Abstract
Phthalate acid esters (PAE), commonly named phthalates, are toxics classified as endocrine-disrupting compounds; they are primarily used as additives to improve the flexibility in polyvinyl chloride. Many studies have reported the occurrence of phthalates in different environmental matrices; however, none of these studies has yet established a complete overview for those compounds in the water cycle within an urban environment. This review summarizes PAE concentrations for all environmental media throughout the water cycle, from atmosphere to receiving waters. Once the occurrences of compounds have been evaluated for each environmental compartment (urban wastewater, wastewater treatment plants, atmosphere, and the natural environment), we reviewed data in order to identify the fate of PAE in the environment and establish whether geographical and historical trends exist. Indeed, geographical and historical trends appear between Europe and other countries such as USA/Canada and China, however they remain location dependent. This study aimed at identifying both the correlations existing between environmental compartments and the processes influencing the fate and transport of these contaminants into the environment. In Europe, the concentrations measured in waterways today represent the background level of contamination, which provides evidence of a past diffuse pollution. In contrast, an increasing trend has actually been observed for developing countries, especially for China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most studies, published during the last three decades, have reported several categories of man-made chemicals, classified as “endocrine-disrupting compounds” (EDCs). Among the most frequently cited EDCs, phthalates are of particular concern due to their ubiquity and generally higher levels found in environment comparatively to other EDCs (Staples et al. 1997; Fatoki and Mathabatha 2001; Fauser et al. 2003; Sanchez-Avila et al. 2009). The predominant use of phthalic acid esters (PAE) is for improving flexibility in polyvinyl chloride (PVC; Giam et al. 1984). For instance, the amount of di-(2-ethylhexyl) phthalate (DEHP) in PVC depends on plastic composition. Some products can contain up to 50 %, but typically there will be approximately 30 % DEHP in most PVC products (Ranke 2005). Moreover, their applications extend to industrial and/or domestic sectors, depending on their molecular weight (Table 1). Low molecular weight, especially dimethyl phthalate (DMP) and diethyl phthalate (DEP) have therefore been incorporated into cosmetics, fragrance, and other personal care products. Besides, di-n-butyl phthalate (DnBP) is also used in epoxy resins, cellulose esters, and special adhesive formulations. Additionally, high molecular weight and branching alkyl chain PAEs, primarily butyl benzyl phthalate (BBP), di-n-octyl phthalate (DnOP), and DEHP are being incorporated into food packaging, building materials, home furnishing, clothing, and medical products (Staples et al. 1997; Cespedes et al. 2004; Jackson and Sutton 2008; Liu et al. 2010a). Despite their various uses, PAE have in recent studies leveled off in American, Canadian, and European production, mainly as a result of highly restrictive regulations (EU 2004, 2005, 2007, 2008; CPSI 2008), while worldwide output has been stagnating to 6,000,000 t in 2006 (Peijnenburg and Struijs 2006). This difference may stem from the increase in consumption from emerging nations, such as Brazil, Russia, India, and China. The European and North American productions have however evolved because of the replacement of DEHP by heavier and more stable phthalates (di-iso-nonyl phthalate and di-iso-decyl phthalate) therefore less subject to release into the environment.
Despite their societal and economical benefits, PAE consumption has lead to the release of PAE into the environment, where they are now ubiquitous and can be found in air, water, sediments and soils. This study focuses more specifically on six most commonly studied congeners of this family (Table 1): DMP, DEP, DnBP, BBP, DnOP, and DEHP. Most studies in the literature examine the occurrence and behavior of these six compounds in various environmental matrices (surface water, wastewater, atmosphere, and treated water) given that these compounds are regularly found in urban and environmental compartments at significant levels (tens or more milligram per liter; Fauser et al. 2003; Cespedes et al. 2004; Vethaak et al. 2005; Peijnenburg and Struijs 2006; Abb et al. 2009).
Strategy of this review
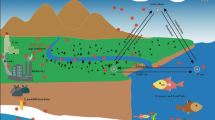
Most of the literature on PAEs in the environment, as published since 1990 (Staples et al. 1997; Vethaak et al. 2005; Deblonde et al. 2011) have been concerned with one or more compartments, primarily downstream compartments such as wastewater treatment plant (WWTP) effluent and receiving waters, but none of these works has dealt with all environmental compartments, in particular urban wastewater, nor with PAE behavior throughout the whole water cycle. As similarly done for the meta-analysis of environmental contamination by alkylphenols (Bergé et al. 2012a), the purpose of this review is to collect a substantial dataset for all compartments (Fig. 1) from the atmosphere (gaseous phase and rain water) and extending to urban areas (industrial, residential, and man-made wastewater, WWTP influent and effluent, WWTP sludge, and storm water) and the natural environment (surface water, sediments, and soils). Unfortunately, the set of congeners reported in the various papers has not been consistent and equivalent between compartments. Some papers have reported on as few as one or two congeners (DEHP, DnBP, or DEP), while others have reported on four or six (DMP, DEP, DnBP, BBP, DEHP, and DnOP). In this review, PAE refers systematically to the sum of these six congeners. From the database we built, statistical calculations have been derived for certain sample sets. The year of sample extraction was not specified in all references, so it was assumed that the samples were extracted on the article publication date. It was also decided that this review focused exclusively on environmental contamination and not on biota or bioaccumulation processes. Moreover, the concentration ranges reported in all tables correspond to minimal and maximal concentrations of each compound for each compartment. The other values represent median concentrations calculated by authors. This review is also aimed at determining whether temporal and/or geographical trends can be drawn in PAE contamination as observed for alkylphenols (Bergé et al. 2012a). To achieve this objective, the data were examined from geographical perspective. Recommendations for further research will also be provided.
Interactions between the various environmental compartments. Cited from Bergé et al. (2012a)
Atmosphere
Air contamination
PAE in the atmosphere or in urban areas are generated by various emission sources, including volatilization from materials, industrial processes, waste combustion, and wastewater treatment processes (Salapasidou et al. 2011). It has been calculated that approximately 4 % of the quantity of DEHP can evaporate from materials such as floorings, rain clothes, toys, and soles of shoes (Ranke 2005). DEHP was indeed detected in aerosols emitted from the aeration tank of a WWTP ranging from 71.1 to 228 ng/m3 (Lepri et al. 2000). As shown in Table 2, a small number of air samples (10 references in all) has been reported for PAE. From data collected, PAE contamination globally lies in the 1–50 ng/m3 range for all congeners, except for DEHP presenting higher levels (up to 3,640 ng/m3). The analysis of this database reveals that European samples were consistently above the values reported in the world (USA, China, etc.), and DEHP was the most abundant phthalate in air, with concentrations ranging from 0.08 to 3,640 ng/m3 (Müller et al. 2003). Recent studies conducted by Tlili et al. (2010) and Teil et al. (2006), in the Parisian area (France), underscored that phthalates are preferentially associated with the gaseous phase rather than aerosols. For instance, Tlili et al. (2010) reported that between 60–70 % and 40–30 %, respectively, of phthalates are associated to gaseous phase. In addition, the same authors reported that the phthalates exhibiting short alkyl chains and high vapor pressures (especially, DMP and DEP) are predominant in the gaseous phase and inversely for the others compounds. Study conducted by Xie et al. (2005) showed that the air–sea exchange was preferentially dominated by the deposition, especially for DnBP (−60 to −686 ng/m2/day) and BBP (−4 to −28 ng/m2/day). Additionally to this latter point, the fluxes calculated for DEHP (−95 to 686 ng/m2/day) highlighted a more complicated mechanism. Moreover, DnBP and DEHP total (dry and wet) depositions, to the Great Lakes (Canada), provided by Eisenreich et al. (1981) were estimated at 3.7 and 16 t per year, respectively. This indicates that the atmosphere could be one of the major contamination sources of PAEs. However, according to Staples et al. (1997) and more recently to Xie et al. (2007), phthalates are subject to photodegradation and therefore generally do not persist in the atmosphere. Authors reported photodegradation half-lives of common phthalates ranging from 0.3 to 15 days. A study led by Salapasidou et al. (2011) underlined that concentrations of DEHP were significantly higher on urban traffic areas (4.63–45.0 ng/m3; median, 19.4 ng/m3) than on urban industrial site (up to 6.50 ng/m3; median, 2.80 ng/m3) implying generally an input from vehicular emissions. Similarly, Wang et al. (2008) reported that concentrations were about 3.5 higher above urban sites than above suburban sites. These authors attributed this difference to both many point sources and environmental recycling. Additionally, Dargnat (2008) underlined the presence of seasonal variability above the Parisian area, with smaller concentrations for spring and during summer. This was previously observed by Guidotti et al. (2000) in Italy and Teil et al. (2006) in France above the same area. Due to the limited number of studies available, no distinct temporal or geographical trend could be drawn.
