Abstract
A total of 129 groundwater samples were collected in the Jangseong region of South Korea to characterize and evaluate groundwater quality and its suitability for irrigation and domestic uses. Samples were chemically analyzed for major ions, pH, electrical conductivity, and total dissolved solids following standard methods. The AquaChem 2014.2 model linked with PHREEQC was used for the statistical analysis and characterization of the hydrochemistry of the groundwater. The analysis showed that in all samples Ca–HCO3 was the leading water type and that the abundance of major cations was in the order Ca > Na > Mg > K, and of anions in the order HCO3 > Cl > SO4 > F. According to the correlation analysis, Ca showed strong interdependence with HCO3, suggesting that these parameters may have originated from common sources. Saturation index calculations indicated that all samples were undersaturated with respect to aragonite, calcite, dolomite, fluorite, gypsum, halite, and siderite, and oversaturated with respect to goethite and hematite. The irrigation suitability analysis revealed that groundwater in the Jangseong area can be used for irrigation without any restrictions based on EC, sodium adsorption ratio, percent sodium, residual sodium carbonate, Kelley ratio, permeability index, and the US Salinity Laboratory diagram analysis. The drinking water suitability analysis made for major parameters by comparison with the WHO guidelines indicates that the groundwater in the area is suitable for drinking except in some samples with high nitrate–N concentrations. The elevated nitrate concentrations in the groundwater are likely an indicator of agricultural pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater has long been used as a source of water for domestic, agricultural, and industrial activities all over the world. Recently, the demand for groundwater has increased greatly due to increased population, industrialization, and urbanization, and this has led to problems such as deterioration of water quality, land subsidence, and groundwater salinization (Zubari 1999; Park et al. 2011; Haque et al. 2013; Kabir et al. 2014).
In the Republic of Korea, groundwater has been a key water resource to supplement the increasing demand for water supply related to growing economic development and agricultural advances (Lee and Kwon 2016). This is largely because significant changes in annual and seasonal precipitation and more frequent occurrences of drought due to climate change, have affected surface water resources. This has attracted a nationwide focus on activity to search for and secure another water supply source (Nam et al. 2015).
Recently, the occurrence of more frequent and extended droughts, in particular, has focused more attention onto groundwater resources, and groundwater wells have been exploited to secure the water supply. Lee and Kwon (2016) reported that in the years 1996–2013 the number of wells used for the abstraction of groundwater had gradually been increased from 779,438 to 1,506,352 with an average annual increase rate of 36,583 wells. As a result, the density of wells (number of wells/km2) also increased from 7.8 to 15.1, and the groundwater use in volume (m3) per unit area (km2) increased from 28,461 to 40,822 for the same period. Although the sudden increase in groundwater use has allowed the water supply demand to be met, it has also created problems like the decline in groundwater levels and the use of wells in areas that are vulnerable to pollution from land-use activities.
To manage groundwater quality properly and to make it a sustainable long-term supply for the intended uses, it is critically important that investigations are undertaken to assess the current status of groundwater quality and to assess factors that might cause future changes in quality (Madhnure et al. 2015). Generally, a groundwater quality study includes an assessment of the physical, chemical, and biological quality parameters of the water. Because groundwater generally has no specific color, odor, or taste, the most concerning parameters are the chemical and biological qualities of the water (Harter 2003).
Naturally, groundwater contains some salts formed from ions dissolved due to weathering or dissolution of the rocks and soil from the saturated and unsaturated zones of the aquifer where the water travels in the pores or fractures. The presence of excessive amounts of these dissolved ions in water has the potential to affect human health, the growth of plants, and the chemical and physical properties of the soil (Todd and Mays 2005). Chemical constituents from anthropogenic activities on the land surface may also be introduced into groundwater by the infiltration of water from rainfall or other sources of recharge (Bartram and Ballance 1996).
Generally, the motion of groundwater along its flow paths underground increases the concentration of many chemical constituents through chemical reactions of groundwater with minerals in the aquifer (Domenico and Schwartz 1998). The extent to which these hydrogeochemical interactions take place along a groundwater flow path depends on aquifer mineralogy and groundwater residence time, and these factors determine the extent of variation in the chemical composition of groundwater (Sharif et al. 2008). Inverse geochemical modeling using models like PHREEQC is commonly used to reconstruct geochemical change in the groundwater between two points in an aquifer in the direction inverse to the groundwater flow path (Sharif et al. 2008; Li et al. 2010).
