Abstract
The concentrations of O3 are increasing, which may have potential adverse effects on crop yield. This paper deals with assessing the intraspecific variability of two wheat cultivars (PBW 343 and M 533) at different growth stages using open top chambers. Mean O3 concentrations were 50.2 and 53.2 ppb, and AOT40 values were 9 and 12.1 ppm h, respectively, in 2008–2009 and 2009–2010. Reproductive stage showed higher AOT40 values (6.9 and 9.2 ppm h) compared to vegetative (2.23 and 2.9 ppm h). Critical levels of a 3-month AOT 40 of 3 ppm h led to 6 % yield reduction in two wheat cultivars for two consecutive years. Variations in photosynthesis rate, stomatal conductance (gs), Fv/Fm ratio, photosynthetic pigments, primary and secondary metabolites, morphological parameters, and yield attributes were measured at vegetative and reproductive stages. Reductions in number of leaves, leaf area, total biomass, root/shoot ratio, RGR, photosynthetic pigments, protein content, and Fv/Fm ratio in PBW 343 were more than M 533 at reproductive stage. Photosynthetic rate did not vary between the cultivars, but gs was higher in PBW 343 compared to M 533 under ambient O3. Higher total phenolics and peroxidase activity were recorded in M 533 at reproductive stage conferring higher resistance at latter age. Results of O3 resistance showed that M 533 was sensitive compared to PBW 343 during vegetative stage but developed more resistance at reproductive stage. PBW 343 with larger leaf area and high gs is more sensitive than M 533 with smaller leaf area and low gs. The study suggests that the sensitivity varied with plant growth stage, and the plant showing higher sensitivity during vegetative period developed more resistance during reproductive period due to higher defense mechanism. Though the yield reductions were same in both cultivars under ambient O3, the mechanism of acquiring the resistance is different between the cultivars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ground level O3 is one of the most important phytotoxic pollutant, and there is abundant evidence that the present ambient O3 concentrations are high enough to cause significant yield loss in agricultural crops (Teixeira et al. 2011). A recent global impact assessment for major agriculture commodities has estimated annual production losses of US$ 14 to 26 billion under the present air quality legislation (Van Dingenen 2009). The scenario is, however, entirely different for Asia due to tremendous increase in anthropogenic activities and rapid expansion of economy, leading an increased emission of O3 precursors. Asia is the world’s biggest emitter of NOx and is predicted to further increase over the coming decades (Royal Society 2008). Asia is also projected to increase in the proportion of the population from 40 % in 2007 to 66 % by 2050. A key feature of economic policies in Asian countries such as China, India, and Thailand is to maintain continued economic growth, leading to higher pollution burden which will continue to rise unless strict emission control policies are introduced and implemented (Emberson and Buker 2011). In India, there are currently no air quality standards to protect agriculture from tropospheric O3. Rai et al. (2011) reported economic losses of Rs 1,208–30,550 ha−1 for major agricultural crops wheat, rice, mustard, urad, soybean, pea, and mung bean grown at ambient O3 through different study approaches like open top chambers (OTCs), use of ethylene diurea (EDU), and field transect study.
The magnitude of O3 impact depends on the severity, frequency and duration of O3 exposure, and the stage of plant growth. Plant responses to O3 are complex and specific and include increase in the activities of antioxidative enzymes and metabolites associated with general plant defense mechanisms (Kangasjärvi et al. 2005). Crop sensitivity to O3 is typically assessed by the decline in growth and/or the appearance of O3 injury (Singh et al. 2005; Agrawal et al. 2005). Growth depends upon the availability of photosynthate supply to the growing organs. Variations in resource allocation to the vegetative and reproductive organs of different species have been reported (Cooley and Manning 1987; Davison and Barnes 1998; Leisner and Ainsworth 2012).
Though there are no successful measures to reduce O3 pollution, hence, it is important that future crop productivity may depend on the development of O3 tolerant varieties capable of maintaining yield under elevated O3 conditions. Wheat is an important agriculture crop and is kept under sensitive category for O3 (Mills et al. 2007). Emberson et al. (2009) also concluded that Asian cultivars are more sensitive than American/European cultivars of different crops. Differences among cultivars in response to O3 are widely reported (Drogoudi and Ashmore 2002; Sarkar and Agrawal 2010) due to differences in stomatal conductance and net photosynthetic rate (Rai and Agrawal 2008), rate of senescence (Feng et al. 2010), induction of antioxidative defense (Biswas et al. 2008), etc.
Keeping the above facts in view, the present study was undertaken with the objective to evaluate the sensitivity of two commonly grown Indian cultivars (M 533 and PBW 343) of wheat in relation to different developmental stages under ambient O3. Ozone sensitivity in a European cultivar of wheat (Pleijel et al. 1998) varied between flowering and seed maturity. But studies are not available for Indian wheat cultivars response to ambient O3 under different stages of development and to correlate these with yield response of the experimental cultivars. The critical levels of AOT40 for the experimental wheat cultivars under ambient O3 were also calculated.
Materials and methods
Study area
The field experiments were conducted during winter under natural field conditions at a rural site of Varanasi city, situated in the eastern Gangetic plains of India at 25°14′ N latitude, 82°03′ E longitude, and 76.19 m above sea level. During the experimental period from December, 2008–March, 2009, the mean monthly minimum and maximum temperature varied from 7.8–21.3 and 17.9–39.9 °C in December, 2009–March, 2010, respectively. Total rainfall was 1.2 mm in December, 2008–March, 2009 and 17.4 mm in December, 2009–March, 2010. Minimum and maximum mean relative humidity ranged from 33.8–70.5 % in December, 2008–March, 2009 to 55.7–95.5 % in December, 2009–March, 2010.
