success with pine needle treatment is of great significance for bioremediation in cold environments: (1) since Aroclor 1260 has been considered as recalcitrant for aerobic degradation and for any microbial degradation previously, (2) the treatment working best at 10 °C gives good indications for remediation being successful at higher latitudes and (3) terpenes, which are present in pine needles, may be seen as a feasible and sustainable stimulation method since they are natural materials, easily available in the northern circumpolar region and they can promote bioavailability of PCBs. Therefore, further research should focus on field-scale biostimulation trials with pine needles in order to optimise the method for onsite remediation.
References
Abraham W-R, Nogales B, Golyshin PN, Pieper DH, Timmis KN (2002) Polychlorinated biphenyls-degrading microbial communities in soils and sediments. Curr Opin Microbiol 5:246–253
Aislabie J, Saul DJ, Foght JM (2006) Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles 10:171–179
Alexander M (1999) Biodegradation and bioremediation, 2nd edn. Academic, California, USA
Anderson MJ, Robinson J (2003) Generalized discriminant analysis based on distances. Aust New Z J Stat 45:301–318
Arnalds O (2004) Volcanic soils of Iceland. Catena 56:3–20
Arnalds O, Óskarsson H, Gísladóttir FO, Grétarsson E (2009) Soil map of Iceland. The Agricultural University of Iceland, Reykajvik, Iceland
BEST (2001) A risk management strategy for PCB-contaminated sediments. Washington D. C., USA: Committee on Remediation of PCB-Contaminated Sediments, Board on Environmental Studies and Toxicology, National Research Council
Björnsson H, Sveinbjörnsdóttir AE, Danielsdóttir AK, Snorrason Á, Viggósson G, Sigurjónsson J, Baldursson S, Þorvaldsdóttir S, Jónsson T (2008) Hnattrænar loftlagsbreytingar og áhrif þeirra á Íslandi (Global climate change and its influence in Iceland). Icelandic Ministry for the Environment. 118 p. [In Icelandic]
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils: New Zealand Soil Bureau Science Report 80. New Zealand Soil Bureau, New Zealand
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated biphenyls and their biodegradation. Process Biochem 40:1999–2013
Crawford RL, Crawford DL (eds) (2005) Bioremediation: Principles and Applications. Cambridge University Press, New York, USA
Donnelly PK, Hedge RS, Fletcher JS (1994) Growth of PCB-degrading bacteria on compounds from photosynthetic plants. Chemosphere 28(5):981–988
Erickson MD (1997) Analytical chemistry of PCBs, 2nd edn. CRC Press LLC, Florida, USA
Fagervold SK, May HD, Sowers KR (2007) Microbial Reductive Dechlorination of Aroclor 1260 in Baltimore Harbor Sediment Microcosms Is Catalyzed by Three Phylotypes within the Phylum Chloroflexi. Appl Environ Microbiol 73(9):3009–3018
Fava F, Bertin L, Fedi S, Zannoni D (2003) Methyl-beta-cyclodextrin-enhanced solubilization and aerobic biodegradation of polychlorinated biphenyls in two aged-contaminated soils. Biotech Bioeng 81:381–390
Gomes HI, Dias-Ferreira C, Ribeiro AB (2013) Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci Total Environ 445–446:237–260
Guicharnaud R, Paton GI (2006) An evaluation of acid deposition on cation leaching weathering rates of an Andosol and Cambisol. J Geochem Explor 88:279–283
Guicharnaud R, Arnalds O, Paton GI (2010) Short term changes of microbial processes of Icelandic soils to increasing temperatures. Biogeosciences 7:671–682
Hallgren P, Westbom R, Nilsson T, Sporring S, Björklund E (2006) Measuring bioavailability of polychlorinated biphenyls in soil to earthworms using selective supercritical fluid extraction. Chemosphere 63:1532–1538
Hernandez BS, Koh S-C, Chial M, Focht DD (1997) Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation 8:153–158
ÍSOR – Iceland Geosurvey (2008) Sýnataka og efnagreiningar á grunnvatni úr fjórum holum á Keflavíkurflugvelli (sampling and analyses of groundwater from four holes at the Kaflavik airport). ÍSOR [In Icelandic], Reykjavík
Jensen S, Reutergårdh L, Janson B (1983) Analytical methods for measuring organochlorines and methyl mercury by gas-chromatography. FAO/SIDA Manual of methods in aquatic environment research. Part 9. Analysis of metals and organochlorines in fish. FAO Fish Tech Paper 212:21–33