Knowledge of the hydrogeochemical processes that control groundwater chemistry can lead to improved understanding of hydrochemical systems, and this can in turn contribute to the effective utilization and sustainable management of the groundwater resource by revealing the associations among other hydrogeological parameters (Merkel and Planer-Friedrich 2008). According to the Ministry of Environment of the Republic of Korea (2017), monitoring of the national groundwater quality in 2012 indicated that 6.5% of all samples (4952) considered, and 12.2% of wells used for drinking purposes, exceeded groundwater quality limits for drinking. This was mostly due to high concentrations of total coliforms and nitrate–nitrogen in the groundwater. Park et al. (2011) evaluated the national groundwater data obtained from the National Groundwater Monitoring Station (NGMS) for 1996–2008 and found that the electrical conductivity (EC) values of groundwater in metropolitan and industrial areas were very high. This indicates gradual deterioration of the groundwater resources due to anthropologic activities. Kim et al. (2005) studied the geochemistry of a small spa area in Korea (the Onyang Spa area) and reported that, for the last 30 years, shallow groundwater quality has gradually been degraded due to the expansion of urbanization. They found that the effects included pollution of deep groundwater separated by zones of low permeability. Another study conducted in the Wonju area of South Korea, near a livestock farming area, revealed that the concentrations of ammonium–N, nitrate–N, and a bacterial indicator of pollution in boreholes located downstream of the livestock waste disposal site increased with rising groundwater levels (Cho et al. 2000). Hwang et al. (2017) also assessed the geochemical characteristics and suitability of groundwater using 486 samples collected in some rural areas in the middle and southern parts of South Korea. They found that Ca–(Cl–NO3) and Ca–HCO3 were the predominant water types and that the groundwater was in an excellent range of suitability with respect to the sodium adsorption ratio (SAR), % Na, permeability index (PI), and magnesium hazard (MH), and in a good class with respect to residual sodium carbonate (RSC) and Kelly ratio (KR).
As reported by Kim and Park (2016), groundwater contamination by nitrate (NO3 −) from non-point sources (mainly from the application of fertilizers and animal wastes) has been a serious concern in South Korea. In their study conducted to characterize the groundwater quality of the agriculture-dominated Hongseong area of Korea, Kim and Park (2016) found that there was extensive contamination of groundwater by nitrate (NO3 −) and that this ion had a positive correlation with chloride (Cl−) with a surface origin.
Although the Jangseong region has sufficient groundwater to support the increased demand for water in the region, the characteristics of the hydrogeochemistry and its suitability for the intended uses are not well understood. Factors that have the ability to influence groundwater quality in the region include: naturally occurring dissolved salts and minerals in the groundwater, pollution from surface land-use activities due to the shallow groundwater table, the high permeability of soils in the region, and the intensive nature of agriculture in the region. Water consumption in the area is high; so it is crucial that groundwater quality in the region is investigated and monitored in order to maintain the sustainability of the groundwater resources and related development activities. In view of these factors, the current study was conducted to determine the status of the physical and chemical characteristics of the groundwater, to categorize the major factors affecting the groundwater quality, and to evaluate the suitability of the groundwater for irrigation as well as for domestic uses.
Materials and methods
Description of the study area
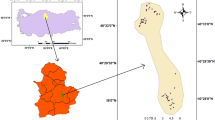
The Jangseong region is located in South Jeolla Province, South Korea, and is situated on the southwestern part of the Korean peninsula approximately 252 km from the capital city, Seoul. The region includes the Hwangryong River Basin, which forms the headwaters of the Youngsan River. It covers an area of about 518.5 km2 and is located between 35°11′ and 35°29′N and 126°35′ and 126°55′E (Fig. 1). The climate of the study area is categorized as a southern inland climate with comparatively heavy rain and wide seasonal variation in temperature. According to meteorological data from the two stations of the Automated Surface Observing System (ASOS) collected adjacent to the study area during 1983–2012, the average annual temperature in Jangseong was 13.1–13.7 °C, and the mean annual precipitation was 1473.4–1720.5 mm (MLIT 2014).
Geology and hydrogeology
The geological strata of the Jangseong area comprise the Precambrian Sobaeksan gneiss complex, the age-unknown leucocratic granites and Okchon Formation group, the Jurassic Daebo granites, the Cretaceous Yuchon Formation group, the Bulguksa granites and dikes, and Quaternary alluvium (MLIT 2014). The Precambrain Sobaeksan gneiss complex in the Jangseong area generally consists of schists and granitic gneisses. This schist (consisting of biotite, muscovite, plagioclase, orthoclase, and quartzite) underlies the northwestern part of the area. Granitic gneisses that also contain these minerals underlie a large proportion of the southern part of the area (Chough et al. 2000).
The age-unknown leucocratic granites (consisting of quartzite, orthoclase, microcline, and plagioclase) occur in a limited area in Parksanri, the northern part of the Jangseong region. The strata of the age-unknown Okchon formation group consist of schists, the Ryongamsan Group, and the Sulokri Group (in order of decreasing age). The Ryongamsan group generally consists of quartzites and sericite schists, whereas the Sulokri group consists of sericite schists and limestone. The Jurassic Daebo granites were formed by plutonic intrusions distributed throughout the Jangseong area. The granites contain quartz, orthoclase and plagioclase feldspars, biotite with minor hornblende, zircon, apatite, and magnetite. The strata of the Cretaceous Yuchon formation group are divided into breccia with tuff, andesite, and rhyolite (bottom to top) (Chough et al. 2000).