Rain water
A few measurements of PAE in rain water have been undertaken (see Table 3, ten references in all). Outdoor levels of DEHP lie on the order of 0.02–39.0 and 0.03–11.0 μg/l for DnBP. At the scale of Europe, data have shown that PAE concentrations in rain water seem to decline, which implies that the environment in European countries is exposed to decreasing contamination. In addition, data show that PAE concentrations in rain water are more important in Northern European countries than Southern European countries, which implies that the fate of PAE in the atmosphere could be governed by atmospheric circulation. A similar phenomenon has been reported for alkylphenols (Bergé et al. 2012a). Dargnat (2008) emphasized that passive volatilization from buildings, in urban areas, was the prevailing source of phthalate in rain water. Author therefore concluded that phthalate uses in building materials constitutes a significant source of contamination. Finally, Vethaak et al. (2005), in the Netherlands, reported that most of phthalates present in rain water were at concentrations comparable to those in surface water.
Urban areas
Sewer contamination
Industrial, man-made, and residential wastewater
As reported in Table 4 and Fig. 2, only a few measurements of PAE have been performed in wastewater (four to five references for each type of wastewater). Industrial DEHP levels were in the 0.01–4,400 μg/l range (median, 34.6 μg/l), i.e., twice as low as levels in residential wastewater (3.30–160 μg/l; median, 61.3 μg/l) and in man-made wastewater (0.60–470 μg/l; median, 66.0 μg/l). In Europe and North America, it has been reported that high DEHP and other phthalate concentrations were measured in industrial wastewater. In the USA, for instance, DEHP levels of 4,400 μg/l were measured in untreated industrial wastewater (Clark et al. 2003). Similarly, Jackson and Sutton (2008) quoted DEHP, DEP, and DnBP levels reaching 2,700, 100, and 120 μg/l in industrial laundry and adhesive manufacturer wastewaters. Both studies led by Vethaak et al. (2005) and Sanchez-Avila et al. (2009) showed that levels between 45 and 100 μg/l have also been measured in residential wastewater in the Netherlands and Spain. Some authors mentioned that the high concentrations measured in Maresme wastewater (Catalonia, Spain) and Parisian sewer network (France) would point out that PAE are still being produced and used in industrial, household, and agricultural formulations despite the implementation of European regulations (EU 2004, 2005, 2007) restricting their use within the EU to levels <0.1 % (Sanchez-Avila et al. 2009; Bergé et al. 2012c).
Wastewater treatment plant influent
As opposed to industrial, man-made, and residential wastewater, a large number of WWTP samples have been analyzed for PAEs (see Table 5, i.e., 13 references). Moreover, according to these concentrations, the PAE median levels reported for WWTP influent were in the same order of magnitude than residential and man-made wastewater but in the lower range of industrial wastewater (PAE, 50.7 μg/l in WWTP influent vs 72.6, 42.8, and 139.9 μg/l in these types of wastewater, respectively (Fig. 2; Jackson and Sutton 2008; Sanchez-Avila et al. 2009; Bergé et al. 2012b). The variations in PAE concentrations among WWTP influent have been further investigated using published data, which have provided sufficient data for geographical areas including Europe and North America (Fig. 3). Surprisingly, no data was available for Chinese plants. This figure also points out the 10th and 90th percentiles (i.e., “the whiskers”) as well as the 25th and 75th percentiles (“boxes”); the medians are also highlighted. PAE median concentrations in samples from North America and Europe were quite similar; 49.5 and 51.8 μg/l, respectively. It is also interesting to note that European countries display only a few outliers to the high side (see Table 5). In Spain and France, these outlier concentrations typically exceed 160 and 100 μg/l, i.e., two to three times the average level, respectively. These outliers are not yet explained, although the presence of untreated loaded industrial effluent could be hypothesized. Additionally, the database analysis indicates that the PAE concentrations measured in French influent were quite similar to those measured in Europe except in Spain, where DEHP concentrations in influent were significantly lower: i.e., five times the average level (Martin-Ruel et al. 2010; Reyes-Contreras et al. 2011; Bergé et al. 2012b). This difference could be due to the lower DEHP consumptions in Spain where this congener was found in lower levels than in other European countries.
Distributions of total PAE (DMP + DEP + DnBP + BBP + DEHP + DnOP) concentrations (in microgram per liter) and contents (in microgram per kilogram per dry weight) in WWTP influent and effluent, surface water, sediments, sludge and soils from various locations (WFD Water Framework Directive, North Am North America)
Wastewater treatment plant effluent
Understanding the biodegradation processes of phthalic compounds proves to be a critical factor in predicting the fate of these compounds in the environment. From an overall standpoint, the removal of PAE by conventional activated sludge WWTPs has been well documented (Marttinen et al. 2003; Tan et al. 2007; Barnabé et al. 2008; Dargnat et al. 2009). Most of the studies reported removal up to 90 %. Additionally, recent studies conducted by Bergé et al. (2012b) and Gasperi et al. (2010) explained that biofiltration coupled to physicochemical lamellar clarification could be a promising alternative to activated sludge tanks for today plant built in large urbanized areas where the building pressure makes available land scarce. A large number of samples from WWTP effluent have been analyzed with respect to PAE (see Table 6). Generally speaking, effluent concentrations lie in the range of 0.02–49.9 μg/l (mean, 0.80 μg/l) for DEP and 0.02–69.0 μg/l (mean, 2.44 μg/l) for DEHP, with PAE ranges extending from 0.07 to 108 μg/l. As with WWTP influents, no clear difference has been observed for PAE concentrations in final effluent whatever the location (Fig. 3). Median PAE concentrations in the samples from North America and Europe were quite similar, lying in the 4.85–5.35 μg/l range. As previously mentioned for WWTP influent, European effluent has exhibited a few high outliers. In Spain, these outlier concentrations typically exceeded 50 μg/l; i.e., ten times the average level. The little availability of phthalate data before 2000 does not allow defining a trend of these compounds in the discharges of WWTP. Surprisingly, the PAE median concentration measured in Spanish WWTP effluent exceeded that in WWTP influent (Sanchez-Avila et al. 2009, 2012; de los Rios et al. 2012; Bizkarguenaga et al. 2012). However, no explanation could be brought by authors to elucidate this difference, but the WWTP efficiency could however be subject to questioning.
According to our review, PAE can display moderate (50 %) to high (94 %) removal rates. In addition, a study conducted by Vogelsang et al. (2006) explained that chemical and biological treatment eliminates from 50 to 60 % of phthalates in a Norwegian WWTP. In Spain, Sanchez-Avila et al. (2009) reported PAE removal efficiency of 68 %. Finally, a study led by Marttinen et al. (2003) explained that DEHP has been removed from wastewater with an efficiency of 94 %. In addition, both studies conducted by Fauser et al. (2003) and in EU (2008) reported similar removal efficiencies for DEHP. Ordinarily, phthalates are removed by different processes occurring within WWTP, including solid settlement, sorption to sludge, volatilization, biodegradation, hydrolysis, and/or photolysis (Rogers 1996). Sorption on sludge is considered to be one of the major pathways for PAE removal in WWTP. Moreover, even if DEHP may be considered inherently biodegradable under aerobic conditions (Staples et al. 1997), it was not removed by the biological pathway. A study conducted by Gavala et al. (2004) showed that enzymatic pretreatment increased DEHP biodegradation rate in secondary sludge. Furthermore, it was showed that recirculating sludge and thus retaining specific micro-organisms could stabilize removal capacity up to 86 % whereas continuous flow had a varying removal of 77–88 % (Oliver et al. 2005, 2007). Alternatively, Vogelsang et al. (2006) and Staples et al. (1997) reported that DEP, a soluble and biodegradable compound under aerobic conditions, has been removed during biological treatment process, although it has not been significantly eliminated by any chemical or mechanical sedimentation processes. As a general rule, in WWTP effluent, DEHP is expected to be the most abundant congener. Phthalates' distribution is however lightly different, compared to WWTP effluents, with a higher proportion of DnBP and DEP (Fig. 4).