Open top chambers
Six OTCs were established at the experimental site for each cultivar. Two different chamber treatments were used, charcoal filtered chambers (FCs) (n = 3) and non-filtered chambers (NFCs) (n = 3) for each cultivar. Three open plots (OPs) (n = 3) for each cultivar was kept to study the chamber effects. Replicates of FCs, NFCs, and OPs of different cultivars were arranged in a randomized complete block design within the main plot.
The detailed design of OTC was described in Tiwari et al. (2006). Each of the OTCs was attached to a high-speed blower for a continuous air supply at three changes per minute. It was observed that temperature and relative humidity were higher by 0.1–0.2 °C and 2–4 %, respectively, in OTCs compared to OPs. The light intensity was 94–95 % of the ambient levels within the chambers.
Plant material
Two cultivars of wheat (Triticum aestivum L.) namely PBW 343 and M 533 were chosen for the experiment. PBW 343 is a single dwarf long duration line, which completes its life cycle in 135 days. It was released in the year 1996 with high yield potential. M 533 was a modern variety released in 2001 and completes its life cycle in 135 days. It is a tall variety. Both the cultivars are highly resistant against rust.
Seeds of wheat were hand sown in chambers and OPs in December. Recommended dose of fertilizers (120 kg ha−1 N as urea, 60 kg ha−1 P as single superphosphate, and 40 kg ha−1 K as muriate of potash, respectively) were given to the plant. Half dose of N and full doses of P and K were given as basal dressing, and another half dose of N was given as one top dressing. Plants were thinned to 1 plant every 15 cm. Manual weeding was performed three times over the course of the experiment. Field was irrigated from time to time to maintain the soil moisture uniformly.
O3 monitoring
Air sample were drawn through polytetrafluoroethylene tube (0.25-cm diameter) at canopy height from different chambers and open plots between 0700 and 1900 hours throughout the growth of the plants. O3 monitoring for 12 h during growing season was characterized as M 12. The sampling tube moved with the increase in plant height; O3 concentrations were monitored using a UV absorption photometric ozone analyzer (Model 400 API, Inc., USA). AOT40 was calculated using Mills et al. (2007).
Growth parameters
For growth and biomass determination, two monoliths (10 × 10 × 20 cm3) containing intact roots were carefully dug at random from each chamber and open plot at 20, 30, and 40 days after germination (DAG) for vegetative stages and 50, 60, and 70 DAG for reproductive stages. The data for vegetative and reproductive stages are mean of three sampling conducted during respective stages. Monoliths were thoroughly washed by placing them on a sieve of 1-mm mesh size under running tap water to remove the soil particles. Growth parameters analyzed were root and shoot length, leaf area, number of tillers, and number of leaves. Leaf area was measured using a portable leaf area meter (model LI-3100, LI-COR, Inc., USA). Component plant parts were separated and oven dried at 80 °C till constant weight was achieved for total biomass determination. For understanding the dry matter production and allocation pattern, growth indices such as relative growth rate (RGR) and root/shoot ratio (RSR) were calculated from the formulae given by Hunt (1982).
Photosynthetic pigments, antioxidative enzymes, and metabolites
Three plants from each chamber and of each cultivar were sampled at 20, 30, and 40 DAG for vegetative and 50, 60, and 70 DAG for reproductive stage for analyses of photosynthetic pigments, antioxidative enzyme, and metabolites. Total chlorophyll and carotenoid contents were measured by using the methods of Maclachlan and Zalik (1963) and Duxbury and Yentsch (1956), respectively. Total phenolics content was determined in acetone extract by using the methodology of Bray and Thorpe (1954). Peroxidase activity and ascorbic acid (AA) content were estimated by the methods of Britton and Mehley (1955) and Keller and Schwager (1977), respectively. The extent of lipid peroxidation (LPO) was estimated as malondialdehyde (MDA) content following the protocol of Heath and Packer (1968).
Gas exchange measurements
Leaf gas exchange rates, i.e., net photosynthesis rate (Ps), stomatal conductance (gs), and internal CO2 were measured using portable photosynthetic system (Model LI-6200, LI-COR, Lincoln, Nebraska, USA), was recorded at 50, 60, and 70 DAG for reproductive stage. Portable photosynthetic system monitors the rate at which the CO2 concentration in the air changes for a short interval. The net photosynthetic rate is calculated using rate of change, amount of leaf area enclosed in leaf chamber, the volume of the enclosure, temperature, and pressure. Measurements were recorded between 0800 and 1000 hours local time on the third fully expanded and mature leaf oriented to normal irradiation during cloud-free days at reproductive stage. The system was calibrated using a known CO2 source of 509 ppm concentration. During measurements of photosynthesis, the photosynthetically active radiation ranged between 400–1100 μmol m−2 s−1, mean temperature varied from 22.1–32.7 °C, relative humidity ranged between 52–72 %, and CO2 concentration was 383–385 ppm. Three replicate measurements were conducted on three plants of each chamber and open plot.
Chlorophyll fluorescence was determined between 0900 and 1100 hours using a portable plant efficiency analyzer (Model, MK29414, Hansatech Instruments Ltd., UK) on the same flag leaves, where Ps measurements were taken. Leaf clips for dark adaptation were placed on the adaxial side of the leaves 30 min before measurement and then exposed to red light of 650 nm through LED at excitation irradiance of 3,000 μmol m−2 s−1. Minimum fluorescence (Fo) and maximum fluorescence (Fm) were measured, from which variable fluorescence (Fv) and ratio of variable and maximal fluorescence (Fv/Fm) were calculated.
Relative resistance
Ozone resistance (R%) were calculated using values of RGR at vegetative and reproductive stage in NFCs and FCs (control) as given by Reiling and Davison (1992)
Yield attributes
Plants were harvested at maturity to assess different yield parameters. Ten plants were sampled from each replicate OPs, FCs, and NFCs. Number and weight of ears per plant, number and weight of grains per plant, and weight of 1,000 grains (test weight) were recorded. Harvest index (HI) was calculated as the ratio of the weight of grains per plant and total above ground biomass of the plant. Data for yield is given for both the years of experiments, while other parameters and yield attributes were given for 2008–2009.
