Abstract
Characterization of groundwater quality allows the evaluation of groundwater pollution and provides information for better management of groundwater resources. This study characterized the shallow groundwater quality and its spatial and seasonal variations in the Lower St. Johns River Basin, Florida, USA, under agricultural, forest, wastewater, and residential land uses using field measurements and two-dimensional kriging analysis. Comparison of the concentrations of groundwater quality constituents against the US EPA’s water quality criteria showed that the maximum nitrate/nitrite (NO x ) and arsenic (As) concentrations exceeded the EPA’s drinking water standard limits, while the maximum Cl, SO 2 −4 , and Mn concentrations exceeded the EPA’s national secondary drinking water regulations. In general, high kriging estimated groundwater NH +4 concentrations were found around the agricultural areas, while high kriging estimated groundwater NO x concentrations were observed in the residential areas with a high density of septic tank distribution. Our study further revealed that more areas were found with high estimated NO x concentrations in summer than in spring. This occurred partially because of more NO x leaching into the shallow groundwater due to the wetter summer and partially because of faster nitrification rate due to the higher temperature in summer. Large extent and high kriging estimated total phosphorus concentrations were found in the residential areas. Overall, the groundwater Na and Mg concentration distributions were relatively more even in summer than in spring. Higher kriging estimated groundwater As concentrations were found around the agricultural areas, which exceeded the EPA’s drinking water standard limit. Very small variations in groundwater dissolved organic carbon concentrations were observed between spring and summer. This study demonstrated that the concentrations of groundwater quality constituents varied from location to location, and impacts of land uses on groundwater quality variation were profound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater pollution is a growing concern everywhere in the world. Groundwater quality degradation in an aquifer is a result of natural conditions and human activities. Natural conditions affect water quality in an aquifer by means of recharge to and discharge from the aquifer, dissolution of minerals, and mixing of fresh groundwater with residential water or intruded seawater (Canter 1996; Boniol 1996; Ouyang 2012). Human activities influence groundwater quality through the vadose zone leaching and ditch seepages of contaminants due to accidental spill, leakage, and inappropriate application of contaminants and fertilizers at the land surface; the upcoming of water with high dissolved solids from the deep zone due to groundwater withdrawals; and the introduction of irrigation water from deep aquifers to surficial aquifers (Boniol 1996; Ouyang 2012).
Despite a need to understand groundwater quality status in the Lower St. Johns River Basin (LSJRB), Florida, and its potential adverse environmental impacts upon surface water quality, there are few data sets that have comprehensively summarized the shallow groundwater quality status. Boniol (1996) reported that the maximum concentrations of nitrate were 16.1 and 11.1 mg/L, respectively, in the Floridan aquifer and surficial aquifer of the LSJRB. In a preliminary study, however, we found that the maximum NO x concentration can be up to 43 mg/L in a residential septic tank disposal area. Although these studies have provided good insights into the shallow groundwater N contamination status in the LSJRB, the spatial and seasonal variations of shallow groundwater nutrients and other constituents and their potential adverse environmental impacts upon surface water quality are still poorly understood. With an increased understanding of the importance of groundwater resources for human consumption, agricultural and industrial uses, and ecosystem health, there also is a greater need to evaluate groundwater quality.
The goal of this study was to characterize the shallow groundwater quality under four major land uses, namely agricultural, forest, wastewater, and residential areas in the LSJRB. The specific objectives were to (1) determine the spatial and seasonal variations of the shallow groundwater constituents such as nutrients, cations, anions, heavy metals, and redox potential using ArcGIS geostatistic package in conjunction with field measurements and (2) evaluate the shallow groundwater quality using the EPA’s water quality criteria.
Materials and methods
Study area and sampling
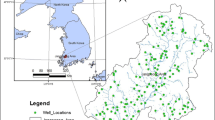
In this study, water quality data collected from the shallow groundwater system in the north area of the LSJRB during spring and summer of 2005 and 2007 were used for the analyses. The LSJRB is located in northeast Florida, between 29° and 30° N and between 81.13° and 82.13° W (Fig. 1), with an area of about 7,192 km2. Land uses within the basin largely consist of residential, commercial, industrial, mining, ranching, row crop, forest, and surface water. A series of water quality problems including point and nonpoint source pollutants such as nutrients, hydrocarbons, pesticides, and heavy metals (Campbell et al. 1993; Durell et al. 2001) have been identified and addressed since the 1950s.