Wastewater treatment plant sludge
As mentioned above, sorption on sludge is considered to be one of the major pathways for PAE removal in WWTP (Kinney et al. 2006; Cai et al. 2007; Barnabé et al. 2008; Clara et al. 2010; Fig. 5). A large number of samples have been analyzed for PAEs (see Table 7, i.e., 30 references). In accordance with its predominance in wastewater, Harrison et al. (2006) and Tan et al. (2007) showed that DEHP was the most abundant phthalate in sludge (Fig. 4). In addition, most studies have reported significant DEHP levels in sludge, i.e., in the range of 150–600 mg/kg dry weight (dw). For example, levels up to 3,514 mg/kg dw in digested sludge from Spain (Abad et al. 2005), 661 mg/kg dw in digested sludge from Sweden (Sweetman 1994), and 578 mg/kg dw in digested sludge from USA (Staples et al. 1997) have been recorded. Additionally, a recent study conducted by Clara et al. (2010) explained that PAE sorption importance and phthalate removals via sludge increased with the molecular weight and is therefore depending on the molecular weight (Table 1). As a complement, authors reported that proportional mass fraction removed with the sludge amounts to 3 % for DMP, 1 % for DEP, 76 % for DnBP, 21 % for BBP, and 78 % for DEHP. Generally speaking, sludge contents lie in the range of 0.02–2.00 mg/kg dw (mean, 0.19 mg/kg dw) for DMP, 0.01–11.0 mg/kg dw (mean, 0.45 mg/kg dw) for DEP and 0.32–3,514 mg/kg dw (mean, 60.3 mg/kg dw) for DEHP. It is interesting to note that certain phthalates display only a few outliers to the high side. For DnBP and BBP, the outlier contents typically exceed 260 and 35 mg/kg dw, respectively, i.e., 490 and 150 times the average level (0.53 and 0.23 mg/kg dw, respectively). The little availability of phthalate removal efficiencies in biosolids suggested that heat drying and anaerobic digestion were less effective at reducing phthalates, particularly DEHP, than composting. As a complement, both studies conducted by Gibson et al. (2005) and Williams (2007) reported that composting removes between 64 and 70 % of DEHP, respectively. Based on our database, PAE contents are statistically close regardless of the location considered, especially between European and Chinese samples, and moreover DEHP contents have been decreasing in both anaerobically digested sludge and final sludge.
Storm water
Although it has been established that storm water is responsible for the spread of pollutants, especially in urban areas, data concerning the emission, occurrence and fate of PAE in storm water remains poorly reported (Makepeace et al. 1995; Pitt et al. 1999; Rule et al. 2006; Björklund et al. 2009; Clara et al. 2010; Zgheib et al. 2012). According to these authors, phthalates in storm water originated from plasticizers in PVC, paints, building materials, etc. For instance, wastewater from car washes can also make a significant contribution to the emission of DEHP (Vikelsoe et al. 1998). Interestingly, PAEs are not chemically bound to the material and may migrate from products during use and disposal (Cadogan et al. 1993). DEHP has been measured at concentrations between 0.45 and 24.0 μg/l in urban storm water in Austria (Clara et al. 2010). DEHP was also measured in all urban and suburban samples between 3 and 58 μg/l (Zgheib et al. 2012). Such levels were higher to those previously reported for storm water in Sweden (5 μg/l; Björklund et al. 2009) and in London (0.75–1.25 μg/l; Rule et al. 2006). Finally, screening performed in the 1990s revealed higher concentrations of phthalates in storm water compared to results from studies performed in 2000s, from less than ten times higher for DnOP and DEHP (Makepeace et al. 1995) up to 140 times higher for BBP (Pitt et al. 1999; Björklund et al. 2009). The higher concentrations measured in previous studies may be explained by the difference between phthalate uses a decade ago and the current situation.
Natural environment
Surface water
Surface water is commonly considered as the natural compartment most affected by human pressures, since this water is subjected to the discharges of treated and/or untreated wastewater and/or storm water (Lin et al. 2009). Therefore, throughout the world, the occurrence and fate of PAE in surface water have been well documented, as demonstrated by the 28 references pertaining to surface water contamination (see Table 8). First, close attention must be paid when comparing concentrations reported in literature, since industrialized and urbanized watershed have been monitored (Tan 1995; Vitali et al. 1997; Long et al. 1998; Yuan et al. 2002; Sha et al. 2007; Dargnat 2008; Zhu and Qiu 2011). Globally and at the world scale, PAE contamination in surface water varies from few micrograms per liter to several tens of micrograms per liter. The variations in PAE concentrations in surface water can be examined using published data, which provide sufficient details for determining the statistical distribution of concentrations. Figure 3 summarizes data for three geographical areas, namely Europe, North America, and China. The median DEHP concentrations for Europe and China are very similar, i.e., 1.05 and 1.11 μg/l, respectively; the median concentration in the North American samples is relatively lower (0.27 μg/l). Moreover, median PAE concentrations in the North American samples (0.29 μg/l) are notably lower than the Chinese (1.24 μg/l) and European samples (1.18 μg/l). It is also interesting to note that all geographical areas displayed a few high outliers. In the UK (Long et al. 1998), these outlier concentrations typically exceeded 15 μg/l (i.e., 12 times the average level). In addition, in China (Sha et al. 2007), they were above 28 μg/l (i.e., 20 times the average level).
It is obvious from the database that PAE concentrations in European surface water first increased before exhibiting a decrease due to tighter regulations (EU 2004, 2005, 2007). It would be appropriate to treat these datasets as two separate time series (Fig. 6). The first series contains the samples from European countries before adoption of the Water Framework Directive (Thüren 1986; Ernst et al. 1988; Fatoki and Vernon 1990; Law et al. 1991; Vitali et al. 1997; Long et al. 1998; Belfroid et al. 1999), all of them exhibit PAE concentrations from 0.04 to 15.8 μg/l. The second series is composed of samples from European countries after the Water Framework Directive (WFD) implementation (Penalver et al. 2001; Fromme et al. 2002; Vethaak et al. 2005; Bendz et al. 2005; Prieto et al. 2007; Sanchez-Avila et al. 2012), revealing lower concentrations, i.e., of about 1.16 μg/l for the sum of PAE. The PAE concentrations plotted as a function of time for these two series are shown in Fig. 6. In contrast, concentrations measured in the Chinese surface water have been increasing for the last decade (see Table 8). This is the result of the constant increase of industrialization, consumptions of phthalates, and absence of regulation in China and more generally in developing countries (Li et al. 2006; Sha et al. 2007; Zeng et al. 2008; Zhu and Qiu 2011; Chen et al. 2012).
Historical trend lines for the median total PAE (DMP + DEP + DnBP + BBP + DEHP + DnOP) concentrations (in microgram per liter) in European surface water. The dots represent samples collected before implementation of the Water Framework Directive, whereas the squares depict samples collected after its implementation
It is obvious from the literature that underground water was recognized to be the most reachable, safe, and the most important source of drinking water supply in many regions. Only a few measurements of PAE in underground water have however been undertaken (Mihovec-Grdic et al. 2002; Zhang et al. 2009; Liu et al. 2010b; Huang et al. 2012). The main reason must be the very low levels of these contaminants expected in underground water and the analytical difficulties to quantify them (at nanogram per liter level). Another reason could be the deficiency of defined regulatory limits by authorities as we can found for both industrial and domestic activities and/or surface water (EU 2004, 2005, 2007, 2008; CPSI 2008). Generally speaking, worldwide concentrations of PAEs in underground water lie in the range of below detection limit to 14.1 μg/l (Zhang et al. 2009; Liu et al. 2010b; Huang et al. 2012). During agricultural irrigation and landscape sludge application, phthalates migrate to the soil and may eventually attain the aquifer, causing deterioration of underground water quality. A recent study conducted by Liu et al. (2010b) reported that water coming from a Chinese landfill was responsible on the spread of phthalates in the area's underground water.