The groundwater in the study area largely occurs in a shallow unconfined aquifer. The water table is located 0.50–41.60 m below the surface, at an average depth of 4.85 m. The water table depth only exceeds 10 m in wells in upland areas (9.6% of the dataset). In the study area, groundwater is the major water source for agriculture, industry, and communities (MLIT 2014).
Sampling and chemical analysis
Groundwater samples were collected from 129 wells in the study area from June 2013 to August 2014. Figure 1 indicates the well locations sampled in the study area. Water samples were collected from pumping wells after water standing in the wells was removed by pumping until steady-state conditions were obtained in field measurements of pH, EC, and temperature. Some parameters (electrical conductivity (EC), TDS, temperature, and pH) of the groundwater were measured immediately after sampling (in the field) using digital conductivity and pH meters. Additional analysis of the groundwater samples collected was carried out following standard methods for water analysis by the American Public Health Association (Clescerl et al. 1999).
AquaChem 2014.2, produced by Waterloo Hydrogeologic (2015), was used to characterize the water quality in this study. This software package, developed for graphical and numerical analysis (including statistical analysis) and modeling of water quality data, was linked with PHREEQC (Appelo and Postma 2004) in this study. Statistical analysis and plotting of Piper diagrams, Wilcox diagrams/US Salinity Laboratory (USSL) diagrams, Schoeller diagrams, and spatial map plots of major cations and anions were done using the AquaChem software package.
The saturation index (SI) at sample temperature and electroneutrality (EN) was determined using the hydrogeochemical equilibrium model (PHREEQC) linked to AquaChem. The electroneutrality (EN) assessment was used as part of the data quality-assurance process. The balance of dissolved cations and anions principle was used in AquaChem for verification of the water quality of samples. Samples having EN up to ±3% were considered (Appelo and Postma 2004; Madhnure et al. 2015; Li et al. 2016).
In order to evaluate the suitability of groundwater for irrigation uses, values of the sodium adsorption ratio (SAR) (Richards 1954; Bauder et al. 2011), sodium percentage (% Na) (Todd and Mays 2005), residual sodium carbonate (RSC) index (Hopkins et al. 2007), permeability index (PI) (Doneen 1964), Kelly ratio (KR) (Kelley 1963), and magnesium hazard (MH) (Paliwal 1972) were calculated. The corresponding values for each method were calculated for every sample collected using Eqs. 1–6 (all ionic concentrations are in meq/L). The suitability class of each water sample was also described using a USSL diagram that indicates the combined effect of salinity and sodium hazard.
The suitability of groundwater for drinking and public health purposes was evaluated based on the basic parameters most important for drinking water quality, like total hardness (TH), total dissolved solids (TDS), pH, and cation and anion concentrations (Singh et al. 2015; Amiri et al. 2015). To estimate the total hardness (TH) of the groundwater, the Sawyer et al. (2003) formula (Eq. 7) was used.
where the values of TH are given in mg/L and those of Ca2+ and Mg2+ concentrations are given in meq/L.
In all samples, the suitability of water for domestic use was determined by comparing the concentration of the specific parameter with that standard specified as the limit most desirable for drinking water by the WHO (2004).
Results and discussion
Hydrogeochemical characteristics of groundwater
The statistical summary results of the measured and calculated water quality parameters including maximum (Max), minimum (Min), average (Mean), standard deviation (SD), and interquartile range (IQR) values of the physicochemical parameters, are reported in Table 1. The concentration of some parameters analyzed (P, F, NH4, As, Mn, Pb, KMnO4, and toluene) or calculated using AquaChem (total organic carbon: TOC) was very low. The percent of non-detection (%ND) by the respective instruments used for analysis is presented in Table 1. Because the concentrations of those parameters were very low and problems associated with high concentrations of them would not be expected, those parameters were not given more attention in this study.
The chemical composition of groundwater is influenced by the characteristics of recharge and by geochemical processes that take place as water reacts with the geologic materials through which it flows (Appelo and Postma 2004). The pH of water is one of the most important operational water quality parameters. In this study, the measured pH ranged from 5.5 to 8.7 with a mean ± SD value of 7.23 ± 0.59 (Tables 1, 3). About 66.7% of the samples had pH > 7. Of the 27.1% samples with pH < 7, 10.9% had values below the WHO standard limit for drinking water (6.5). According to WHO (2008), the acceptable pH range for drinking water is 6.5–8.5. Certain water chemistry parameters such as dissolved oxygen (DO), solubility, density, pH, and conductivity are influenced by temperature (Bartram and Ballance 1996). In this study area, the temperature of the groundwater samples ranged from 13.8 to 23.3 °C, with an average value of 17.1 °C.
