Abstract
Chronic exposure to arsenic (As) in rice has raised many health and environmental problems. As reported, great variation exists among different rice genotypes in As uptake, translocation, and accumulation. Under hydroponic culture, we find that the Chinese wild rice (Oryza rufipogon; acc. 104624) takes up the most arsenic among tested genotypes. Of the cultivated rice, the indica cv. 93-11 has the lowest arsenic translocation factor value but accumulates the maximum concentration of arsenic followed by Nipponbare, Minghui 86, and Zhonghua 11. Higher level of arsenite concentration (50 μM) can induce extensive photosynthesis and root growth inhibition, and cause severe oxidative stress. Interestingly, external silicate (Si) supplementation has significantly increased the net photosynthetic rate, and promoted root elongation, as well as strongly ameliorated the oxidative stress by increasing the activities of antioxidant enzymes superoxide dismutase, ascorbate peroxidase, and peroxidase in roots and/or leaves of 93-11 seedlings. Notably, 1.873 mM concentration of Si considerably decreases the total As uptake and As content in roots, but significantly increases the As translocation from roots to shoots. In contrast, Si supplementation with 1.0 mM concentration significantly increases the total As uptake and As concentrations in roots and shoots of 93-11 seedlings after 50 μM arsenite treatment for 6 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a carcinogenic metalloid that is ubiquitous and abundant in Earth’s crust. The continual release of As into the environment either by natural or anthropogenic activities elevates its concentration in surface soil and eventually into plants (Moreno-Jiménez et al. 2012). It is reported that nearly 150 million people from over 70 countries have suffered from extensive As exposure by drinking As-contaminated water and the food chain (Brammer and Ravenscroft 2009; Zhao et al. 2010a), of which approximately 110 million people reside in the South and Southeast Asia, where rice has become the major exposure route of As (Dave et al. 2012). For example, in India, rice As contamination may contribute to more than half of dietary total As intake (Meharg 2004; Mondal and Polya 2008), given that rice can take up more As than other cereal plants (Su et al. 2010). Accordingly, arsenic contamination in rice raises much concerns and becomes of a widespread interest in further uncovering the physiological mechanisms of As tolerance and responses in plants.
Extensive variation in As uptake and accumulation exists among plant species, and even among different genotypes within a species (Moreno-Jiménez et al. 2012). For example, the rice cultivars that are tolerant and sensitive to As stress accumulate greater and less arsenic, respectively (Dwivedi et al. 2010; Rai et al. 2011). In nature, arsenic can exist in four valency states: −3, 0, +3, and +5. Under aerobic and anaerobic conditions, the oxidation state of As tends to be +5 and +3, respectively (Moreno-Jiménez et al. 2012). Thus, plants are mainly exposed to inorganic arsenate As(V) and arsenite As(III). However, Verbruggen et al. (2009) revealed that the arsenic toxicity effects to plants are mainly due to As(III) because once absorbed into the cell, As(V) is rapidly reduced to As(III) by arsenate reductase (Xu et al. 2007). As(III) can be efficiently taken up by roots through a number of NIP aquaporins (Ma et al. 2008; Ali et al. 2009), and arsenite has high affinity with −SH groups of enzymes and tissues proteins, and thereby inhibiting many key metabolic processes in the cell (Tripathi et al. 2007).
The visual effect of arsenic toxicity is the impairment of plant development, such as reduced root and shoot length, chlorosis in leaves, and shrinking or necrosis in aerial plant parts (Shri et al. 2009; Choudhury et al. 2011; Moreno-Jiménez et al. 2012), owing to the physiological and morphological disorders caused by As exposure that includes the induction of oxidative stress (Shri et al. 2009; Rai et al. 2011), changes of nutritional patterns (Dwivedi et al. 2010), photosynthetic inhibition (Azizur Rahman et al. 2007), and metabolic and genetic alterations (Tripathi et al. 2007). In order to overcome such toxicity, plants evolved various molecular mechanisms to adapt to environmental arsenic stress. The primary detoxification mechanism is to form phytochelatines– (PCs) or glutathione–As complexes and further compartmentalize them into vacuoles of root cells (Zhao et al. 2009; Moreno-Jiménez et al. 2012). To protect plants against oxidative stress by regulation of expression of antioxidant enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), and catalase (CAT), is another efficient detoxification mechanism (Rai et al. 2011). Of these enzymes, SOD catalyzes the detoxification of the active superoxide radicals (Bowler et al. 1992) and is likely to be central in the defense (Rai et al. 2011). CAT and APX are responsible for the subsequent conversion of H2O2 into water in peroxisomes, and in cytosol and chloroplasts, respectively (Noctor and Foyer 1998), while POD plays an important role in degradation of lipid peroxides (Shri et al. 2009). In addition, active efflux of arsenic, mainly in the form of As(III) (Xu et al. 2007), also plays a role in partial protection plants from As toxicity in non-hyper-accumulating plants (Zhao et al. 2009). In rice, OsNIP2;1 (Lsi1) that was firstly identified to function in permeability to silicic acid and arsenite (Ma et al. 2006, 2008) is crucial for mediating the efflux of As(III) from roots (Zhao et al. 2010b).