Most studies focusing on surface water analyze only the dissolved phase, hence only a few results are available for suspended solids (Table 9; four references available). According to these four studies, levels of phthalates in suspended solids range from a few to 630 mg/kg dw (Table 9). Such contamination levels may be explained by the high hydrophobicity of DEHP and DnBP (with respect to their log K ow; see Table 1), which leads to sorption and accumulation of these compounds on suspended solids (Gounaris et al. 1993; Sha et al. 2007). For less hydrophobic compounds, contents are lower, i.e., about between 0.05 and 0.20 mg/kg for all compounds, except DEHP (0.70–630 mg/kg). Finally, suspended solids may, however, play a key role in aquatic systems since, under low-flow conditions, particles can settle and contribute to sediment formation, thus yielding contaminant stocks (Figs. 1 and 5). This phenomenon has been demonstrated for some heavy metals, pesticides and PCBs by Zgheib et al. (2012).
Sediments
Several sediment samples have been collected from rivers and lakes across Europe, North America, and developing countries (particularly China and India; Vitali et al. 1997; Fromme et al. 2002; Yuan et al. 2002; Vethaak et al. 2005; Sha et al. 2007; Zeng et al. 2008; Liu et al. 2010a). The amount of DnBP and DEHP measured in these samples (see Table 10, 23 references) varied from 0.01 to 115 mg/kg dw (median, 0.44 and 1.90 mg/kg dw, respectively). Generally speaking, DMP and DEP were not detected in sediments (Fig. 4), mainly due to their low hydrophobicity. In addition, a recent study led by Liu et al. (2010a) reported that DEP is easily degraded in top material and cannot be eluted in deep sediments. Moreover, under anaerobic conditions, DnBP and BBP are easily degraded whereas DEP and DEHP were poorly removed (Yuan et al. 2002). From the exhaustive data in literature, it is clear that PAE are often present in sediment regardless of the location (Fig. 2). The variation in phthalate contents among sediments was examined using published data, which provided sufficient details for determining the statistical distribution of these concentrations. Huang et al. (2005) examined the evolution of DEHP sediment content along a river transects densely populated. They revealed the existence of dynamic processes occurring in surface water, such as sorption to settleable particles and atmospheric volatilization governing the fate of phthalates in the environment and could lead to the formation of sinks in sediments and atmospheric compartment.
A comparison between Tables 9 and 10 indicates that the PAE contents found in sediments are close to those found in suspended solids (Fig. 5). As previously mentioned and highlighted in Fig. 1, sediments and suspended solids are linked through sedimentation during low-flow periods and through resuspension during high-flow periods (Mitsunobu and Takahashi 2006). Presumably, the high K ow of certain phthalates explains their sorption to particles in water, which in turn settle to form sediment (Staples et al. 1997). Additionally, this phthalate accumulation in sediments is fed and promoted by anthropogenic suspended solids, such as particles released by municipal WWTP and untreated water (Huang et al. 2005; Srivastava et al. 2009).
Soils
Several measurements of phthalates in soils have been undertaken (see Table 11, 12 references); these have mainly concerned agricultural and urban soils. DEHP and DnBP levels ranged, respectively, from 0.02 to 264 mg/kg dw (median, 3.33 mg/kg dw) and 0.01 to 30.1 mg/kg dw (median, 0.96 mg/kg dw) for both types of soils. A study performed by Michael et al. (1984) highlighted that these two compounds could enter soils via irrigation and pesticide application. In addition, both studies conducted by Wang et al. (2003) and Dolgen et al. (2007) reported that sewage sludge application could also lead to soil contamination, phytotoxicity, and could cause the accumulation of phthalates in the food supply. Some studies have observed high biodegradation rates of DnBP and DEHP in soils (Juneson et al. 2001; DiGennaro et al. 2005). Biodegradation however is not the only pathway for eliminating DEHP and DnBP in soils. Other processes, such as hydrolysis and photolysis may affect DEHP and DnBP concentrations and occurrence rates. These processes however are recognized to be less important than biodegradation (Yan et al. 1995). A study led by Chang et al. (2004) explained that optimal PAE degradation is enhanced when DnBP and DEHP are present simultaneously. It may be due to the large carbon source and energy provided by both compounds. In addition, in specific physicochemical conditions, DEP biodegradation can lead to the formation of DMP and its monoester (Cartwright et al. 2000). Interestingly and according to the available literature on soils (Table 11), similar contents for agricultural and urban soils have been observed. This homogeneity is quite surprising but could be explained by a global contamination through atmospheric deposits (Fig. 1). Nonetheless, a direct comparison of soil contamination is rather difficult and subtle, since various processes may be occurring. The differences or homogeneity observed can in fact reflect different inputs, environmental factors, including oxygen availability and nutrient amounts in soil, and sources or either different dynamic pollutants in the soils (Semple et al. 2001; Namkoong et al. 2002).
Compost particle sizes have been investigated by several studies (Amellal et al. 2001; Delhomenie et al. 2002; Chang et al. 2009). Authors explained that the smaller the particle was the higher the specific surface was and the lower the porosity was. This favored the microorganism settlement and therefore promoted microbial degradation activity. Finally, the use of straw compost in remediating contaminated soil by phthalates is therefore effective to solid wastes recycling. The variation in PAE contents among soils was examined using published data, which provided sufficient details for determining the statistical distribution of these contents. Figure 3 shows the data from Europe and China. Soils in China (3.09 mg/kg dw) seem to be slightly more contaminated than European soils (0.17 mg/kg dw). This difference may be linked to several factors: (1) greater use or consumption of phthalate-containing goods in China, (2) a higher contamination of air in China, and (3) an absence of pertinent regulations on sludge amendment.
Discussion and recommendations for further study
It is now obvious from the concentrations and contents measured in the different environmental compartments that phthalates are ubiquitous environmental contaminants. Their concentrations and trends in most environmental compartments however remain location dependent. This review has highlighted some geographical disparities, especially between Europe, North America, and China. In Europe, historical trends were also highlighted. In pursuit of the WFD implementation, more specific data for most congeners are needed in upstream compartments to enable source elucidation and to elaborate potential source control action. It is obviously important to measure all industrially significant phthalates across all samples. It is also important to monitor chemicals used to replace phthalates after the implementations of restrictive regulations worldwide, such as di-iso-nonyl phthalate and di-iso-decyl phthalate. Finally, phthalate metabolites have to be investigated, which are supposed to be the active molecule of the estrogenic effect (Rais-Bahrami et al. 2004).
As mentioned above, DEHP is always present in all environmental compartments. It is moreover the abundant phthalate in all matrices, particularly in solid matrices, such as sludge and suspended solids (Fig. 4). The result is that what is left over from many industrial and commercial products of this compound leads to the contamination of the wastewater of urban areas.
In Europe, regulations banning products containing certain phthalates (DEHP, DnBP, and BBP) have been implemented since 2000. It is therefore relevant to evaluate the effectiveness of these regulations by means of reliable and accurate measurements of the PAE contamination levels for sediments, soils, and surface water as a function of time. Indeed, each day without consistent restrictions means that phthalates are produced in huge quantities and it can subsequently result on a widespread use in a lot of products leading to uncontrolled environmental discharges and dissemination. Recent observations in Europe have however shown that PAE concentrations have not been increasing. Moreover, a decreasing trend has actually been observed just over the last few years. Nevertheless, it is important to track these changes closely. As previously noted however, sediments and atmosphere behave like major sinks for PAE in the environment. The role of atmosphere has to be better understood since atmosphere seems to play a key role in PAE widespread. Recent measurements in China, whatever the media, have indicated increasing phthalate contents and concentrations. In addition, the environmental data exhibit a large spatial variability, reflecting differences in phthalate contaminations in various areas of China, which might be caused by uneven development of heavy industry, the imbalance of enforcement or the specific geographical location. Finally, even if trends show decreases across industrialized countries, especially in Europe and North America, the levels being recovered are still significant (up to 100 μg/l) and still contribute to build the background level. A similar trend has been observed for alkylphenols (Bergé et al. 2012a). In addition, and based on the data collected, there are a few samples with very high levels as compared to the average (see the high outliers in all environmental media). A closer attention needs to be paid to these data—why are these few samples so highly contaminated? Is it related to industrial activities or to unknown diffuse sources? Understanding these outliers may contribute to elucidate the mechanisms by which these samples have become contaminated.