The water quality parameters analyzed in the study area samples included the major anions (HCO3, SO4, F, Cl, and NO3) and cations (Ca, Na, K, and Mg). As shown in the statistical summary (Table 1), the groundwater in the Jangseong Aquifer generally has very low mineral content. Of the concentrations (mg/L) of the major cations, Ca ranged from 5 to 82 with a mean value of 28, Mg varied from 0 to 21 with a mean of 5, Na ranged from 4 to 42 with a mean of 14, and K ranged from 0 to 17 with an average of 2. Among the anions, the concentration (mg/L) of HCO3 ranged from 9 to 231.9 with a mean of 82, SO4 2− ranged from 0 to 90 with a mean of 10, F ranged from 0 to 2 with a mean of 0.1, and NO3 − ranged from 0.1 to 182 with a mean of 23.
Chemical composition of groundwater
The abundance of the major cations and anions found were in the order Ca2+ > Na+ > Mg2+ > K+ and HCO3 − > Cl− > SO4 2−, respectively (Figs. 3, 4). From the AquaChem analysis, it was determined that the major water type (i.e., occurring in ~67.5% of the groundwater wells sampled) was water in which Ca–HCO3 was predominant. The remaining hydrochemical facies found were Ca–Cl, Ca–Na–HCO3, and Ca–Na–HCO3–Cl.
Piper diagrams (Fig. 2) can be used for the graphical clustering of the hydrochemistry facies to indicate samples with similar compositions (Piper 1944; Karmegam et al. 2011). In the piper diagram, most of the water samples fall into the left quadrant of the diamond plot, which indicates that calcium bicarbonate water was the most common type. The concentration of most of the samples in one area in the piper diagram indicates the uniformity of the major cation and anion distribution in the study area. The hydrochemical facies are a function of lithology, reaction kinetics in solution, and the flow pattern of water in the aquifer (Fetter 2001; Cloutier et al. 2006).
A spatial distribution plot (Fig. 3) was created to display the spatial distribution of the dominant parameters (cations and anions) in the water using a pie chart at all the sample locations. An ArcGIS shape file base map was imported into AquaChem to enable the plot to be produced. The pie charts showed the variation in the concentration of the major cations (Na+, K+, Mg2+, Ca2+) and anions (HCO3 −, SO4 2−, Cl−) throughout the study area. The map plot with pie charts allows rapid visualization of the most abundant ions throughout the entire study area. In Fig. 3, the predominant components on the majority of the pie charts are orange (Ca2+) and blue (HCO3 −) for the entire study area. At the next lower level of abundance, light green (Na+) and light blue (Cl−) were typical anions and cations in the study area. The spatial distribution of major cations and anions on the map plot indicates the uniformity of the chemical composition of the groundwater throughout the study area.
The major cation and anion concentrations of the groundwater samples were also plotted on a Schoeller diagram (Fig. 4) to provide a visual assessment of the composition of groundwater in the area. This plot also shows that calcium is the dominant cation and that bicarbonate is the dominant anion in all the samples analyzed. The graph also indicates a similar trend in the spatial variation of the concentrations of all cations and anions in most of the samples. This result is consistent with results from the piper diagram and map plot (Figs. 2, 3).
Correlation analysis
The chemical constituents of the groundwater were characterized based on the major hydrochemical parameters analyzed. A Pearson’s correlation matrix was used to examine the relationships between the hydrochemical parameters. The numerical values of the correlation coefficient, R, for the major water quality parameters were tabulated as a correlation matrix and are presented in Table 2.
The matrix shows that Ca2+ shows a strong positive correlation with EC (R = 0.925) and HCO3 − (R = 0.805) and moderately positive correlation with Cl− (R = 0.668) and SO4 2− (R = 0.598). Magnesium also shows a strong positive correlation with EC (R = 0.78), moderately positive correlation with Cl− (R = 0.673) and HCO3 − (R = 0.630), and weak positive correlation with SO4 2− (R = 0.455). Sodium shows a strong positive correlation with Cl− (R = 0.704) and a moderate positive correlation with EC (R = 0.642). Electrical conductivity (EC) shows a strong correlation with Ca2+ (R = 0.925), Mg2+ (R = 0.780), Cl− (R = 0.768), and moderate correlation with HCO3 − (R = 0.677) and SO4 2− (R = 0.583). DO shows a negative correlation with most of the parameters. The strong correlation observed between some parameters suggests the extent of interdependence and also suggests that these ions may be derived from a common source.