Jensen S, Häggberg L, Jörundsdóttir H, Odham G (2003) A quantitative lipid extraction method for residue analysis of fish involving nonhalogenated solvents. J Agric Food Chem 51:5607–5611
Jones A, Stolbovoy V, Tarnocai C, Broll G, Spaargaren O, Montanarella L (eds) (2010) Soil Atlas of the Northern Circumpolar Region. European Commission, Publications Office of the European Union, Luxemburg. 144 pp
Jörundsdóttir H (2009) Temporal and spatial trends of organohalogens in guillemot (Uria aalge) from North Western Europe. Dissertation, Stockholm University, Sweden
Jota MA, Hassett JP (1991) Effects of environmental variables on binding of a PCB congener by dissolved humic substances. Environ Toxicol Chem 10:483–491
Kalinovich IK, Rutter A, Rowe RK, Poland JS (2012) Design and application of surface PRBs for PCB remediation in the Canadian Arctic. J Environ Manag 101:124–133
Karstensen KH, Ringstad O, Rustad I, Kalevi K, Jörgensen K, Nylund K, Alsberg T, Ólafsdóttir K, Heidenstam O, Solberg H (1997) Nordic Guidelines for Chemical Analysis of Contaminated Soil Samples. NORDTEST Technical Report 329. Oslo, Norway: SINTEF Applied Chemistry
Karstensen KH, Ringstad O, Rustad I, Kalevi K, Jörgensen K, Nylund K, Alsberg T, Ólafsdóttir K, Heidenstam O, Solberg H (1998) Methods for chemical analysis of contaminated soil samples - tests of their reproducibility between Nordic laboratories. Talanta 46:423–437
Kuipers B, Cullen WR, Mohn WW (2003) Reductive dechlorination of weathered Aroclor 1260 during anaerobic biotreatment of Arctic soils. Can J Microbiol 49:9–14
Lambo AJ, Patel TR (2007) Biodegradation of polychlorinated biphenyls in Aroclor 1232 and production of metabolites from 2,4,4′-trichlorobiphenyl at low temperature by psychrotolerant Hydrogenophaga sp strain IA3-A. J Appl Microbiol 102:1318–1329
Margesin R (2007) Alpine microorganisms: useful tools for low-temperature bioremediation. J Microbiol 4:281–285
Master ER, Mohn WW (1998) Psychrotolerant bacteria isolated from Arctic soil that degrade polychlorinated biphenyls at low temperatures. Appl Environ Microbiol 64:4823–4829
Meyles CA, Schmidt B (2005) Report on Soil Protection and Remediation of Contaminated Sites in Iceland: A preliminary study. Environment and Food Agency of Iceland (UST), Reykjavik, Iceland
Michel Jr FC, Quensen J, Reddy CA (2001) Bioremediation of a PCB-contaminated soil via composting. Compost Science & Utilization 9:274–284
Mohn WW, Westerberg K, Cullen WR, Reimer KJ (1997) Aerobic biodegradation of biphenyl and polychlorinated biphenyls by Arctic soil microorganisms. Appl Environ Microbiol 63:3378–3384
Ohtsubo Y, Kudo T, Tsuda M, Nagata Y (2004) Strategies for bioremediation of polychlorinated biphenyls. Appl Microbiol Biotechnol 65:250–258
Ólafsdóttir K, Petersen A, Thórdadóttir S, Jóhannesson T (1995) Organochlorine residues in Gyrfalcons (Falco rusticolus) in Iceland. Bull Environ Contam Toxicol 55:382–389
Ólafsdóttir K, Petersen A, Magnusdóttir EV, Björnsson T, Jóhannesson T (2005) Temporal trends of organochlorine contamination in Black Guillemots in Iceland from 1976 to 1996. Environ Pollut 133:509–515
Parfitt RL, (1990) Allophane in New Zealand – A review. Aust J Soil Res 28:343–360
Parfitt RL, Childs CW, (1988) Estimation of forms of Fe and Al: A review, and analysis of contrasting soils by dissolution and moessbauer methods. Aust J Soil Res 26:121–144
Park Y-I, So J-S, Koh S-C (1999) Induction by carvone of the polychlorinated biphenyl (PCB)-degradative pathway in Alcaligenes eutrophus H850 and its molecular monitoring. J Microbiol Biotechnol 9(6):804–810
Pieper DH (2005) Aerobic degradation of polychlorinated biphenyls. Appl Microbiol Biotechnol 67:170–191
Rodriques JLM, Maltseva OV, Tsoi TV, Helton RR, Quensen JF III, Fukuda M, Tiedje JM (2001) Development of a Rhodococcus recombinant strain for degradation of products from anaerobic dechlorination of PCBs. Environ Sci Technol 35:663–668
Rodriques JLM, Kachel CA, Aiello MR, Quensen JF, Maltseva OV, Tsoi TV, Tiedje JM (2006) Degradation of Aroclor 1242 dechlorination products in sediments by Burkholderia xenovarans LB400(ohc) and Rhococcus sp. Strain RHA1(fcb). Appl Environ Microbiol 4:2476–2482
Ross G (2004) The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Saf 59:275–291
Safe SH (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemicals and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87–149