The mechanisms involving these contaminants between the environmental compartments cannot be easily distinguished; possibilities include biodegradation, volatilization, sorption to biomass, and particle sedimentation. The three latter mechanisms may be important in explaining the distribution changes throughout the whole water cycle from wastewater to the natural environment. This change is featured by an increase of DnBP and a decrease of DEHP and DEP (Fig. 4). Research on these mechanisms, particularly the processes acting in WWTP, is required in order to improve better understanding and to quantify what proportion is being volatilized into the atmospheric compartment and how much is adsorbed to biomass. The latest research on WWTP has shown high efficiency of the processes acting on sewage treatment plants as well as an insignificant contribution from discharges (Dargnat et al. 2009; Martin-Ruel et al. 2010; Bergé et al. 2012b). Additionally, latest research showed that DEHP removal was dependent on various parameters, but that the type of biomass could significantly enhance removal (Oliver et al. 2005, 2007). The removal of phthalates from wastewater can, however, be improved by adding tertiary treatment such as nanofiltration, reverse osmosis, ozone oxidation, UV irradiation, or activated carbon filters to existing processes, in spite of the high cost of these processes (Agenson et al. 2003; Bodzek et al. 2004; Oh et al. 2006; Verliefde et al. 2007).
A serious element dictating the fate of micropollutants in urban areas is their release into the environment during storms. During wet-weather periods, the operations of wastewater treatment plants are modified and may influence the quality of effluent discharged into the environment (Gilbert et al. 2011; Bergé et al. 2012a, b). Additionally, combined sewer overflow discharges can happen (Gasperi et al. 2008; Zgheib et al. 2012). During wet-weather events, the resuspension of in-sewer deposits provides for additional pollution at the WWTP. This additional contamination can then increase the load of phthalates and disturb usual plant operations and, in return, efficiency. Finally, nowadays, no information is available to evaluate the runoff contribution to wastewater.
References
Abad E, Martinez K, Planas C, Palacios O, Caixach J, Rivera J (2005) Priority organic pollutant assessment of sludges for agricultural purposes. Chemosphere 61(9):1358–1369
Abb M, Heinrich T, Sorkau E, Lorenz W (2009) Phthalates in house dust. Environ Int 35(6):965–970
Agenson KO, Oh JI, Urase T (2003) Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membrane: controlling parameters of process. J Membr Sci 225(1–2):91–103
Amellal N, Portal JM, Vogel T, Berthelin J (2001) Distribution and location of polycyclic aromatic hydrocarbons (PAHs) and PAH-degrading bacteria within polluted soil aggregates. Biodegradation 12:49–57
Aparicio I, Santos JL, Alonso E (2009) Limitation of the concentration of organic pollutants in sewage sludge for agricultural purposes: a case study in South Spain. Waste Manage 29(5):1747–1753
Atlas E, Giam CS (1988) Ambient concentrations and precipitation scavenging of atmospheric organic pollutants. Water Air Soil Pollut 38:19–36
Barnabé S, Beauchesne I, Cooper DG, Nicell JA (2008) Plasticizers and their degradation products in the process streams of a large urban physicochemical sewage treatment plant. Water Res 42(1–2):153–162
Belfroid CA, Van der Horst A, Vethaak AD, Schafer AJ, Rijs GBJ, Wegener J (1999) Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands. Sci Total Environ 225:101–108
Bendz D, Paxéus NA, Ginn TR, Loge FJ (2005) Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Haje River in Sweden. J Hazard Mater 122(3):195–204
Bergé A, Cladière M, Gasperi J, Coursimault A, Tassin B, Moilleron R (2012a) Meta-analysis of environmental contamination by alkylphenols. Env Sci Poll Res. doi:10.1007/s11356-012-1094-7
Bergé A, Gasperi J, Rocher V, Coursimault A, Moilleron R (2012b) Phthalate and alkylphenol removal within wastewater treatment plants using physicochemical lamellar clarification and biofiltration. In: Institute W (ed) Water pollution XI. Weissex Institute, New Forest, UK
Bergé A, Gasperi J, Rocher V, Coursimault A, Moilleron R (2012c) Occurrence and fate of phthalate in urban area: case of Parisian sewer network and wastewater treatment plant (in French). Technics Sci Methods 4:21–29
Bizkarguenaga E, Ros O, Iparraguirre A, Navarro P, Vallejo A, Usobiaga A, Zuloaga O (2012) Solid-phase extraction combined with large volume injection-programmable temperature vaporization-gas chromatography–mass spectrometry for the multiresidue determination of priority and emerging organic pollutants in wastewater. J Chromatogr A 1247:104–117
Björklund K, Cousins AP, Strömvall A-M, Malmqvist P-A (2009) Phthalates and nonylphenols in urban runoff: occurrence, distribution and area emission factors. Sci Total Environ 407(16):4665–4672
Bodzek M, Dudziak M, Luks-Betlej K (2004) Application of membrane techniques to water purification. Removal of phthalates. Desalination 162(121–128)
Boutrup S, Plesner T (2001) Anthropogneic substances in Arhus County 1998–2001. Wastewater, runoff from impervious surfaces and atmospheric deposition. Arhus Amt, Natur-og Miljokontoret, Hojbjerg
Cadogan DF, Papez M, Poppe AC, Pugh DM, Scheubel J (1993) An assessment of the release, occurrence and possible effects of plasticizers in the environment. In: PVC 93: The future, Brighton, UK
Cai Q-Y, Mo C-H, Li YH (2005) The study of PAEs in soils from typical vegetable fields in areas of Guanzhou and Shenzhen, South China. Acta Ecol Sin 25(2):283–288
Cai Q-Y, Mo C-H, Wu Q-T, Zeng Q-Y, Katsoyiannis A (2007) Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere 68(9):1751–1762
Cartwright CD, Owen SA, Thompson IP, Burns RG (2000) Biodegradation of diethyl phthalate in soil by a novel pathway. FEMS Microbiol Lett 186(1):27–34
Cespedes R, Petrovic M, Raldua D, Saura U, Pina B, Lacorte S, Viana P, Barcelo D (2004) Integrated procedure for determination of endocrine-disrupting activity in surface waters and sediments by use of the biological technique recombinant yeast assay and chemical analysis by LC-ESI-MS. Anal Bioanal Chem 378:697–708
Chang BV, Chang CM, Cheng CH, Yuan SY (2004) Biodegradation of phthalate esters by two bacteria strains. Chemosphere 55:533–538
Chang BV, Lu YS, Yuan SY, Tsao TM, Wang MK (2009) Biodegradation of phthalate esters in compost-amended soil. Chemosphere 74:873–877
Chee KK, Wong MK, Lee HK (1996) Microwave extraction of phthalate esters from marine sediment and soil. Chromatographia 42:378–384
Chen L, Zhao Y, Li L, Chen B, Zhang Y (2012) Exposure assessment of phthalates in non-occupational populations in China. Sci Total Environ 427–428:60–69
Cheng HF, Chen SY, Lin JG (2001) Hazardous organic matters in municipal sewage sludge in Taiwan. Water Sci Technol 44(10):65–70
Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, Scheffknecht C, Chovanec A (2010) Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 78(9):1078–1084
Clara M, Windhofer G, Weilgony P, Gans O, Denner M, Chovanec A, Zessner M (2012) Identification of relevant micropollutants in Austrian municipal wastewater and their behaviour during wastewater treatment. Chemosphere 87(11):1265–1272
Clark K, Cousins I, MacKay D (2003) Assessment of critical exposure pathways. In: Staples CA (ed) The handbook of environmental chemistry. Springer, New York
Cortazar E, Bartolomé L, Delgado A, Etxebarria N, Fernandez LA, Usobiaga A, Zuloaga O (2005) Optimisation of microwave-assisted extraction for the determination of nonylphenols and phthalate esters in sediment samples and comparison with pressurised solvent extraction. Anal Chim Acta 534(2):247–254
CPSI (2008) Public Law 110-314-AUG. 14, 2008: establishing consumer product safety standards and other safety requirements for children's products. The Senate and House of Representatives of the United States of America
Dargnat C (2008) Sources, transfer and fate of phthalate esters in the Seine river basin. Characterization of hazards to the environment and ecosystems. Paris VI-Pierre and Marie Curie, Paris
Dargnat C, Teil M-J, Chevreuil M, Blanchard M (2009) Phthalate removal throughout wastewater treatment plant: case study of Marne Aval station (France). Sci Total Environ 407(4):1235–1244
DeLeon I, Byrne CJ, Peuler EA, Antoine SR, Schaeffer J, Murphy RC (1986) Trace organic and heavy metal pollutants in the Mississippi River. Chemosphere 15:795–805
de los Rios A, Juanes JA, Ortiz-Zarragoitia M, de Alda Lopez M, Barcelo D, Cajaraville MP (2012) Assessment of the effects of a marine urban outfall discharge on caged mussels using chemical and biomarker analysis. Mar Pollut Bull 64(3):563–573
Deblonde T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 214:442–448
Delhomenie M-C, Bibeau L, Heitz M (2002) A study of the impact of particle size and adsorption phenomena in a compost-based biological filter. Chem Eng Sci 57(24):4999–5010
DiGennaro P, Collina E, Franzetti A, Lasagni M, Luridiana A, Pitea D, Bestetti G (2005) Bioremediation of diethylhexyl phthalate contaminated soil: a feasibility study in slurry- and solid-phase reactors. Environ Sci Technol 39(1):325–330
Dolgen D, Necdet Alpaslan M, Delen N (2007) Agricultural recycling of treatment-plant sludge: a case study for a vegetable-processing factory. J Environ Manage 84(3):274–281
Eisenreich SJ, Looney BB, David JB (1981) Airborne organic contaminants in the Great Lakes ecosystem. Environ Sci Technol 15:30–38
Ernst W, Boon JP, Weber K (1988) Occurrence and fate of organic micropollutants in the North Sea. In: Salomons W, Baynes BL, Duursma EK, Förstner U (eds) Pollution of the North Sea: an assessment. Springer, Berlin, pp 284–299
EPA (2009) U.S. Environmental Protection Agency. Toxics Release Inventory (TRI) Program: Get TRI Data and Tools. 2007 Public Data Release, Released March 14, 2009. http://www.epa.gov/tri/tridata/index.htm#pdr. Accessed 9 Dec 2009
EU (2004) Directive 2004/93/CEE restricting the use of phthalate. Official Journal of the European Union, Luxembourg
EU (2005) Directive 2005/84/CEE limiting the use of phthalates in toys. Official Journal of the European Union, Luxembourg
EU (2007) Directive 2007/19/CEE restricting the use of phthalate in plastics for food packaging. Official Journal of the European Union, Luxembourg
EU (2008) Summary risk assessment report for DEHP, Final Report, European Commission, EUR 23384EN. Office for Official Publications of the European Communities, Luxembourg
Fatoki OS, Mathabatha S (2001) An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water South Africa 27(2):233–240
Fatoki OS, Vernon F (1990) Phthalate esters in rivers of the greater Manchester area, UK. Sci Total Environ 95:227–232
Fauser P, Vikelsoe J, rensen PB SÃ, Carlsen L (2003) Phthalates, nonylphenols and LAS in an alternately operated wastewater treatment plant-fate modelling based on measured concentrations in wastewater and sludge. Water Res 37(6):1288–1295
Fragermann HJ (2003) Contamination of sewage sludge with organic pollutants —results of an investigation program in NRW (in German). Paper presented at the Uses of waste in/on floors, Duisburg, June
Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36(6):1429–1438
Gangl M, Sattelberger R, Kreuzinger N (2001) Endocrine Disrupting Compounds in sewage sludge (in austrian). Federal Environmental Agency office ofAustria, Vienna
Gasperi J, Garnaud S, Rocher V, Moilleron R (2008) Priority pollutants in wastewater and combined sewer overflow. Sci Total Environ 407(1):263–272
Gasperi J, Garnaud S, Rocher V, Moilleron R (2009) Priority pollutants in surface waters and settleable particles within a densely urbanised area: case study of Paris (France). Sci Total Environ 407(8):2900–2908
Gasperi J, Rocher V, Gilbert S, Azimi S, Chebbo G (2010) Occurrence and removal of priority pollutants by lamella clarification and biofiltration. Water Res 44:3065–3076
Gavala HN, Yenal U, Ahring BK (2004) Thermal and enzymatic pre-treatment of sludge containing phthalate esters prior to mesophilic anaerobic digestion. Biotechnol Bioeng 85(5):561–567
Giam CS, Atlas E, Powers MA, Leonard JE (1984) Phthalic acid esters, 3. The handbook of environmental chemistry, vol part C. Springer, Berlin
Gibson R, Wang M-J, Padgett E, Beck AJ (2005) Analysis of 4-nonylphenols, phthalates, and polychlorinated biphenyls in soils and biosolids. Chemosphere 61(9):1336–1344
Gilbert S, Gasperi J, Rocher V, Lorgeoux C, Chebbo G (2011) Removal of alkylphenols and polybromodiphenylethers by a biofiltration treatment plant during dry and wet-weather periods. Water Sci Tech 65(9):1591–1598
Gounaris V, Andersen PR, Holson TM (1993) Characteristics and environmental significance of colloids in landfill leachate. Environ Sci Technol 27:1381–1387
Guidotti M, Giovinazzo R, Cedrone O, Vitali M (2000) Determination of organic micropollutants in rain water for laboratory screening of air quality in urban environment. Environ Int 26:23–28
Hadjmohammadi MR, Fatemi MH, Taneh T (2011) Coacervative extraction of phthalates from water and their determination by high performance liquid chromatography. J Iran Chem Soc 8(1):100–106
Harrison EZ, Oakes SR, Hysell M, Hay A (2006) Organic chemicals in sewage sludges. Sci Total Environ 367:481–497
Hu XY, Wen B, Shan XQ (2003) Survey of phthalate pollution in arable soils in China. J Environ Monit 5:649–653
Huang PC, Hsieh CY, Tsai YT, Peng KC, Lee CC (2005) The distribution of di-(2-ethyl-hexyl) phthalate (DEHP) in major rivers in Taiwan. In: Accreditation NIoE (ed). The Annual Conference of Environmental Analytical Chemistry, Zhongli City, Taiwan, R.O.C.