Saturation Indices (SI)
Mineral saturation indices indicate whether a mineral will dissolve, precipitate, or remain at thermodynamic equilibrium in the water. If SI is <0, the water is considered undersaturated with respect to the target mineral, and thus it should be dissolving, if present. If SI equals zero, the water is considered at equilibrium with respect to the mineral. If SI > 0, the water is considered supersaturated with respect to the target mineral, and the mineral should be precipitating (Appelo and Postma 2004; Jezerský 2007).
SI values were calculated using PHREEQC for the possible minerals in the database by comparing ion activity products (IAP) of the dissolved ions of the mineral with their solubility product (K sp) in equation SI = log (IAP/K sp). These calculations indicated that anhydrite (CaSO4: Fig. 5a), halite (NaCl: Fig. 5b), gypsum (CaSO4: Fig. 5c), siderite (FeCO3: Fig. 5d), aragonite (CaSO4: Fig. 5e), dolomite (MgCa(CO3)2: Fig. 5f), and calcite (CaCO3: Fig. 5g), in all samples, showed undersaturation (negative values), indicating possible dissolution of the respective minerals from the aquifer materials in contact with the groundwater. However, with respect to goethite [FeO(OH: Fig. 5h)] and hematite (Fe2O3: Fig. 5i), the saturation indices of all samples were positive, suggesting that precipitation of the respective minerals would be expected unless the minerals were not reactive. Even though the SI values of calcite (CaCO3) were out of the range −0.05 to 0.05 in some samples assumed to be saturated (Merkel and Planer-Friedrich 2008), in most of the samples calcite was near saturation (SI = 0: Fig. 5d). The SI analysis indicated the presence of host rock in the aquifer for those minerals found saturated or oversaturated. The dissolution of carbonate minerals could be the process primarily responsible for the chemical composition of the groundwater in the study area.
Groundwater quality assessment
Suitability for irrigation uses
In the Jangseong area, groundwater is the sole source of irrigation water and is widely utilized. Therefore, it is important that the suitability of this groundwater for long-term irrigation is assessed to ensure that this activity will not cause adverse impacts on soil structure or crop health (Hydari et al. 2001; Acosta-Motos et al. 2017). The major indicators used for assessing the potential salinity and sodium hazards associated with the use of groundwater for irrigation include TDS, %Na, SAR, KR, PI, and RSC (Ayers and Westcot 1985; Bauder et al. 2011; Amiri et al. 2015). The results are summarized in Table 1.
The level of salinity in irrigation water is usually measured using EC and TDS values. According to the guideline of the Food and Agriculture Organization (Ayers and Westcot 1985), irrigation water is classified based on the EC value for salinity level and suitability. Based on this guidance, EC values <250 (μS/cm) can be considered “Excellent,” with limited restrictions on irrigation use; values of 250–750 are in the “good” class with moderate restrictions on irrigation use. Values in the range 750–2250 are in the “permissible” class and have substantial restrictions on irrigation use, and if the value is >2250, the water is in “doubtful” class requiring very high degree of restriction on its use for irrigation. In this study, the measured EC values in 59% of the wells were <250 µS/cm (i.e., in “excellent” irrigation class), and in the remaining 41%, the EC values were in the range 250–750 µS/cm (i.e., in “good” irrigation class). The TDS values measured in all samples were in the range 29–335 mg/L, which is far less than the maximum permissible limit of 2000 mg/L for irrigation use.
The sodium hazard analysis of groundwater in the Jangseong area for irrigation based on SAR indicated that the SAR values in all the 129 samples analyzed were in the range 1–9, which is considered as the “excellent” quality class (Bauder et al. 2011). The analysis based on the calculated %Na values also showed that 20% of the analyzed samples were in the range 0–20% (“excellent” class), 67% were in 20–40% (“good” class), and the remaining 13% were in the “permissible” quality class. The RSC results of all the samples were <1.25 (safe irrigation water class). Regarding the KR values, in only four samples (3.1%) was the KR value <1 (indicates the presence of excess sodium); in the others, the values were in the desirable range. The PI value is a measure of the effect of the Na concentration in irrigation water on soil permeability due to long-term use. The PI values in this study indicated that all samples except one were in Class I (>75%) and II (25–50%) (i.e., suitable for irrigation), that one having a PI value <25% (the maximum value for Class III: not suitable for irrigation). The MH values (%) for this study ranged from 0.42 to 41 with a mean value of 24.3. In all samples, the values were less than the maximum suitability class of 50%, indicating the suitability of all samples for irrigation uses. Thus, according to the analysis results of all the indices of sodium hazard, the risk of sodium concentration in groundwater should not be a concern for irrigation except in a very few cases, where ongoing monitoring is likely to be required.