Yield response regression
The relationship between AOT40 and relative yield of PBW 343 and M 533 was determined for each crop by linear regression using SPSS software (SPSS Inc., version 16).
Statistical analysis
The data of the photosynthetic pigments, antioxidative enzymes, metabolites, growth parameters, biomass and RGR, and RSR were subjected to three-way analyses of variance (ANOVA) to examine the effects of cultivar, age and treatment, and their interactions on various variables. The data for yield attributes were subjected to two-way ANOVA tests to examine the individual and combined effects of cultivar and treatment. Yield data of two years were subjected to three-way ANOVA for examining the effects of individual factor year, cultivar, treatment, and their interaction on weight of seeds per plant. Duncan’s multiple range tests were performed as post hoc on all the parameters subjected to various ANOVA tests. All the statistical tests were performed using SPSS software (SPSS Inc., version 16). Pearson’s correlation test was used to explore the correlations among various parameters at different developmental phases influencing the yield.
Results
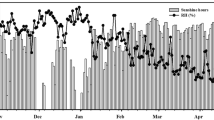
Mean day time (12 h) ambient O3 concentration (M 12) was 50.2 ppb in the year 2008–2009 with a range of 46.1–54.6 ppb (December–March). During the second year (2009–2010), mean O3 concentration was 53.2 ppb (Table 1) with variations from 49.6–56.4 ppb. Exceedance hours above 40 ppb O3 concentration were 72 and 82 h and 126 and 114 h, respectively, in 2008–2009 and 2009–2010 at vegetative stage. During the reproductive stage, exceedance hours were 117 and 115 h 2008–2009 and 144 and 176 h in 2009–2010. AOT40 values recorded in both growing season were 9.0 and 12.1 ppm h, respectively, in 2008–2009 and 2009–2010, and AOT40 values recorded at vegetative and reproductive stage were 2.23 and 6.9 ppm h in 2008–2009 and 2.9 and 9.2 ppm h in 2009–2010 (Table 1).
Biochemical analyses and photosynthetic pigments
Results presented in the study is mean value of 20, 30, and 40 DAG for vegetative stage and 50, 60, and 70 DAG for reproductive stage for all the parameters assessed. Lipid peroxidation measured as MDA concentration was significantly higher in plants of NFCs and OPs compared to FCs in both the cultivars (Fig. 1). In M 533, significant increase in MDA concentration was recorded at vegetative stage, but in PBW 343, LPO increased significantly at both the ages. Three-way ANOVA test showed MDA concentration varied significantly due to cultivar, treatment, cultivar × age, and age × treatment (Table 2). POD activity varied significantly due to cultivar, age, treatment, cultivar × age, and age × treatment interactions (Table 2). Higher POD activity was observed in M 533 than PBW 343 at both the ages in NFCs compared to FCs (Fig. 2). Ascorbic acid content increased significantly at vegetative phase in M 533 but at latter age increase of 4–5 %. In PBW 343, AA content increased at both the ages in NFCs (Fig. 2). Three-way ANOVA test for AA content showed significant variations due to cultivar and interaction between cultivar × age and age × treatment (Table 2).
Total phenolics increased significantly in NFCs by 72 and 110 % in M 533 and 50.3 and 47.3 % in PBW 343, respectively, at vegetative and reproductive stage (Fig. 2). Higher reductions in protein content were observed in M 533 compared to PBW 343 at both the ages of samplings. Variations in total phenolics were significant due to individual factors and their interactions (Table 2). Protein content varied significantly due to individual factors and interaction between cultivar and treatment (Table 2).
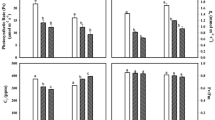
Total chlorophyll and carotenoid contents reduced significantly by 16.9 and 9.4 % in PBW 343 and 13.4 and 5.1 % in M 533 at vegetative stage and 22.7 and 19.7 % in PBW 343 and 18.6 and 10.1 % in M 533, respectively, in NFCs as compared to FCs at reproductive stage (Fig. 1). Significant variations in photosynthetic pigments was observed due to individual factors like cultivar, age, and treatment, and its interaction and carotenoid content varied significantly due to individual factors (Table 2). Ps decreased significantly in both the cultivars of wheat growing in NFCs as compared to FCs. The percent reductions recorded in Ps were 19 and 18.4 % in PBW 343 and M 533, respectively, at reproductive stage (Fig. 3). Results of stomatal conductance (gs) showed reductions of 33.2 % in PBW 343 and 43.6 % in M 533 in NFCs compared to FCs (Fig. 3). Results of two-way ANOVA test showed that Ps varied significantly due to cultivar and treatment, whereas significant variations in gs was observed due to individual factors and their interactions. In both the wheat cultivars, internal CO2 concentrations were higher in plants of NFCs with respect to the FCs (Fig. 3). Transpiration rate decreased by 17.2 and 15.5 % in PBW 343 and M 533, respectively, in NFCs. Significant reductions were also recorded in WUE 2.5 % in PBW 343 and M 533, grown in NFCs compared to FCs (Fig. 3). Results of two-way ANOVA test showed that internal CO2 concentration varied significantly due to cultivar, treatment, and their interaction; however, transpiration rate and water use efficiency varied significantly due to cultivar and treatment (Fig. 3).
Results of chlorophyll fluorescence kinetics showed significant increase in Fo grown in NFCs compared to FCs (Fig. 4). Increments recorded were 9.5 and 17.9 % in PBW 343 and M 533, respectively. Lower values of Fm (2 and 11 %) and Fv (7.1 and 15 %) in PBW 343 and M 533, respectively, were observed in NFCs compared to FCs. Reductions were also recorded in Fv/Fm ratio in PBW 343 and M 533. Variations in Fm and Fv were significant due to individual factors of cultivar, treatment, and their interactions; however, Fo and Fv/Fm varied significantly due to individual factors (Fig. 4).