Shoji S, Nanzyo M, Dahlgren R (1993) Volcanic ash soils: Genesis, Properties, and Utilization. Developments in Soil Science: 21. Elsevier, The Netherlands
Sigurgeirsson MA, Arnalds O, Palsson SE, Howard BJ, Gudnason K (2005) Radioceasium fallout behaviour in volcanic soils in Iceland. J Environ Radioact 79(1):39–53
Smith KA, Mullins CE (eds) (2001) Soil and Environmental Analysis: Physical methods, 2nd edn. Marcel Dekker, New York
Tharakan J, Tomlinson D, Addagada A, Shafagati A (2006) Biotransformation of PCBs in contaminated sludge: potential for novel biological technologies. Eng Life Sci 6:43–50
Tiedje JM, Quensen JF III, Chee-Sanford J, Schimel JP, Cole JA, Boyd SA (1993) Microbial reductive dechlorination of PCBs. Biodegradation 4:231–240
Trevors JT (1984) Dehydrogenase activity in soil: a comparison between the INT and TTC assay. Soil Biol Biochem 16:673–674
Ulbrich B, Stahlmann R (2004) Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol 78:252–268
UNEP/AMAP (2011) Climate Change and POPS : Predicting the Impacts. Report of the UNEP/AMAP Expert Group. Secreteriat of the Stockholm Convention, Geneva, p 62
Vasilyeva GK, Strijakova ER (2007) Bioremediation of soils and sediments contaminated by polychlorinated biphenyls. Microbiology 76:639–653
Ville P, Roch P, Cooper EL, Masson P, Narbonne J-F (1995) PCBs increase molecular-related activities (Lysozyme, Antibacterial, Hemolysis, Proteases) but inhibit macrophage-related functions (Phagocytosis, Wound Healing) in earthworms. J Invertebr Pathol 65:217–224
Wågman N, Strandberg B, Tysklind M (2001) Dietary uptake and elimination of selected polychlorinated biphenyls congeners and hexachlorobenzene in earthworms. Environ Toxicol Chem 8:1778–1784
Wagner A, Adrian L, Kleinsteuber S, Andreesen JR, Lechner U (2009) Transcription analysis of genes encoding homologues of reductive dehalogenases in “Dehaloccoides” sp. Strain CBDB1 by using terminal restriction fragment length polymorphism and quantitative PRC. Appl Environ Microbiol 7:1876–1884
Watts JEM, Fagervold SK, May HD, Sowers KR (2005) A PCR-based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151:2039–2046
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Welander U (2005) Microbial degradation of organic pollutants in soil in a cold climate. Soil Sed Contam 14:281–291
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated buphenyls. FEMS Microbiol Ecol 32:1–15
Wilke B-M, Bräutigam L (1992) Effects of polychlorinated biphenyls on soil microbial activity. Zur Pflanzenernährung och Bodenkultur 155:483–488
Witzig R, Junca H, Hecht H-J, Pieper DH (2006) Assessment of toluene/biphenyl dioxygenase gene diversity in benzene-polluted soils: link between benzene biodegradation and genes similar to those encoding isopropylbenzene dioxygenases. Appl Environ Microbiol 5:3504–3514
Wu Q, Bedard DL, Wiegel J (1997) Temperature determines the pattern of anaerobic microbial dechlorination of Aroclor 1260 primed by 2,3,4,6-tetrachlorobiphenyl in Woods Pond sediment. Appl Environ Microbiol 63:4818–4825
Zharikov GA, Borovick RV, Kapranov VV, Kiseleva NI, Krainova OA, Dyadishcheva VP, Shalanda AV, Zharikov MG (2007) Study of contamination and Migration Polychlorinated biphenyls in the environment. Bioremediation of contaminated soils and assessment of their impact on the Serpukhov population health. In: Heipieper HJ (ed) Bioremediation of Soils Contaminated with Aromatic Compounds. Springer, Amsterdam, The Netherlands, pp 93–104
Acknowledgments
The authors are grateful for Kadeco, Rio Tinto Alcan, Nordplus Express Mobility Grant, University of Iceland Graduate Travel Grant and SoilSoc–Nordic Network on Soils and Society for financial support for the project. Elin V. Magnusdóttir at the University of Iceland and Sunna Áskelsdóttir at the Agricultural University of Iceland are acknowledged for assistance and guidance in the laboratory work. Kristina Lindström at the University of Helsinki is thanked for senior advice and microbiological laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Rights and permissions
About this article
Cite this article
Lehtinen, T., Mikkonen, A., Sigfusson, B. et al. Bioremediation trial on aged PCB-polluted soils—a bench study in Iceland. Environ Sci Pollut Res 21, 1759–1768 (2014). https://doi.org/10.1007/s11356-013-2069-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2069-z