The suitability of groundwater for irrigation based on its cationic composition was also assessed for the study area. In all samples, the maximum value of Ca, 83 mg/L (4.13 meq/L), was within the suitable range 0–20 meq/L for irrigation use. With respect to Mg values, in all the wells sampled, its concentration was in the range 0.1–21 mg/L (0–1.74 meq/L) with an average of 5 mg/L (0.44 meq/L). This is less than the maximum limit of the desirable range (5 meq/L) (Ayers and Westcot 1985). The potential toxicity of irrigation water with respect to Na and Cl ion concentrations was checked by comparison with the FAO guideline (Ayers and Westcot 1985). According to the FAO guideline, water with Na ion concentration <3 meq/L and Cl concentration <0.4 meq/L can be used for irrigation without impacts on crop health or soil structure. In the Jangseong region, the maximum concentrations found for Na and Cl were 42 mg/L (1.8 meq/L) and 103 mg/L (2.91 meq/L), respectively, suggesting that groundwater can be safely used for irrigation without adverse effects from its Na and Cl content.
The USSL diagram, which indicates the combined effect of total salinity and sodium hazards, is also used to determine the suitability of water quality for irrigation purposes (Fig. 6). It is a simple scatter plot of sodium hazard (as SAR) on the y axis versus salinity hazard (in terms of electrical conductivity on log scale) for the x axis. The plot has sections for conductivity and SAR values to classify the water quality based on its suitability. The result from the plot illustrates that all of the groundwater samples analyzed in this study fell in the classes C1S1 and C2S1. This indicated that the groundwater in the study area mostly fell in the “good” quality class and could be used for irrigation with little danger of problems with exchangeable sodium in the soil profile.
Suitability for domestic uses
The suitability of groundwater quality for drinking use was evaluated by comparing total hardness (TH), total dissolved solids (TDS), and major cation and anion concentrations in samples analyzed in relation to the standard desirable limits of those parameters specified for drinking water by the WHO (2004).
According to the WHO guideline, even though TH > 200 mg/L may cause scale formation in water supply pipes, and values >500 mg/L may not be acceptable to a community based on esthetic factors, there is no health-based guideline limit for TH. In this study, the values of TH (mg/L) ranged from 326 to 4792 with a mean value of 1576. The spatial distribution of TH shows that the higher values are concentrated in the northern part of the area (Fig. 7a). This means that groundwater in the area is hard, and that it should be softened to meet the requirements of communities. It is crucial to soften the water to reduce the effects from scale formation in the distribution system in the cases of drinking and industrial uses.
Generally, concentrations of TDS < 500 mg/L are accepted as satisfactory for domestic as well as for industrial purposes. TDS > 1000 mg/L may give water an unpleasant taste, making it unsuitable for use as drinking water (WHO 2003). The TDS values of samples measured in this study were in the range 34–269 mg/L with a mean value of 106. Consequently, the TDS of the groundwater in the study area (Table 3) is considered acceptable for domestic use based on its TDS content. The EC analysis also showed that the groundwater quality was suitable for domestic use, with the exception of five samples (3.9% of the total dataset). Moreover, even these were still within the maximum permissible limit (MPL). However, the pH values of 14 wells (10.85%) were found to be <6.5, the lower limit of the WHO standard. Even though pH has no direct impact on human health, water with low pH values may need to be treated with an acid-neutralizing agent to minimize adverse effects such as the corrosion of pipes.
The assessment of major cation and anion concentrations (Table 3) indicated that the groundwater quality in the study area is within the desirable limits (DL) of the WHO guideline with respect to Ca, Na, Mg, Cl, and SO4 ions in all the samples analyzed. In one sample each, K and F were found at concentrations higher than the respective recommended maximum permissible limit (MPL).
By contrast, with respect to NO3–N, only 38% of groundwater samples from the study area were below the WHO recommended drinking water limit of 10 mg/L, and 10.1% of the samples were above the 50 mg/L of the maximum WHO (2004) guideline. The highest nitrate concentration measured in the study area was 230 mg/L. The concentrations of NO3–N in the remaining 51.9% of the samples were in the range of 10–50 mg/L. Although the kriged distribution of nitrate concentrations (Fig. 7b) did not indicate the highest values measured in the study area, the map clearly shows that the southern part of the study area is most affected by nitrate contamination of the groundwater. This part of the study area is predominantly used for agricultural activities. The higher concentrations of nitrate observed in groundwater in this part of the study area are likely to be sourced from agricultural land-use practices in this area (Cho et al. 2000; Kim and Park 2016).
Because the suitability analysis of the groundwater quality was carried out based on a limited number of measured chemical parameters in the study area, and because the constituents of other parameters may also cause health impact on groundwater users, further investigation of potentially harmful chemical constituents is crucial for long-term use of this groundwater as drinking water.