Growth parameters
Reductions in number of leaves were 6.8 and 24.6 % in PBW 343 (Table 3) and 47 and 25.8 % in M 533 (Table 4) at vegetative and reproductive stage, respectively, in NFCs compared to FCs. Leaf area decreased at all the ages, but higher reduction was observed in PBW 343 than M 533 at reproductive stage. Variations in number of leaves per plant were significant due to cultivar, age, treatment, and interactions between cultivar × age and cultivar × age × treatment. Leaf area varied significantly due to all the individual factors and their interactions (Table 2).
A significant reduction in total biomass was observed in both the cultivars of wheat in NFCs compared to FCs (Tables 3 and 4). In wheat cultivars M 533 and PBW 343, the accumulation of biomass varied with age. Growth analysis showed significant reduction in RGR of PBW 343 grown in NFCs at both the ages. Reduction recorded in RGR (26 and 8 %) was higher in PBW 343 compared to M 533 between vegetative and reproductive stage (Tables 3 and 4). In M 533, RGR decreased at vegetative stage, but significant increase was recorded at reproductive stage in NFCs compared to FCs (Table 4). RGR did not show significant interactions between cultivar and treatment. Ozone resistance (R%) values showed higher resistance in M 533 at reproductive stage and at vegetative stage in PBW 343 (Table 5). RSR reduced significantly at vegetative stage in M 533 and reproductive stage in PBW 343 (Tables 3 and 4). Response of RSR varied significantly due to age, treatment, and interactions of cultivar × age and age × treatment (Table 2).
Yield attributes
At the time of harvest, the number and weight of ears per plant decreased significantly in both the cultivars PBW 343 and M 533 (Fig. 5). Number and weight of ears per plant reduced more in PBW 343 than in M 533 (17.8 %) (Fig. 5). Reductions recorded in number of grains per plant was 15.8 and 8.7 %, respectively, in PBW 343 and M 533 in NFCs compared to FCs. Similar trend was observed for test weight. Variation observed in HI was significant due to treatment. Results of two-way ANOVA showed that all parameters of yield attributes varied significantly due to individual factors except harvest index which did not vary significantly due to cultivar (Fig. 5). Number of grains per plant varied significantly due to individual factors and their interactions. Number and weight of grains per plant reduced significantly by 15.8 and 16.2 % in PBW 343 and 8.7 and 14.1 % in M 533, respectively, compared to FCs in 2008–2009. In the year 2009–2010, the weight of grains per plant reduced significantly by 19 and 18.8 %, respectively, in PBW 343 and M 533 (Table 6). Three-way ANOVA test showed significant variations in yield due to cultivar and treatment (Table 6).
Yield response regression
Results showed that with increasing O3 concentration, yield is negatively affected (Fig. 6). Linear regression between relative yield and AOT40 showed 6 % yield loss at AOT40 of 3 ppm h (95 % confidence interval) for both the cultivars (Fig. 6).
Discussion
The results of the present study clearly indicate the adverse effects of ambient O3 on two recent Indian wheat varieties PBW 343 and M 533, commonly grown in central Indo-Gangetic plain. Responses of both the cultivars, however, did not vary significantly between non-filtered chambers and open plots, suggesting that chamber effects are not significant.
Ambient daytime O3 (M 12) concentration varied from 51.3 to 53 ppb during wheat growing season between 2008–2009 and 2009–2010. The O3 concentrations recorded during the present study were higher than earlier reported values for eight hourly mean of 44 ppb (M 8) in 2006–2007 (Singh and Agrawal 2009), 45.3 ppb (M 12) in 2007–2008, and 47.3 ppb in 2008–2009 (Sarkar and Agrawal 2010) at the same experimental site. In the present study, results of cumulative O3 exposure indices above 40 ppb (AOT40) also showed higher values (1.4 times) than reported by Sarkar and Agrawal (2010) for the year 2007–2009 (7.9 ppm h). These monitoring data clearly depicts the increasing trend of O3 in rural areas of Indo-Gangetic plains. Roy et al. (2009) have also recorded a substantially higher simulated AOT40 values (12–14 ppm h) in Gangetic plains compared to other regions of India, having potentially higher adverse impact on vegetation. Higher occurrence of O3 concentration was recorded during reproductive compared to vegetative stage.
In the present study, LPO measured as MDA content increased in both the cultivars in NFCs compared to FCs, but higher increment was recorded in PBW 343 than M 533. Degree of LPO denotes the state of integrity of membrane, which is correlated with the level of antioxidants modifying the sensitivity of plants to O3 (Calatayud and Barreno 2004). Significant variations in MDA content due to cultivar × age and age × treatment interactions suggest that the response of cultivars to O3 varied with development stage. Rai et al. (2007) have reported higher increase in MDA concentration (47.4 %) in a sensitive wheat cultivar M 234 at a mean O3 concentration of 41 ppb compared to increase in PBW 343 (34.6 %) and M 533 (4.5 %) during the present study. Lipid peroxidation was more negatively correlated with yield in PBW 343 (r = −0.89, p < 0.01) than M 533 (r = −0.54, NS) at reproductive stage (Table 7), while lipid peroxidation was more influenced at vegetative stage in M 533 (Table 7).
Increase in MDA content was accompanied by reductions in photosynthetic pigments under ambient O3 concentration (NFCs) in both the cultivars. Generally, the loss of membrane integrity induces chlorophyll degradation, a general feature in plants subjected to oxidative stress as induced by O3 in the present study. O3 treatment may prevent chlorophyll synthesis leading to net decline in photosynthesis (Castagna et al. 2001). In the present study, chlorophyll content increased with age in M 533, while declined in PBW 343, which also showed higher MDA content. Significant interaction between cultivar × age × treatment suggests that chlorophyll content varied with age and O3 concentration between the cultivars. Carotenoids, an accessory pigment, play an important role in the protection of chlorophyll pigments under stress, also decreased in NFCs, but the range of reduction was higher in PBW 343 at reproductive age than M 533.