Fifty-nine shallow groundwater wells (Fig. 1) were installed or activated in 2003–2004 for the purpose of monitoring groundwater quality under agricultural, forest, wastewater, and residential land uses in the LSJRB. Well casing depths range from 4 to 7 m, which are considered shallow groundwater wells in Florida. The groundwater samples were collected seasonally and/or bi-weekly for a 3-year period by a contractor from 2005 to 2007. All sampling activities were conducted in accordance with the standard operating procedures for the collection and analysis of water quality samples and field data (SJRWMD 2010). These standard operating procedures are in compliance with the US EPA’s standard methods for groundwater sampling and analysis. Statistical analysis was performed with SAS 9.0, and all of the experimental data were statistically evaluated at α = 0.05.
Kriging analysis
Field data provide information on groundwater quality constituent concentrations at the specific sampled sites but do not provide the same information on other unsampled locations. Therefore, the spatial and seasonal variations of the constituents across the entire study area within the LSJRB cannot be thoroughly determined. Since it is difficult and expensive to perform field measurements for every location in the LSJRB, the kriging estimate was employed in this study to provide a quantitative estimate of spatial and seasonal variations in groundwater quality constituent concentrations. This information is useful for identifying the locations of the highly contaminated spots in the study area.
Spatial distributions of groundwater quality constituent concentrations in the north area of the LSJRB were determined by ordinary kriging estimation using the ArcGIS geostatistical analyst tool. The ordinary kriging is a weighted-linear-average estimator where the weights are chosen to minimize the estimated (kriged) variance. It uses data from a single data type to predict values of that same data type at unsampled locations. The details for mastering the art of kriging are published elsewhere (Cooper and Istok 1988; ASCE 1989; Isaaks and Srivastava 1989; Rouhani et al. 1996; Goovaerts 1999; Ouyang et al. 2002).
Kriging procedures used in this study include: (1) preliminary data analysis, (2) data structural analysis, and (3) kriging estimation. Prior to kriging estimation, descriptive statistics were performed to examine the groundwater quality data collected from the LSJRB. Histogram plots of the data showed that the groundwater quality constituents were somewhat abnormally distributed. In general, a normal distribution requirement in kriging analysis may not be so critical unless the data set is too skewed or contains outliers. If that is the case, some kind of transformation is needed.
A data structural analysis was performed to determine the spatial correlation of the groundwater quality data, including experimental variogram, structural variogram model, and cross validation analyses. The experimental variogram is an inverse measure of the two-point covariance function for a stationary stochastic process. A variogram map was constructed to determine if the spatial correlation structure of the groundwater quality data is dependent upon direction. Since the spatial correlated distribution of the water quality data did not apparently depend on direction, an isotropic spherical model was selected to fit the experimental variograms. The model-fitting procedure was performed graphically in order to find a structure that would be as close as possible to the experimental variogram curves.
Cross validation is a general procedure that checks the compatibility between a set of data and a structural model. The difference between the measured value and the cross validation estimated value is the estimation error, which gives an indication of how well the data value fits into the neighborhood of the surrounding data values. The cross validation standardized errors between −2.5 and 2.5 represent robust data and indicate that a model can correctly predict the estimated values. The kriging domain used in this study was 30 km × 40 km, which encompassed the entire study area within the LSJRB.
Results and discussion
General groundwater quality assessment
The shallow groundwater quality in the LSJRB can be characterized by chemical constituents and other properties. The chemical constituents selected for this study include major cations such as calcium (Ca), magnesium (Mg), manganese (Mn), and sodium (Na); major anions such as chloride (Cl), sulfate (SO 2 −4 ), and carbonate alkalinity (HCO −3 + CO −3 ); nutrients such as total nitrogen (TN), ammonium (NH +4 ), nitrate (NO −3 ), nitrite (NO −2 ), total phosphorus (TP), and phosphate (PO 3 −4 ); and heavy metals such as arsenic (As) and lead (Pb). Other properties used for this study include dissolved organic carbon (DOC) and redox potential (ROP).