Huang PC, Tien CJ, Sun YM, Hsieh CY, Lee CC (2008) Occurrence of phthalates in sediment and biota: relationship to aquatic factors and the biots-sediment accumulation factor. Chemosphere 73:539–544
Huang G, Sun J, Chen Z, Chen X, Jing J, Liu J, Zhang Y (2012) Levels and sources of phthalate esters in shallow groundwater and surface water of Dongguan city, South China. Geochem J 46(5):421–428
INERIS (2007) Regional action of search and reduction discharges of dangerous substances in water by the classified installations and the urban wastewater treatment plants (in French). INERIS, INERIS, Verneuilen-Halatte, Oise
IPCS (2003) International Chemical Safety Card —Diethyl phthalate. World Health Organization. International Program on Chemical Safety (ISCS 0258), Geneva
Jackson J, Sutton R (2008) Sources of endocrine-disrupting chemicals in urban wastewater, Oakland, CA. Sci Total Environ 405(1–3):153–160
Jaganyi D (2007) Methodology and survey of organic pollutants in South African sewage sludges, volume 1. Research Report No.1339/1/07 from the South African Water Research Commission on the project “Survey and Methodology for Analyzing Organic Pollutants in South African Sewage Sludges”, 153 p
Juneson C, Ward OP, Singh A (2001) Biodegradation of bis(2-ethylhexyl)phthalate in a soil slurry-sequencing batch reactor. Process Biochem 37(3):305–313
Keil R, Salemme K, Forrest B, Neibauer J, Logsdon M (2011) Differential presence of anthropogenic compounds dissolved in the marine waters of Puget Sound, WA and Barkley Sound, BC. Mar Pollut Bull 62(11):2404–2411
Kinney CA, Furlong ET, Zaugg SD, Burkhardt MR, Werner SL, Cahill JD, Jorgensen GR (2006) Survey of organic wastewater contaminants in biosolids designated for land application. Environ Sci Technol 40:7207–7215
Kjohlholt J, Poll C, Kofoed JF (1997) Anthropogenic sustances in runoff from impervious surfaces. Literature review and measurements, vol 355. The Danish EPA, Kobenhavn
Laturnus F, Gron C (2007) Organic waste products in agriculture —Monitoring the waste constituents phthalate esters in soil-crop system by gas chromatography and ion trap tandem spectrometry. J Environ Eng Landsc Manag 15:252–260
Law RJ, Fileman TW, Matthiessen P (1991) Phthalate esters and other industrial organic chemicals in the North and Irish Seas. Water Sci Technol 14:127–134
Lepri L, Del Bubba M, Masi F, Udisti R, Cini R (2000) Particle size distribution of organic compounds in aqueous aerosols collected from above sewage aeration tanks. Aerosol Sci Technol 32(5)
Li XH, Ma LL, Liu XF, Fu S, Cheng HX, Xu XB (2006) Phthalate ester pollution in urban oil of Beijing, People's Republic of China. Bull Environ Contam Toxicol 77:252–259
Ligocki MP, Leuenberger C, Pankow JF (1985) Trace organic compounds in rain —II. Gas scavenging of neutral organic compounds. Atmos Environ 19(10):1609–1617 (1967)
Lin C, Lee C-J, Mao W-M, Nadim F (2009) Identifying the potential sources of di-(2-ethylhexyl) phthalate contamination in the sediment of the Houjing River in southern Taiwan. J Hazard Mater 161(1):270–275
Liu H, Liang H, Liang Y, Zhang D, Wang C, Cai H, Shvartsev SL (2010a) Distribution of phthalate esters in alluvial sediment: a case study at JiangHan Plain, Central China. Chemosphere 78(4):382–388
Liu H, Liang Y, Zhang D, Wang C, Liang H, Cai H (2010b) Impact of MSW landfill on the environmental contamination of phthalate esters. Waste Manage 30:1569–1576
Long JLA, House WA, Parker A, Rae JE (1998) Micro-organic compounds associated with sediments in the Humber rivers. Sci Total Environ 210–211:229–253
Ma LL, Chu SG, Xu XB (2003) Phthalate residues in greenhouse soil from Beijing suburbs, People's Republic of China. Bull Environ Contam Toxicol 71:394–399
Makepeace DK, Smith DW, Stanley SJ (1995) Urban stormwater quality: summary of contaminant data. Crit Rev Environ Sci Technol 25(2):93–139
Martin-Ruel S, Esperanza M, Choubert JM, Valor I, Budzinski H, Coquery M (2010) On-site evaluation of the efficiency of conventional and advanced secondary processes for the removal of 60 organic micropollutants. Water Sci Technol 62(12):2970–2978
Marttinen SK, Kettunen RH, Rintala JA (2003) Occurrence and removal of organic pollutants in sewages and landfill leachates. Sci Total Environ 301(1–3):1–12
Michael PR, Adams WJ, Werner AF, Hicks O (1984) Surveillance of phthalate esters in surface waters and sediments in the US. Environ Toxicol Chem 3:377–389
Mihovec-Grdic M, Smit Z, Puntaric D, Bosnir J (2002) Phthalates in underground waters of the Zagreb area. Croat Med J 43(4):493–497
Mitsunobu S, Takahashi Y (2006) Study of the water solubility and sorption on particulate matter of phthalate in the presence of humic acid using 14C labeled di-(2-ethylhexyl) phthalate. Water Air Soil Pollut 175:99–115
Müller AK, Nielsen E, Ladefoged O (2003) Human exposure to selected phthalates in Denmark by the Danish Veterinary and Food Administration. http://www.food.dtu.dk/Default.aspx?=ID22630. Accessed 12 May 2010.
Namkoong W, Hwang E-Y, Park J-S, Choi J-Y (2002) Bioremediation of diesel-contaminated soil with composting. Environ Pollut 119(1):23–31
Oh S, Oh BS, Jung YJ, Oh YJ, Yoo YS, Kang JW (2006) Application of ozone, UV and ozone/UV processes to reduce diethyl phthalate and its estrogenic activity. Sci Total Environ 367(2–3):681–693
Oliver R, May E, Williams J (2005) The occurrence and removal of phthalates in a trickle filter STW. Water Res 39(18):4436–4444
Oliver R, May E, Williams J (2007) Microcosm investigations of phthalate behaviour in sewage treatment biofilms. Sci Total Environ 372:605–614
Paulsrud B, Wien A, Nedland KT (1998) A survey of toxic organics in Norwegian sewage sludge, compost, and manure. In 8th Annual International Conference of the FAO ESCORENA Network of Recycling of Agricultural, Municipal and Industrial Residues in Agriculture (Formerly Animal Waste Management), Rennes, France, 10 p
Peijnenburg WJGM, Struijs J (2006) Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol Environ Saf 63(2):204–215
Penalver A, Pocurull E, Borrull F, Marcé RM (2001) Comparison of different fibers for the solid-phase microextraction of phthalate esters from water. J Chromatogr A 922(1–2):377–384
Petrovic M, Barcelo D (2000) Determination of anionic and nonionic surfactants, their degradation products, and endocrine-disrupting compounds in sewage sludge by liquid chromatography/mass spectrometry. Anal Chem 72:45-60
Pitt R, Clark S, Field R (1999) Groundwater contamination potential from stormwater infiltration practices. Urban Water 1(3):217–236
Preston MR, Al-Omran LA (1989) Phthalate esters speciation in estuarine water, suspended particulates and sediments. Environ Pollut 62:183–193
Prieto A, Zuloaga O, Usobiaga A, Etxebarria N, Fernandez LA (2007) Development of a stir bar sorptive extraction and thermal desorption-gas chromatography–mass spectrometry method for the simultaneous determination of several persistent organic pollutants in water samples. J Chromatogr A 1174(1–2):40–49
Quenea K, Derenne S, Gonzalez-Vila FJ, Mariotti A, Rouzaud JN, Largeau C (2005) Study of the composition of the macromolecular refractory fraction from an acidic sandy forest soil (Landes de Gascogne, France) using chemical degradation and electron microscopy. Org Geochem 36(8):1151–1162
Rais-Bahrami K, Nunez S, Revenis ME, Luban NLC, Short BL (2004) Follow-up study of adolescents exposed to di(2-ethylhexyl) phthalate (DEHP) as neonates on extracorporeal membrane oxygenation (ECMO) support. Environ Heal Perspect 112(13):1339–1340
Ranke J (2005) Classification and risk assessment of chemicals: the case of DEHP in the light of REACH. J Transdiscipl Environ Stud 4(3):15
Ray LE, Murray HE, Giam CS (1983) Organic pollutants in marine samples from Portland, Maine. Chemosphere 12(7–8):1031–1038
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R (2008) Ultrasound-assisted emulsification-microextraction of emergent contaminants and pesticides in environmental waters. J Chrom A 1190(1–2):27–38
Reyes-Contreras C, Matamoros V, Ruiz I, Soto M, Bayona JM (2011) Evaluation of PPCPs removal in a combined anaerobic digester-constructed wetland pilot plant treating urban wastewater. Chemosphere 84(9):1200–1207
Rogers HR (1996) Sources, behaviour and fate of organic contaminants during sewage treatment and in sewage sludges. Sci Total Environ 185(1–3):3–26
Rule KL, Comber SDW, Ross D, Thornton A, Makropoulos CK, Rautiu R (2006) Sources of priority substances entering an urban wastewater catchment-trace organic chemicals. Chemosphere 63(4):581–591
Russel DJ, Mc Duffie B (1986) Chemodynamic properties of phthalate esters: partitioning and soil migration. Chemosphere 15:1003–1021