Reductions in photosynthetic pigments have been found associated with decrease in Ps following modification of chlorophyll fluorescence kinetics during the present study. The results further indicated that ambient O3 induced reductions in Ps rate may also be attributed to stomatal limitation as evidenced by reductions in gs and simultaneous increase in intercellular CO2 (Ci). Increase in Ci suggests reduced mesophilic activity (Feng et al. 2003), which was more pronounced in PBW 343 than M 533. Stomatal conductance is a key factor in determining O3 damage in plants. Stomatal conductance besides being dependent on metereological variables is influenced by the growth stage of the plants. Lehnherr et al. (1988) and Frederick (1997) found that maximum stomatal opening in wheat occurs at anthesis. Higher stomatal conductance was observed in PBW 343 at reproductive phase, suggesting more uptake of O3. Results of correlation matrix showed that yield in PBW 343 were influenced by impact of O3 concentrations on stomatal conductance than M 533 at reproductive stage. Both gs and Ci also varied significantly due to cultivar, treatment, and cultivar × treatment, thus suggesting coordinated response.
Photosynthetic rate varied significantly due to treatment, showing negative impact of ambient O3 on photosynthetic rate, but no cultivar variation was recorded. Reductions in transpiration rate during the present study can be directly correlated to gs response under O3 stress. Reductions observed in WUE of M 533 (2–5 %), and PBW 343 (2–4 %) were lower compared to sensitive wheat cultivar M 234 (10 %) (Rai et al. 2007). Lower magnitude of reductions in WUE of PBW 343 and M 533 is mainly due to higher magnitude of reductions in transpiration rate. No significant variations in WUE due to treatment and cultivar × treatment suggest that the cultivars did not vary in their response to ambient O3. The trend of WUE suggests that gs responded more negatively to O3 compared to Ps in both the cultivars.
The reduction in Ps may also occur due to structural damage of thylakoids, which may affect the photosynthetic electron transport, as observed in form of reductions in Fv/Fm ratio (maximum quantum yield). The activity of PS II is also closely associated with lipid peroxidation. The rate of electron transport reflects the efficiency of the light reactions in overall photosynthetic performance (Guidi et al. 2009). O3 exposure may negatively affect one or several of the steps in the electron transport process and oxidative stress caused by excessive excitation energy via higher photon capture by leaves, which can enhance the negative effects of O3 on photosynthetic apparatus (Feng et al. 2011). Reduction in Fv/Fm ratio (maximum quantum yield) under ambient O3 did not follow the same trend for both the cultivars. Reductions in Fv/Fm ratio was mainly due to increase in Fo without significant impact on the other chlorophyll fluorescence parameters (Fm, maximal fluorescence and Fv, variable fluorescence) in M 533, while in PBW 343, increase in Fo was associated with simultaneous decrease in Fm, suggesting greater structural modification in the later compared to former cultivar. Both the cultivars showed variations in light-harvesting activities under O3 impact as observed by significant variations in Fm and Fv due to O3 × cultivar interaction.
Plants have elaborative antioxidative system to combat general oxidative stress and are also linked with O3 stress tolerance (Fuhrer and Booker 2003). Apoplastic ascorbate has been proposed to constitute a first line of defense against O3 (Fuhrer and Booker 2003). An increase in AA at initial age of sampling in M 533 under ambient O3 suggests higher extracellular ascorbic acid involved in O3 detoxification, but a decrease at a later stage suggest that ascorbate is used as a substrate by ascorbate peroxidase to reduce H2O2 to water. This trend further suggests a higher detoxification capacity at the earlier stages of development to delay the effect of O3 on leaf senescence at vegetative phase. While in PBW 343, AA content did not change with age. The significant interaction of O3 × cultivar for ascorbic acid clearly indicates cultivar differences in response to ambient O3. Feng et al. (2010) also found that ascorbate increased by 33.5 % in Y16, a tolerant cultivar, than Y2 (26.7 %), a sensitive cultivar, of wheat at elevated O3 above ambient (66 ppb). Rai et al. (2007) reported more increase in AA at flag leaf stage compared to vegetative stage in a sensitive cultivar M 234, at a mean O3 concentration of 41 ppb.
An increase in POD activity in both the varieties in NFCs compared to FCs denotes higher production of H2O2 under oxidative stress caused by ambient O3, but a higher magnitude of increase was observed in M 533 than in PBW 343. When the results of POD activity was compared with an old and sensitive cultivar M 234, a higher magnitude of induction was recorded in present cultivars, suggesting that the recent cultivars had higher efficiency of scavenging H2O2.
Total phenolics and peroxidases increased, and ascorbic acid did not vary in M 533 at reproductive stage as compared to PBW 343, conferring greater protection against O3, as H2O2 is efficiently scavenged by peroxidases in plant vacuoles in the presence of phenolics and reduced ascorbate (Zancani and Nagy 2000). Total phenolics function as antioxidants either by enzymatic or direct radical scavenging mechanisms, when oxidized to their respective phenoxyl radicals through peroxidases. Phenoxyl radicals can be reduced to their parent compounds by nonenzymatic reactions with ascorbate (Takahama and Oniki 1992). In M 533, higher activity of peroxidases may be correlated with higher total phenolics at reproductive stage.