Analytical results show that concentrations of chemical constituents and values of other properties varied from location to location as well as from season to season. Table 1 summarizes the descriptive statistics, including the number of samples; the minimum, maximum, and mean concentrations; and the standard deviations of the selected chemical constituents and other properties. The US EPA’s water quality criteria are also given in the table. Comparison of the concentrations of groundwater quality constituents with EPA’s water quality criteria shows that the maximum NO x and As concentrations exceeded the EPA’s drinking water standard limits, while the maximum Cl, SO 2 −4 , and Mn concentrations exceeded the EPA’s national secondary drinking water regulations (NDWRs). The NDWRs are nonenforceable guidelines regulating contaminants that may cause cosmetic effects or aesthetic effects in drinking water. Table 1 further reveals that the maximum TN and TP concentrations exceeded the ambient water quality criteria recommendations for rivers and streams in nutrient ecoregion XII (southeastern area). Discharge of this groundwater with high TP and TN concentrations into the Lower St. Johns River (LSJR) would degrade the ambient water quality.
Further analysis of the groundwater quality data shows that there were 14 and 2 wells, respectively, with NO x and As concentrations exceeding the EPA’s drinking water limits, whereas there were 3, 4, and 59 wells, respectively, with the SO 2 −4 , Cl, and Mn concentrations exceeding the EPA’s secondary drinking water regulations. However, it should be pointed out that the groundwater quality data were collected from the shallow groundwater system, which is within 7 m in depth. Most of the Florida residents who use the well water as a drinking water normally have wells with more than 33 m in depth. However, contamination of shallow groundwater with these constituents could pose threats to deeper groundwater aquifer through percolation as well as to the adjacent surface waters through discharge.
This study further reveals that there were 14 and 23 wells, respectively, with TN and TP exceeding the ambient water quality criteria recommendations for rivers and streams in nutrient ecoregion XII (Table 1), which could contaminate the LSJR due to the groundwater discharge.
Spatial and seasonal variations of nutrients
Figures 2 through 5 show the spatial distributions of groundwater nutrient concentrations (in milligram per kilogram) in spring and summer. In general, higher kriging estimated concentrations of NH +4 were found around the Julington Creek (agricultural) area (Fig. 2). Within this area, the kriging estimated NH +4 concentration was higher in summer than in spring. For example, the estimated concentration of NH +4 was about 1.3 mg/L in summer around well HL-WM2, but it was about 1.1 mg/L in spring at the same location. The former was about 18 % higher than the latter. Figure 2 further reveals that the extent of the estimated NH +4 with high concentrations was larger in summer than in spring in the area around well HL-WM2. Results indicate that seasonal variations in groundwater NH +4 concentrations are significant although the exact reasons remain unknown. A possible explanation of this phenomenon would be more NH +4 leaching from the vadose zone into the shallow groundwater due to the wetter summer.
Changes in kriging estimated NO x concentrations in spring and summer are shown in Fig. 3. Unlike the case of NH +4 , high estimated NO x concentrations were observed at the Strawberry Creek and Red Bay Branch areas. These were the residential areas with a high density of septic tank distribution. The high concentration and large extent of NO x distribution in the areas were presumably attributed to the leakage of NO x from the septic tanks into the shallow groundwater. Under aerobic conditions, NH +4 can be oxidized into NO x by certain microorganisms in the soil. This negatively charged NO x would leach through the vadose zone into the shallow groundwater. Septic tank system is the most common form of on-site wastewater management system. It has been reported that among all of the groundwater pollution sources, septic tank systems discharge the greatest total volume of wastewater directly into soils overlaying groundwater and are the second largest source of groundwater nutrient contamination in the USA (Ouyang and Zhang 2012).