Salapasidou M, Samara C, Voutsa D (2011) Endocrine disrupting compounds in the atmosphere of the urban area of Thessaloniki, Greece. Atmos Environ 45(22):3720–3729
Sanchez-Avila J, Bonet J, Velasco G, Lacorte S (2009) Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci Total Environ 407(13):4157–4167
Sanchez-Avila J, Tauler R, Lacorte S (2012) Organic micropollutants in coastal waters from NW Mediterranean Sea: sources distribution and potential risk. Environ Int 46:50–62
Schaecke B, Kape E (2003) Discussion of new pollution limits for sewage sludge recycling to agricultural (in German). Paper presented at the WABKA, Rostock, October
Schnaak W, Küchler T, Kujawa M, Henschel KP, Subenbach D, Donau R (1997) Organic contaminants in sewage sludge and their ecotoxicological significance in the agricultural utilization of sewage sludge. Chemosphere 35(1–2):5–11
Semple KT, Reid BJ, Fermor TR (2001) Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ Pollut 112(2):269–283
Seriki K, Gasperi J, Castillo L, Scholes L, Eriksson E, Revitt M, Meinhold J, Atanasova N (2008) Priority pollutants behaviour in end of pipe wastewater treatment plants. ScorePP (www.scorepp.eu), (Project no. 037036; No. 037036), 91 p
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAE in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124:277–287
Sheldon LS, Hites RA (1979) Environmental occurrence and mass spectral identification of ethylene glycol derivatives. Sci Total Environ 11(3):279–286
Shi W, Zhang F-X, Hu G-J, Hao Y-Q, Zhang X-W, Liu H-L, Wei S, Wang X-R, Giesy JP, Yu H-X (2012) Thyroid hormone disrupting activities associated with phthalate esters in water sources from Yangtze River Delta. Environ Int 42:117–123
Solis ME, Liu CC, Nam P, Niyogi DK, Bandeff JM, Huang YW (2007) Occurrence of organic chemicals in two rivers inhabited by Ozark Hellbenders (Cryptobranchus alleganiensis bishopi). Arch Environ Contam Toxicol 53:426–434
Srivastava A, Sharma VP, Tripathi R, Kumar R, Patel DK, Mathur PK (2009) Occurrence of phthalic acid esters in Gomti River Sediment. India Env Monit Asses. doi:10.1007/s10661-009-1182-4
Staples CA, Peterson DR, Parterton TF, Adams WJ (1997) The environmental fate of phthalate esters: a literature review. Chemosphere 25:667–749
Suzuki Y, Yamaguchi Y, Suzuki S, HIrata S, Aihara M, Hiraki K (2001) Characteristics of aquatic humic substances in natural waters by synchronous and derivative synchronous fluorescence spectrum. Anal Sci 17:1605–1607
Sweetman AJ (1994) Development and application of a multi-residue analytical method for the determination of n-alkanes, linear alkylbenzenes, polynuclear aromatic hydrocarbons and 4-nonylphenol in digested sewage sludges. Water Res 28:343–353
Tan GH (1995) Residue levels of phthalate esters in water and sediment samples from the Klang River Basin. Bull Environ Contam Toxicol 54:171–176
Tan BLL, Hawker DW, Müller JF, Leusch FDL, Tremblay LA, Chapman HF (2007) Modelling of the fate of selected endocrine disruptors in a municipal wastewater treatment plant in South East Queensland, Australia. Chemosphere 69(4):644–654
Teil MJ, Blanchard M, Chevreuil M (2006) Atmospheric fate of phthalate esters in an urban area (Paris-France). Sci Total Environ 354(2–3):212–223
Thüren A (1986) Determination of phthalates in aquatic environments. Bull Environ Contam Toxicol 36:33–40
Thüren A, Larsson P (1990) Phthalate esters in the Sweedish atmosphere. Environ Sci Technol 24:554–559
Tlili K, Labadie P, Blanchard M, Teil MJ, Desportes A, Bourges C, Alliot F, Chevreuil M (2010) Presence of phthalates and PBDE in the air, the atmospheric deposits and sludge from Parisian conurbation (in French). Paper presented at the PIREN-SEINE, Paris
Torslov J, Samsoe-Petersen L, Rasmussen JO, Kristensen P (1997) Use of waste products in agriculture. Contamination level, environmental risk assessment and recommendations for quality criteria. Environmental Project No. 366, Danish Environmental Protection Agency (EPA), Thomas Telford Publishing, Copenhagen, pp 175–189
Verliefde A, Cornelissen E, Amy G, Van der Bruggen B, Van Dijk H (2007) Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment to removal possibilities with nanofiltration. Environ Pollut 146
Vethaak A, Lahr J, Kuiper RV, Grinwis GCM, Rankouhi TR, Giesy JP, Gerritsen A (2002) Estrogenic effects in fish in The Netherlands: some preliminary results. Toxicology 181–182:147–150
Vethaak AD, Lahr J, Schrap SM, AlC B, Rijs GBJ, Gerritsen A, de Boer J, Bulder AS, Grinwis GCM, Kuiper RV, Legler J, Murk TAJ, Peijnenburg W, Verhaar HJM, de Voogt P (2005) An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere 59(4):511–524
Vikelsoe J, Thomasen M, Johansen E (1998) Sources of phthalates and nonylphénols in municipal wastewater. A study in a local environment. NERI Technical report nr. 225
Vikelsoe J, Thomsen M, Carlsen L (2002) Phthalates and nonylphenols in profiles of differently dressed soils. Sci Total Environ 296(1–3):105–116
Vitali M, Guidotti M, Macilenti G, Cremisini C (1997) Phthalate esters in freshwaters as markers of contamination sources—a site study in Italy. Environ Int 23(3):337–347
Vogelsang C, Grung M, Jantsch TG, Tollefsen KE, Liltved H (2006) Occurrence and removal of selected organic micropollutants at mechanical, chemical and advanced wastewater treatment plants in Norway. Water Res 40(19):3559–3570
Wang Y, Fan Y, Gu JD (2003) Microbial degradation of the endocrine-disrupting chemicals phthalic acid and dimethyl phthalate ester under aerobic conditions. Bull Environ Contam Toxicol 71:810–818
Wang P, Wang SL, Fan CQ (2008) Atmospheric distribution of particulate- and gas-phase phthalic esters (PAEs) in a Metropolitan City, Nanjing, East China. Chemosphere 72(10):1567–1572
Webber MD, Lesage S (1989) Organic contaminants in Canadian Municipal sludges. Waste Manag Res 7(1):63–82
Webber MD, Nichols JA (1995) Organic and metal contaminants in Canadian Municipal sludges and a sludge compost. Wastewater Technology Centre, Burlington
Williams (2007) Occurrence of microconstituents in biosolids. In: Water Environment Federation Annual Conference and Technical Exposition, San Diego, CA, USA
Xie Z, Ebinghaus R, Temme C, Caba A, Ruck W (2005) Atmospheric concentrations and air–sea exchanges of phthalates in the North Sea (German Bight). Atmos Environ 39(18):3209–3219
Xie Z, Ebinghaus R, Temme C, Lohmann R, Caba A, Ruck W (2007) Occurrence and air–sea exchange of phthalates in the Arctic. Environ Sci Technol 41(13):4555–4560
Xu G, Li FS, Wang QH (2008) Occurrence and degrade-action characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Sci Total Environ 393:333–340
Yan H, Ye C, Yin C (1995) Kinetics of phthalate ester biodegradation by Chlorella pyrenoidosa. Environ Toxicol Chem 14(6):931–938
Yuan SY, Liu C, Liao CS, Chang BV (2002) Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 49(10):1295–1299
Yuwatini E, Hata N, Taguchi S (2006) Behavior of di(2-ethylhexyl) phthalate discharged from domestic waste water into aquatic environment. J Environ Monit 8:191–196
Zeng F, Cui K, Xie Z, Wu L, Liu M, Sun G, Lin Y, Luo D, Zeng Z (2008) Phthalate esters (PAEs): emerging organic contaminants in agricultural soils in peri-urban areas around Guangzhou, China. Environ Pollut 156(2):425–434
Zeng F, Cui K, Xie Z, Wu L, Luo D, Chen L, Lin Y, Liu M, Sun G (2009) Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. J Hazard Mater 164:1171–1178
Zgheib S, Moilleron R, Chebbo G (2012) Priority pollutants in urban storm water: part 1—case of separate storm sewers. Water Res. doi:10.1016/j.watres.2011.12.012
Zhang YH, Chen BH (2003) Determination of phthalates in environmental samples. J Environ Heal 20(5):283–286
Zhang D, Liu H, Liang Y, Wang C, Liang H, Cai H (2009) Distribution of phthalate esters in the groundwater of Jianghan plain, Hubei, China. Front Earth Sci China 3(1):73–79
Zhang L, Dong L, Ren l, Shi S, Zhou L, Zhang T, Huang Y (2012) Concentration and source identification of polycylic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. J Environ Sci 24(2):335–342
Zhou I, Liu YS (2010) Study on soil organic pollution in agro-ecosystem of Zhenjiang city. Sci Tech Inf 17:1008–1009
Zhu XJ, Qiu YY (2011) Measuring the phthalates of Xiangjiang River using liquid extraction gas chromatography. Adv Mater Res 301–303:752–755
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bergé, A., Cladière, M., Gasperi, J. et al. Meta-analysis of environmental contamination by phthalates. Environ Sci Pollut Res 20, 8057–8076 (2013). https://doi.org/10.1007/s11356-013-1982-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1982-5