In the present study, protein content decreased significantly in both the cultivars especially at latter age in NFCs. Modification of structural proteins by altering their secondary and tertiary structure by ROS enhances their susceptibility to proteolysis under ambient O3. Reductions in protein content of different varieties of wheat (Sonalika and HUW 510) under ambient and elevated O3 concentrations have been reported (Sarkar et al. 2010). In the present study, M 533 showed more reductions in protein than PBW 343, whereas total phenolics increased more in M 533 than PBW 343 in NFCs compared to FCs with increasing age. This trend suggests that there is an inverse correlation between these two metabolites, catalyzed by phenylalanine ammonia lyase and use phenylalanine (PHE) as a precursor. Protein competition model of phenolic allocation suggests that protein–phenolic competition for PHE results in a process level trade-off between rates of protein synthesis and phenolic synthesis, and an inverse relation between protein and phenolic allocation exists (Jones and Hartley 1999).
The alterations at biochemical and physiological levels are translated in forms of morphological changes and yield losses under ambient O3 exposure. The O3-induced reduction in biomass and growth production have been reported for a wide variety of wheat (Pleijel et al. 2006; Rai et al. 2007; Akhtar et al. 2010; Sarkar and Agrawal 2010). In the present study, leaf area and number of leaves reduced significantly in both cultivars under NFCs/OPs compared to FCs. During the vegetative stage, greater magnitude of reductions were recorded in M 533, whereas at reproductive stage in PBW 343. Leaf area not only plays important role in light perception, water and nutrient use, growth, and yield potential but also in pollutant absorption. Higher reduction in leaf area was recorded at flag leaf stage in PBW 343. In M 533, smaller leaf area may have provided lower surface area for O3 uptake. Ozone sensitivity in wheat leaves increased with leaf age (Pleijel et al. 1998). Variations in leaf area and number of leaves were significant due to cultivar, age, treatment, and their interactions suggesting varietal differences in sensitivity at increasing O3 and age. Results of correlation showed that yield was influenced more by the number of leaves (r = 0.78, p < 0.01) and leaf area (r = 0.71, p < 0.001) at reproductive stage in PBW 343 than in M 533, where no significant variations were observed. During vegetative stage, however, significant correlation between the number of leaves (r = 0.88, p < 0.01), leaf area (r = 0.81, p < 0.01), and yield was observed in M 533, suggesting greater sensitivity to ambient O3 at vegetative stage (Table 7). Variations in number of leaves and leaf area at reproductive age influences supply of photosynthates towards developing ears and affects yield.
A significant reduction in total biomass was observed in both the cultivars of wheat in NFCs compared to FCs. Higher reductions at vegetative stage was observed in M 533 (39.6 %), whereas at reproductive stage in PBW 343 (25 %) in NFCs, suggesting larger leaf area and more number of leaves at low ambient levels led to a higher photosynthetic activity and promoted growth in PBW 343. At reproductive stage, higher absorption of ambient O3 due to higher stomatal conductance and large leaf area in PBW 343 compared to M 533 led to more change in functional attributes, hence alteration in photosynthate accumulation and allocation. Reductions in total biomass observed during the present study further confirmed the negative influence of O3 on functional processes of plants. Exposure to O3 may also alter the source–sink balance, decreasing assimilates partitioning to below ground organs, and causing decrease in root/shoot ratio. The root/shoot ratio decreased in both the cultivars. The magnitude of reduction in RSR was higher in M 533 at vegetative stage but no significant difference at reproductive stage. Relative growth rate, a measure of production efficiency of plants, increased in M 533 under ambient O3 and decreased in PBW 343 at reproductive phase. Reductions in RGR of PBW 343 reflect resource limitation and resource imbalance due to more reductions in leaf area and number of leaves leading to changes in assimilatory capacity of plants during reproductive phase. O3 resistance value (R%) showed that M 533 was more sensitive during vegetative stage as observed by higher reductions in the number of leaves, leaf area, total biomass, and alteration in root/shoot ratio; however, at reproductive stage, it showed lower gs, lower reductions in number of leaves, leaf area and biomass, and no alteration in photosynthates partitioning among root and shoot. While in PBW 343, higher stomatal conductance and larger leaf area led to more uptakes of pollutants and hence more negative effects on growth parameters and yield.
Number of ears per plant decreased in NFCs compared to FCs in both the cultivars during the present study with greater reduction in PBW 343. Though the numbers of ears were higher in PBW 343, the reduction under ambient O3 was also higher in the same variety compared to M 533. This may be due to the damage of embryo directly or by affecting the pollen and ovule, which combine to form embryo or due to the reaction products of O3 with plant metabolites, that might impair ovule and/or embryo development, changes in the supply of, or competition for assimilates or in the synthesis and distribution of hormones required for the successful seed development and maturation (Black et al. 2000). Weight of ears, however, showed higher values for M 533 compared to PBW 343 and also lower magnitude of reductions compared to latter cultivar. This suggests that though the number of ears in PBW 343 was more, but supplied assimilate was not enough during ear filling in this cultivar. The assimilate availability in M 533 was enough to fill-up comparatively lower number of ears leading to lower reduction in weight of ears. The same trend continued with the number of grains and weight of grains, which reduced less in M 533 compared to PBW 343. Harvest index is a parameter, which indicates the partitioning of dry matter between grain and aboveground biomass. Significant reductions in HI was recorded in PBW 343 under ambient O3, suggesting that relatively less dry matter partitioned into grain under ambient ozone. The ability of some species to maintain seed yield following O3 exposure might reflect either an intrinsically greater tolerance or a greater availability of assimilates to support compensatory growth. In an experiment conducted by Tiwari et al. (2005) using EDU to assess the O3 impacts on wheat cultivars found M 533 to be more resistant compared to M 234 at 40 ppb ambient O3. Results of correlation showed that more impact on yield in PBW 343 is mainly attributed due to more damage at reproductive phase than vegetative phase on the physiological, biochemical, and growth parameters, but its higher yield potential maintained its yield loss.