Considerable variations in groundwater NO x concentration distribution pattern were also observed between spring and summer. It is apparent from Fig. 3 that more areas were found with high kriging estimated NO x concentrations in summer than in spring. This occurred partially because of more NO x leaching into the shallow groundwater due to the wetter summer and partially because of faster nitrification rate due to the higher temperature in summer.
Kriging estimated TN concentrations showed a similar trend in both spring and summer (Fig. 4). It further appears that high estimated TN concentrations were located in the areas near both ends of the Buckman Bridge. This distribution pattern is similar to that of NH +4 in summer, indicating that TN and NH +4 may come from similar sources and were most likely from the chemical fertilizers in these residential areas with a low density of septic tank distribution.
Figure 5 shows the spatial distribution of groundwater TP concentrations in spring and summer. In general, large extent and high kriging estimated TP concentrations were found from Cedar River to Trout Creek (residential) areas. It is also evident that variations in estimated TP concentrations between spring and summer were discernable as shown in Fig. 5.
Spatial and seasonal variations of cations and DOC
Spatial variations of the kriging estimated groundwater As concentrations and DOC contents in spring and summer are shown in Figs. 6 and 7. Arsenic is a ubiquitous trace metal found in environments throughout the world. The major sources of As pollution are anthropogenic and natural inputs. Anthropogenic sources include mining and smelting of metalliferous ores, municipal waste, landfill leachates, fertilizers, pesticides, and sewage (Forstner 1995; Rio et al. 2002). Natural sources of As pollution include As-rich parent materials as As easily substitutes for Si, Al, or Fe in silicate minerals, volcanic activities, wind-borne soil particles, sea salt sprays, and microbial volatilization (Nriagu 1994; Bhumbla and Keefer 1994). Long-term exposure to As can lead to a variety of skin, neurological and peripheral vascular disorders, and cancers of the skin, bladder, liver, lung, kidney, and colon. In addition, diabetes, ischemic heart disease, reproductive effects, and impairment of liver function have also been linked to As exposure (Bhumbla and Keefer 1994). Figure 6 shows the spatial distribution of groundwater As concentrations in spring and summer. It is apparent from this figure that higher groundwater As contents were found around the Julington Creek and Peters Branch (agricultural) areas with concentrations exceeding the EPA’s drinking water standard limit. Although the exact sources of the arsenic contamination in this area remain to be investigated, the possible sources would be from chromated copper arsenate (CCA), geologic sources (phosphate rock and limestone mining), and arsenic herbicide (monosodium methylarsonate). Solo-Gabriele et al. (2003) performed a comprehensive investigation on arsenic sources within the state of Florida. These authors found that among the arsenic contamination sources, about 70 % is associated with the production of CCA-treated wood, 20 % is associated with geologic sources, and 4 % was associated with the arsenical herbicide monosodium methylarsonate. CCA is a chemical used for wood preservative treatment in the state of Florida and has been accumulated in the surface reservoirs due to the in-service use of the wood product and due to the disposal of CCA-treated wood (Solo-Gabriele et al. 1998). The Julington Creek area has been used for the CCA-treated wood transportation. In addition to the application of arsenical herbicide for agricultural practices, arsenic trioxide has been used to eradicate the cattle tick which carried a microbe (Boophilus annulatus) that caused cattle fever, an illness which resulted in weight loss, reduced milk production, and weakness among cattle. These herbicide and pesticide could be the sources for Peters Branch. While the shallow groundwater is not used as the drinking water by the local residents, further study is warranted to identify the sources of As in the areas. The spatial distribution of groundwater As concentrations was relatively more even in summer than in spring.
Naturally occurring DOC is an important feature of stream water quality. It contributes significantly to the acidity of natural waters through organic acids, biological activities through the absorption of light, and water chemistry through the complexation of metals and production of carcinogenic compounds with chlorine. In addition, by forming organic complexes, DOC can influence nutrient availability and control the solubility and toxicity of contaminants. DOC can also increase the weathering rate of minerals and increase the solubility and thus the mobility and transport of many metals and organic contaminants (Ouyang 2003). Figure 7 shows the spatial distributions of groundwater DOC concentrations in spring and summer. In general, very small variations in groundwater DOC concentrations were observed between spring and summer. The lower DOC concentrations were found around the Browns Creek area, whereas the high DOC concentrations were observed around the Doctors Lake area (more trees coverage).