Except for oxygen, silicon (Si) is considered as the most abundant element in earth’s crust. Increasing evidence shows that Si has favorable effects on plant growth under biotic and abiotic stresses (Shi et al. 2005; Guo et al. 2005, 2007; Gottardi et al. 2012), although its essentiality for higher plants has not been established (Epstein 1999). Rice is known as one of the best Si accumulator (Ma et al. 2006), which can accumulate silicon up to 10 % of shoot dry weight (Savant et al. 1997). Given that silicic acid and arsenite share the similar transporter system (Ma et al. 2006, 2008), significant inhibitory effects of silicon on As uptake and As content in straw and grain were reported either by indigenous silicic acid in the soil solution (Bogdan and Schenk 2008) or by external Si supplementation (Guo et al. 2006; Li et al. 2009; Seyfferth and Fendorf 2012). Addition of silicon fertilizer (in the form of SiO2 gel) to soil significantly decreased the total As concentration in rice straw and grain (Li et al. 2009). A similar case was also reported by Seyfferth and Fendorf (2012) when Si gel was added to soil. However, the addition of diatomaceous earth did increase but not inhibited the As content in rice grain, husk, and straw (Seyfferth and Fendorf 2012). In addition, a significantly negative correlation between external silicon supplementation and arsenate and/or arsenite uptake and concentration in rice seedlings was also established under hydroponic culture conditions (Guo et al. 2005, 2007; Tripathi et al. 2013).
Although some studies have been carried out to investigate the potential interaction between Si supply and As uptake and accumulation, and probably related mechanisms (Guo et al. 2007; Li et al. 2009; Seyfferth and Fendorf 2012; Tripathi et al. 2013), the effects of silicic acid on higher dose of arsenite concentration (50 μM) in relation with photosynthesis, lipid oxidation, and antioxidant enzymes activity have not been clarified in rice seedlings. Here, four cultivated as well as one Chinese wild rice were hydroponically cultured to compare and screen out the cultivar that is adaptive to arsenite stress. Based on this cultivar, the effects of exogenous silicate supplementation on the alleviation of arsenite toxicity were investigated accordingly.
Materials and methods
Rice cultivation and arsenite treatment for five genotypes
One wild rice (Oryza rufipogon) and four widely cultivated rice (Oryza sativa L.) in China were selected to screen for tolerance to arsenite (Table 1). The rice seeds were disinfected in 0.5 % NaOCl for 15 min, and then rinsed thoroughly and soaked in deionized water for 24 h. After that, seeds were separately transferred to filter papers floating on 0.5 mM CaCl2 solution for germination. After 5 days, the seedlings of each genotype were independently transferred to a 10-L container and pre-cultured in a growth chamber under a 16-h-light (30 °C) and 8-h-dark (20 °C) photoperiod for further 21 days. In each container, 8-L half-strength Kimura solution was filled in and renewed every 3 days. The nutrient composition is as follows: 0.091 mM KNO3, 0.183 mM Ca(NO3)2, 0.274 mM MgSO4, 0.1 mM KH2PO4, 0.183 mM (NH4)2SO4, 0.5 μM MnCl2, 3 μM H3BO3, 0.1 μM (NH4)6Mo7O24, 0.4 μM ZnSO4, 0.2 μM CuSO4, 40 μM NaFe(III)–EDTA, and 2 mM 2-morpholinoethanesulfonic acid (MES; pH adjusted to 5.5 with NaOH; Zhao et al. 2010b). Then, the uniform seedlings were transferred to 2-L pots (four plants per pot) for further treatments. For each of the five genotypes examined, two treatments [−As, no added As(III); and +As, 25 μM As(III)] with four replications (pots) were adopted. In other words, in each of the five experiments, four pots were used as control (−As) and four pots were exposed to arsenite in the form of NaAsO2 at 25 μM (+As) for 6 days, respectively. At harvest, the As-treated seedlings roots were immersed into an ice-cold desorption solution containing 1 mM K2HPO4, 0.5 mM Ca(NO3)2, and 5 mM MES (pH 5.5) for 10 min to remove apoplastic As (Zhao et al. 2010b). The harvested plants were further divided into roots and shoots, and oven dried at 105 °C for 30 min, and 75 °C for 48 h for further use.