Results of crop response function data calculated in the present study showed that the critical level of AOT40 was 3 ppm h, leading to yield loss of 6 % (95 % CI; range 0–12.1 ppm h) for two consecutive years for test cultivars of wheat. This data has shown 1 % increase in yield losses than reported by Mills et al. (2007), stating critical level of AOT40 of 3 ppm h for 3 months for European countries leading to 5 % yield loss in wheat, and Sarkar and Agrawal (2010) reported critical level of AOT40 for two wheat cultivars (M 510 and Sonalika), which was 3 ppm h, leading to 5.4 % yield loss based on fumigation study. The most probable cause may be continuous increase in O3 concentrations in the Indo-Gangetic plains of India. The present wheat cultivars PBW 343 and M 533 are more sensitive than Sonalika and M 510 (Sarkar and Agrawal 2010), but less sensitive than M 234 (20.7 %). Emberson et al. (2009) based on pooled data on wheat across Asia using fumigation/filtration and chemical protectant experimental methods suggested that Asian crop and cultivars are more sensitive than the North American under ambient and elevated O3 environment. The present result also showed the same trend at 3 ppm h, leading to 6 % yield reduction.
Conclusions
The present investigation suggests that the tropical wheat cultivars (PBW 343 and M 533) showed similar sensitivity to O3 with respect to yield under ambient O3. Sensitivity to O3, however, varied between vegetative and reproductive phases. The mechanism of interaction with O3 also varied between the cultivar leading to different pathways of acquiring resistance under the ambient O3 stress. Cultivar PBW 343 showed better performance at vegetative stage, while M 533 at reproductive stage. Cultivar PBW 343 with larger leaf area and higher stomatal conductance is more sensitive to O3 compared to those with small leaf area and low stomatal conductance. Critical levels of a 3-month AOT40 of 3 ppm h led to 6 % yield reduction in tropical wheat cultivars, and there is a need to have more dataset establishing critical levels for tropical wheat cultivars due to variations in O3 concentration at different developmental stages and prevalence of higher ambient O3 concentrations in tropical countries.
Abbreviations
- AA:
-
Ascorbic acid
- AOT40:
-
Accumulated O3 over a threshold concentration of 40 ppb
- ANOVA:
-
Analysis of variance
- DAG:
-
Days after germination
- gs :
-
Stomatal conductance
- Fo:
-
Minimal fluorescence
- Fm:
-
Maximal fluorescence
- Fv:
-
Variable fluorescence
- Fv/Fm:
-
Photosynthetic efficiency
- FCs:
-
Filtered chambers
- K:
-
Potassium
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- N:
-
Nitrogen
- NFCs:
-
Non-filtered chambers
- OPs:
-
Open plots
- OTCs:
-
Open top chambers
- ppb:
-
Parts per billion
- POD:
-
Peroxidase
- P:
-
Phosphorus
- Ps:
-
Photosynthetic rate
- PAR:
-
Photosynthetically active radiation
- RGR:
-
Relative growth rate
- WUE:
-
Water use efficiency
References
Agrawal SB, Singh A, Rathore D (2005) Role of ethylene diurea (EDU) in assessing impact of ozone on Vigna radiata L. plants in a suburban area in Allahabad. Chemosphere 61:218–228
Akhtar N, Yamaguchi M, Inada H, Hoshino D, Kondo T, Izutaet T (2010) Effects of ozone on growth, yield and leaf gas exchange rates of two Bangladeshi cultivars of wheat (Triticum aestivum L.). Environ Pollut 158:2970–2976
Biswas DK, Xu H, Li YG, Sun JZ, Wang XZ, Han XG, Jiang GM (2008) Genotypic differences in leaf biochemical, physiological, and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biol 14:46–59
Black VJ, Black CR, Roberts JA, Stewart CA (2000) Impact of ozone on the reproductive development of plants. New Phytol 147:421–447
Bray HG, Thorpe WY (1954) Analysis of phenolic compounds of interest in metabolism. Methods of Biochem Anal 1:27–52
Britton C, Mehley AC (1955) Assay of catalase and peroxidase. In: Colowick SP, Kalpan NO (eds) Methods Enzymol Academic Press Inc., New York vol. 2 pp 764
Calatayud A, Barreno E (2004) Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments, and lipid peroxidation. Plant Physiol Biochem 42:549–555
Castagna A, Nali C, Ciompi S, Lorenzini G, Soldatini GF, Ranieri A (2001) Ozone exposure affects photosynthesis of pumpkin (Cucurbita pepo L.) plants. New Phytol 152:223–229
Cooley DR, Manning WJ (1987) The impact of O3 on assimilate partitioning in plants: a review. Environ Pollut 47:95–113
Davison AW, Barnes JD (1998) Effects of ozone on wild plants. New Phytol 139:135–151
Drogoudi PD, Ashmore MR (2002) Effects of elevated ozone on yield and carbon allocation in strawberry cultivars differing in developmental stage. Phyton 42:45–53
Duxbury AC (1956) Yentsch CS (1956). Plankton pigment monographs. J of Marine Res 15:19–101
Emberson L, Buker P (2011). Current knowledge of the impacts of ozone on food crops in South Asia. In: Mills G, Harmens H (eds) Ozone Pollution: A hidden threat to food security. ICP Vegetation Report, Center for Ecology, Bangor, UK pp 83–92
Emberson LD, Buker P, Ashmore MR, Mills G, Jackson LS, Agrawal M, Atikuzzaman MD, Cinderby S, Engardt M, Jamir C, Kobayashi K, Oanh NTK, Quadir QF, Wahid A (2009) A comparison of North- America and Asian exposure–response data for ozone effects on crop yields. Atmos Environ 43:1945–1953
Feng Z, Jin M, Zhang F (2003) Effects of ground-level (O3) pollution on the yields of rice and winter wheat in the Yangtze River Delta. J Environ Sci 15:360–362
Feng Z, Pang J, Nouchi I, Kobayashi K, Yamakawa T, Zhu J (2010) Apoplastic ascorbate contributes to the differential ozone sensitivity in two varieties of winter wheat under fully open airfield conditions. Environ Pollut 158:3539–3545