In general, the groundwater Na concentration distribution was relatively more even in summer than in spring (figure not shown). We attributed this discrepancy to the dilution effects of rainwater during the wetter summer. Differences in groundwater Mg concentrations developed between spring and summer (figure not shown). In other words, the spatial distribution of groundwater Mg concentrations was relatively more even in summer than in spring. There was a large spot with a higher Mg concentration in the south of well RV-MW3 in spring as compared to that in summer. A wetter summer could explain the phenomenon. As more rainwater leached into the groundwater, more Mg was diluted and resulted in more even distribution of Mg.
Conclusion
Field data analysis shows that concentrations of groundwater quality constituents varied from location to location as well as from season to season. Comparison of the concentrations of groundwater quality constituents with EPA’s water quality criteria shows that the maximum nitrate/nitrite (NO x ) and As concentrations exceeded the EPA’s drinking water standard limits, while the maximum Cl, SO 2 −4 , and Mn concentrations exceeded the EPA’s NDWRs).
It is also apparent that the maximum TN and TP concentrations exceeded the ambient water quality criteria recommendations for rivers and streams in nutrient ecoregion XII (southeastern area). Discharge of this groundwater with high TP and TN concentrations into the LSJR would degrade the ambient water quality.
Further analysis of the groundwater quality data reveals that there were 14 and 2 wells, respectively, with NO x and As concentrations exceeding the EPA’s drinking water limits, whereas there were 3, 4, and 59 wells, respectively, with SO 2 −4 , Cl, and Mn concentrations exceeding the EPA’s secondary drinking water regulations. Furthermore, there were 14 and 23 wells, respectively, with TN and TP concentrations exceeding the ambient water quality criteria recommendations for rivers and streams in nutrient ecoregion XII, which would potentially contaminate the LSJR due to the groundwater discharge.
In general, high kriging estimated groundwater NH +4 concentrations were found around the Julington Creek agricultural area. Within this area, the estimated NH +4 concentrations were higher in summer than in spring. Results indicate that seasonal variations in groundwater NH +4 concentrations were significant although the exact reasons remain unknown. A possible explanation of this phenomenon would be more NH +4 leaching from the vadose zone into the shallow groundwater due to the wetter summer.
Unlike the case of NH +4 , high kriging estimated groundwater NO x concentrations were observed at the Strawberry Creek and Red Bay Branch areas. These were the residential areas with a high density of septic tank distribution. The high concentration and large extent of NO x distribution in the areas were presumably attributed to the leakage of NO x from the septic tanks into the shallow groundwater. It has been reported that among all of the groundwater pollution sources, septic tank systems discharge the greatest total volume of wastewater directly into soils overlaying groundwater and are the second largest source of groundwater nutrient contamination in the USA.
This study further reveals that more areas were found with high estimated NO x concentrations in summer than in spring. This occurred partially because of more NO x leaching into the shallow groundwater due to the wetter summer and partially because of faster nitrification rate due to the higher temperature in summer.
It appears that high estimated TN concentrations were located in the areas near both ends of the Buckman Bridge. This distribution pattern was similar to that of NH +4 in summer, indicating that TN and NH +4 could come from similar sources and were most likely from the chemical fertilizers in these residential areas with a low density of septic tank distribution.
Large extent and high kriging estimated TP concentrations were found from the Cedar River to the Trout Creek areas. It is also evident that variations in kriging estimated TP concentrations were discernable between spring and summer.
Higher kriging estimated groundwater As concentrations were found around the Julington Creek and Peters Branch areas, which exceeded the EPA’s drinking water standard limit. Although the shallow groundwater is not used as drinking water by the local residents, further study is warranted to identify the sources of As and potential migration of As from shallow groundwater into the deep aquifer in the areas. Very small variations in groundwater DOC concentrations were observed between spring and summer.