Conclusions
A groundwater quality investigation was conducted in the Jangseong region of South Korea with the aim of assessing the existing water quality and its suitability for its intended uses. Groundwater samples were collected from 129 wells and then analyzed for major cations and anions, important mineral ions, and physicochemical parameters like EC, TDS, pH, and temperature. For further analysis of the results, the AquaChem model linked with PHREEQC was used to evaluate the groundwater chemistry. This combination was used to assess the degree to which groundwater was saturated with respect to selected minerals, for statistical analysis of the results, and for producing a number of standard geochemical plots.
The results of the analysis show that Ca and HCO3 are the dominant cation and anion, respectively, in groundwater in the study area and that the groundwater has a Ca–HCO3 composition. The correlation analysis also revealed that Ca and HCO3 show a strong association, suggesting that these ions have originated from the same source. In all samples, the water is undersaturated with respect to aragonite (CaSO4), calcite (CaCO3), dolomite (MgCa(CO3)2), fluorite (CaF2), gypsum (CaSO4), halite (NaCl), and siderite (FeCO3); and oversaturated with respect to goethite (FeO(OH) and hematite (Fe2O3).
The suitability of the groundwater for irrigation was evaluated based on salinity (EC), SAR, %Na, RSC, KR, MH, PI, and use of a USSL diagram. In the USSL diagram, all the samples grouped within the C1S1 and C2S1 classes, indicating that the groundwater in the study area is suitable for irrigation without any restriction. Based on the parameters measured, the groundwater appears to be generally suitable for domestic use with the exception of elevated nitrate concentrations, which were measured in about 10% of the samples. The highest nitrate concentrations were measured in groundwater in the southern part of the study area where agriculture is the dominant land use. It is likely that agricultural practices are the source of the nitrate in groundwater. Because the groundwater in the area is shallow and vulnerable to the anthropogenic activities near and at the land surface, regular monitoring of the groundwater quality and analysis of its seasonal variability is needed for sustainable management of the groundwater resources of the area.
References
Acosta-Motos J, Ortuño M, Bernal-Vicente A et al (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. doi:10.3390/agronomy7010018
Amiri V, Sohrabi N, Dadgar MA (2015) Evaluation of groundwater chemistry and its suitability for drinking and agricultural uses in the Lenjanat plain, central Iran. Environ Earth Sci 74:6163–6176. doi:10.1007/s12665-015-4638-6
Appelo CAJ, Postma D (2004) Geochemistry, groundwater and pollution, 2nd edn. CRC Press, Boca Raton
Ayers RS, Westcot DW (1985) Water quality for agriculture. Food and Agriculture Organization of the United Nations, Rome
Bartram J, Ballance R (eds) (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes, 1st ed. Published on behalf of United Nations Environment Programme and the World Health Organization, UNEP/WHO, London, New York
Bauder TA, Waskom RM, Davis JG, Sutherland PL (2011) Irrigation water quality criteria. Colorado State University Extension Fort Collins, CO
Cho J-C, Cho HB, Kim S-J (2000) Heavy contamination of a subsurface aquifer and a stream by livestock wastewater in a stock farming area, Wonju, Korea. Environ Pollut 109:137–146
Chough SK, Kwon S-T, Ree J-H, Choi DK (2000) Tectonic and sedimentary evolution of the Korean peninsula: a review and new view. Earth Sci Rev 52:175–235
Clescerl LS, Greenberg AE, Eaton AD (1999) Standard methods for examination of water & wastewater, 20th edn. American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WEF)
Cloutier V, Lefebvre R, Savard MM et al (2006) Hydrogeochemistry and groundwater origin of the Basses-Laurentides sedimentary rock aquifer system, St. Lawrence Lowlands, Québec, Canada. Hydrogeol J 14:573–590. doi:10.1007/s10040-005-0002-3
Domenico PA, Schwartz FW (1998) Physical and chemical hydrogeology, 2nd edn. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Water Science and Engineering, Department of Water Sciences and Engineering, University of California, paper 4001
Fetter CW (2001) Applied hydrogeology, 4th edn. Pearson Education, London
Haque SJ, Onodera S, Shimizu Y (2013) An overview of the effects of urbanization on the quantity and quality of groundwater in South Asian megacities. Limnology 14:135–145. doi:10.1007/s10201-012-0392-6
Harter T (2003) Groundwater quality and groundwater pollution. UCANR Publications, Oakland
Hopkins BG, Horneck DA, Stevens RG et al (2007) Managing irrigation water quality for crop production in the Pacific Northwest. [Covallis, Or.]: Oregon State University Extension Service