Feng Z, Pang J, Kobayashi K, Zhu J, Ort DR (2011) Differential responses in two varieties of winter wheat to elevated O3 concentration under fully open airfield conditions. Global Change Biol 17:580–591
Frederick JR (1997) Winter wheat leaf photosynthesis, stomatal conductance, and leaf nitrogen concentration during reproductive development. Crop Sci 37:1819–1826
Fuhrer J, Booker F (2003) Ecological issues related to ozone: agricultural issues. Environ Int 29:141–154
Guidi L, Degl’Innocenti E, Martinelli F, Piras M (2009) Ozone effects on carbon metabolism in sensitive and insensitive Phaseolus cultivars. Environ Exper Bot 66:117–125
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hunt R (1982) Growth curves. Edward Arnold Publishers Ltd., London
Jones GG, Hartley SE (1999) A protein competition model of phenol allocation. Oikos 86:27–44
Kangasjärvi J, Jaspers P, Kollist H (2005) Signaling, gene expression and cell death in ozone-exposed plants. Plant Cell Environ 28:1021–1036
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J Forest Pathol 7:338–350
Lehnherr B, Ma¨chler F, Grandjean A, Fuhrer J (1988) The regulation of photosynthesis in leaves of field-grown spring wheat (Triticum aestivum L., cv. Albis) at different levels of ozone in ambient air. Plant Physiol 88:1115–1119
Leisner CP, Ainsworth EA (2012) Qunatifying the effects of ozone on plant reproductive growth and development. Global Change Biol 18:606–616
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can J Bot 41:1053–1062
Mills G, Buse A, Gimeno B, Bermejo V, Holland M, Emberson L, Pleijel H (2007) A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos Environ 41:2630–2643
Pleijel H, Danielsson H, Gelang J, Slid E, Sèllden G (1998) Growth stages dependence of the grain yield response to ozone in spring wheat (Triticum aestivum L.). Agricult. Ecosyst Environ 70:61–68
Pleijel H, Eriksen AB, Danielsson H, Bondesson N, Selldén G (2006) Differential ozone sensitivity in an old and a modern Swedish wheat cultivar grain yield and quality, leaf chlorophyll, and stomatal conductance. Environ Exper Bot 56:63–71
Rai R, Agrawal M (2008) Evaluation of physiological and biochemical responses of two rice (Oryza sativa L.) cultivars to ambient air pollution using open top chambers at a rural site in India. Sci Total Environ 407:679–691
Rai R, Agrawal M, Agrawal SB (2007) Assessment of yield losses in tropical wheat using open top chambers. Atmos Environ 41:9543–9554
Rai R, Agrawal M, Agrawal SB (2011) Effects of ambient O3 on wheat during reproductive development: gas exchange, photosynthetic pigments, chlorophyll fluorescence, and carbohydrates. Photosynthetica 49:285–294
Reiling K, Davison AW (1992) Effects of a short ozone exposure given at different stages in the development of Plantago major L. New Phytol 121:643–647
Roy SD, Beig G, Ghude SD (2009) Exposure–plant response of ambient ozone over the tropical Indian region. Atmos Chem Phys 4:3359–3380
Royal Society (2008) Ground-level ozone in the 21st century: future trends, impacts and policy implications. Science Policy report 15/08. The Royal Society, London
Sarkar A, Agrawal SB (2010) Elevated ozone and two modern wheat cultivars: an assessment of dose-dependent sensitivity with respect to growth, reproductive, and yield parameters. Environ Exper Bot 69:328–337
Sarkar A, Rakwal R, Agrawal SB, Shibato J, Ogawa Y, Yoshida Y, Agrawal GK, Agrawal M (2010) Investigating the impact of elevated levels of O3 on tropical wheat using integrated phenotypical, physiological, biochemical, and proteomics approaches. J Proteome Res 9:4565–4584
Singh S, Agrawal SB (2009) Use of ethylene diurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivarsof Indian wheat (Triticum aestivum L.). Environ Monit Assess 159:125–141
Singh A, Agrawal SB, Rathore D (2005) Amelioration of Indian urban air pollution phytotoxicity in Beta vulgaris by modifying NPK nutrients. Environ Pollut 134:385–395
Takahama U, Oniki T (1992) A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Phsiol Plant 101:845–852
Teixeira E, Fischer G, van Velthuizen H, van Dingenen R, Dentener F, Mills G, Walter C, Ewert F (2011) Limited potential of crop management for mitigating surface ozone impacts on global food supply. Atmos Environ 45:2569–2576
Tiwari S, Agrawal M, Manning JW (2005) Assessing the impact of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylenediurea (EDU). Environ Pollut 138:153–163
Tiwari S, Agrawal M, Marshall F (2006) Evaluation of ambient air pollution impact on carrot plants at a suburban site using open top chamber. Environ Monit Assess 266:15–30
Van Dingenen R, Dentener FJ, Raes F, Krol MC, Emberson L, Cofala J (2009) The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos Environ 43:604–618
Zancani M, Nagy G (2000) Phenol dependent H2O2 breakdown by soybean root plasma membrane bound peroxidase is regulated by ascorbate and thiols. J Plant Physiol 156:295–299
Acknowledgments
The authors are thankful to the Head of the Department of Botany for all the laboratory facilities and to the University Grant Commission, New Delhi and Department of Science and Technology, New Delhi for providing financial support to the work. Richa Rai is grateful to the Council of Scientific and Industrial Research, New Delhi for awarding Research Associate fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Rai, R., Agrawal, M. Assessment of competitive ability of two Indian wheat cultivars under ambient O3 at different developmental stages. Environ Sci Pollut Res 21, 1039–1053 (2014). https://doi.org/10.1007/s11356-013-1981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1981-6