It should be pointed out that in Florida, shallow groundwater is not recommended as drinking water. Our findings (e.g., shallow groundwater is contaminated by nutrients and heavy metals) further strengthen this recommendation. Contamination of shallow groundwater with such pollutants could pose threats to the deeper groundwater aquifer through percolation as well as to the adjacent surface waters through discharge.
Further study is warranted to estimate the discharges of the shallow groundwater quality constituents (with concentrations exceeding the EPA’s water quality criteria) into the LSJR and their potential adverse impacts upon the river water quality.
References
ASCE (American Society of Chemical Engineers) (1989) Review of geostatistics in geohydrology. I: Basic concepts. J Hydr Eng 116:612–632
Bhumbla DK, Keefer RF (1994) Arsenic mobilisation and bioavailability in soil. In: Nriagu JO (ed) Arsenic in the environment, part I: cycling and characterization. Wiley, New York, pp 51–82
Boniol D (1996) Summary of groundwater quality in the St Johns River Water Management District. Special Publication SJ96-SP13. St. Johns River Water Management District, Palatka
Campbell D, Bergman M, Brody R, Keller A, Livingston-Way P, Morris F, Watkins B (1993) SWIM plan for the Lower St Johns River Basin. St. Johns River Water Management District, Palatka
Canter LW (1996) Nitrates in groundwater. Lewis Publishers, New York, p 263
Cooper RM, Istok JD (1988) Geostatistics applied to groundwater contamination. I: Methodology. J Hydr Eng 114:270–286
Durell GS, Seavey JA, Higman J (2001) Sediment quality in the Lower St. Johns River and Cedar-Ortega River Basin: chemical contaminant characteristics. Battelle, Duxbury
Forstner U (1995) Land contamination by metals global scope and magnitude of problem. In: Huang CP, Baley GW, Bowers ER, Allen HE (eds) Metal speciation and contamination of soil. CRC Press, Boca Raton
Goovaerts P (1999) Geostatistics in soil science: state-of-art and perspectives. Geoderma 89:1–45
Isaaks EH, Srivastava RM (1989) An introduction to applied geostatistics. Oxford University Press, New York
Nriagu JO (1994) Arsenic in the environment. Part II: Human health and ecosystem effects. Wiley, New York
Ouyang Y, Higman J, Thompson J, O’Toole T, Campbell D (2002) Characterization and spatial distribution of heavy metals in sediment from Cedar and Ortega Rivers Basin. J Cont Hydrol 54:19–35
Ouyang Y (2003) Simulating dynamic load of naturally occurring TOC from watershed into a river. Water Res 37:823–832
Ouyang Y (2012) Estimation of shallow groundwater discharge and nutrient load into a river. Ecol Eng 38:101–104
Ouyang Y, Zhang JE (2012) Quantification of shallow groundwater nutrient dynamics in septic areas. Water Air Soil Pollut 223:3181–3193
Rio MD, Font R, Almela C, Velez D, Montoro R, Bailon ADH (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J Biotech 98:125–137
Rouhani SR, Srivastava M, Desbarats AJ, Cromer MV, Johnson AI (1996) Geostatistics for environmental and geotechnical applications. ASTM, West Conshohocken
SJRWMD (St. Johns River Water Management District) (2010) Field standard operating procedures for surface water sampling. St. Johns River Water Management District, Palatka
Solo-Gabriele HM, Sakura-Lemessy DM, Townsend T, Dubey B, Jambeck J (2003) Quantities of arsenic within the State of Florida. Florida Center For Solid and Hazardous Waste Management, Gainesville
Solo-Gabriele HM, Townsend T, Penha J, Tolaymat T, Calitu V (1998) Generation, use, disposal, and management options for CCA treated wood. Report #98-01. Florida Center for Solid and Hazardous Waste, Gainesville, FL. http://www.ccaresearch.org/publications.htm. Accessed 2 June 2013
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Ouyang, Y., Zhang, JE. & Parajuli, P. Characterization of shallow groundwater quality in the Lower St. Johns River Basin: a case study. Environ Sci Pollut Res 20, 8860–8870 (2013). https://doi.org/10.1007/s11356-013-1864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1864-x