Hwang JY, Park S, Kim H-K et al (2017) Hydrochemistry for the assessment of groundwater quality in Korea. J Agric Chem Environ 06:1–29. doi:10.4236/jacen.2017.61001
Hydari N, Gupta AD, Loof R (2001) Salinity and sodicity influences on infiltration during surge flow irrigation. Irrig Sci 20:165–173. doi:10.1007/s002710100043
Jezerský Z (2007) Hydrogeochemistry of a deep gas-storage cavern, Czech Republic. Hydrogeol J 15:599–614. doi:10.1007/s10040-007-0162-4
Kabir ML, Park Y, Lee J-Y (2014) Chemical characteristics of groundwater in carbonate rock areas of Korea. J Soil Groundw Environ 19:7–15. doi:10.7857/JSGE.2014.19.2.007
Karmegam U, Chidambaram S, Prasanna MV et al (2011) A study on the mixing proportion in groundwater samples by using Piper diagram and Phreeqc model. Chin J Geochem 30:490–495. doi:10.1007/s11631-011-0533-3
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95:385–391
Kim H, Park S (2016) Hydrogeochemical characteristics of groundwater highly polluted with nitrate in an agricultural area of Hongseong. Korea. Water 8:345. doi:10.3390/w8080345
Kim K, Koo M-H, Moon S-H et al (2005) Hydrochemistry of groundwaters in a spa area of Korea: an implication for water quality degradation by intensive pumping. Hydrol Process 19:493–505. doi:10.1002/hyp.5551
Lee J-Y, Kwon K (2016) Current status of groundwater monitoring networks in Korea. Water 8:168. doi:10.3390/w8040168
Li P, Qian H, Wu J, Ding J (2010) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3:282–291
Li P, Zhang Y, Yang N et al (2016) Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo Health 8:239–252. doi:10.1007/s12403-016-0198-6
Madhnure P, Peddi NR, Allani DR (2015) An integrated hydrogeological study to support sustainable development and management of groundwater resources: a case study from the Precambrian Crystalline Province, India. Hydrogeol J 24:475–487. doi:10.1007/s10040-015-1342-2
Merkel BJ, Planer-Friedrich B (2008) Groundwater geochemistry: a practical guide to modeling of natural and contaminated aquatic systems. Springer, New York
Ministry of Environment (2017) Groundwater quality. http://eng.me.go.kr/eng/web/index.do?menuId=334. Accessed 25 May 2017
Ministry of Land, Infrastructure, and Transport (MLIT) (2014) The basic groundwater investigation in Jangseong Region. Appendix 1: investigation, chapter 2, pp 16–17, 27–35
Nam W-H, Hayes MJ, Svoboda MD et al (2015) Drought hazard assessment in the context of climate change for South Korea. Agric Water Manag 160:106–117. doi:10.1016/j.agwat.2015.06.029
Paliwal KV (1972) Irrigation with saline water. Water Technol Cent Indian Agric Res Inst New Delhi 2:173–189
Park Y-C, Jo Y-J, Lee J-Y (2011) Trends of groundwater data from the Korean National Groundwater Monitoring Stations: indication of any change? Geosci J 15:105–114. doi:10.1007/s12303-011-0006-z
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25:914–928. doi:10.1029/TR025i006p00914
Richards L (ed) (1954) Diagnosis and improvement of saline and alkaline soils. U. S. Government Printing Office, Washington
Sawyer CN, McCarty PL, Parkin GF (2003) Chemistry of environmental engineering and science, 5th edn. The McGraw Hill Inc, New York
Sharif MU, Davis RK, Steele KF et al (2008) Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA). J Hydrol 350:41–55. doi:10.1016/j.jhydrol.2007.11.027
Singh N, Singh RP, Kamal V et al (2015) Assessment of hydrogeochemistry and the quality of groundwater in 24-Parganas districts, West Bengal. Environ Earth Sci 73:375–386. doi:10.1007/s12665-014-3431-2
Todd DK, Mays LW (2005) Groundwater hydrology. Wiley, Hoboken
Waterloo Hydrogeologic (2015) AquaChem readme. http://www.novametrixgm.com/aquachem-readme. Accessed 8 Sept 2016
WHO (2003) Total dissolved solids in drinking-water: background document for development of WHO guidelines for drinking-water quality, vol 2, 2nd edn. Health criteria and other supporting information, Geneva
WHO (2004) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva
WHO (2008) Guidelines for drinking-water quality [electronic resource]: incorporating first and second addenda, vol 1, 3rd edn. Recommendations, Geneva
Zubari WK (1999) The Dammam aquifer in Bahrain–hydrochemical characterization and alternatives for management of groundwater quality. Hydrogeol J 7:197–208. doi:10.1007/s100400050192
Acknowledgements
This work was supported by a grant (17RDRP-B076272-04) from Infrastructure and Transportation Technology Promotion Research Program funded by the Ministry of Land, Infrastructure and Transport of Korean government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolera, M.B., Park, S., Chang, S.W. et al. Spatial assessment of groundwater quality in the Jangseong region, South Korea. Environ Earth Sci 76, 545 (2017). https://doi.org/10.1007/s12665-017-6875-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6875-3