Experimental design and treatments of the As and Si interactions for 93-11 seedlings
The experimental design was set up as a complete, randomized block design with six treatments and four replications. The 21-day-old seedlings of 93-11 were transferred to 24 2-L pots (four plants per pot) for further treatments, consisting of a control [CK, no added As(III) and Si] and five treatments [1.0 mM Si; 1.873 mM Si; 50 μM As(III); 1.0 mM Si plus 50 μM As(III); and 1.873 mM Si combination with 50 μM As(III)], where silicon and arsenite were supplied by the form of Na2SiO3 and NaAsO2, respectively. After different times (3 days and 6 days) of treatment, the roots and leaves were sampled to determinate the total As concentration and the activities of antioxidant enzymes.
Total As quantification and data analysis
After 6 days of the treatments, the seedlings’ roots were soaked in 20 mL ice-cold desorption solution for 10 min to desorb apoplastic As, and then the roots and shoots were separately harvested and oven dried. After that, the rice samples were powdered by using a ball mill (Retsch, MM-301, Germany). The ground rice samples (0.3 g) of each treatment were subsequently digested with 2 mL nitric acid and 0.5 mL H2O2 at 120 °C for 24 h. After cooling, deionized water was added to the digestion solution to make the final volume of 20 mL. Then the levels of total arsenic in samples were quantified by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500a; Agilent Technologies, CA, USA).
Specific arsenic uptake (SAU), a commonly used measure of the ability of As uptake by rice genotypes, was calculated as the ratio of total As uptake in roots and shoots to the root dry weight under arsenic exposure.
In the formula, SAU represents specific arsenic uptake. The T root-As and T shoot-As indicate the average As content in roots and shoots, respectively. The Rootbiomass and Shootbiomass represent the dry weight of roots and shoots examined, respectively.
In addition, the translocation factor (TF) was used to assess the ability of As transfer from roots to shoots, which was calculated as the ratio of total As concentration in shoots to that in roots (Dwivedi et al. 2010).
Determination of photosynthetic inhibition
After 3 and 6 days of the treatments, a portable photosynthesis system (LI 6400; Li-Cor, Lincoln, NE, USA) was employed to measure the net photosynthetic rate (P n) using the seedlings leaves with or without As(III) (NaAsO2, 25 μM) and/or Si (Na2SiO3, 1.0 mM) exposure in 93-11. Stomatal limitation (L s) was calculated as 1 − Ci/Ca to estimate the extent of photosynthetic limitations caused by stomata closure (Berry and Downton 1982).
Estimation of malondialdehyde content
After 6 days of the treatments, the malondialdehyde (MDA) contents in sampled root tissues were determined to estimate the extent of lipid peroxidation, according to the Heath and Packer (1968) method with slight modifications. Briefly, about 0.3 g root tissues was homogenized in 3 mL of 0.1 % trichloroacetic acid (TCA) containing 2 g of thiobarbituric acid (TBA), and then the homogenate was centrifuged at 12,000×g for 20 min. In analysis, 3 mL TCA was mixed with 1 mL of supernatant. The mixture was heated at 95 °C for 30 min and cooled quickly on ice bath, and then centrifuged at 12,000×g for 10 min. The absorbance of the supernatant at 532 and 600 nm was recorded, respectively. Finally, based on the correction of non-specific turbidity, the MDA content was determined using the extinction coefficient of 155 mM−1 cm−1.
Assay of antioxidant enzymes
Fresh root and leaf samples of the cv. 93-11 at 3 and 6 days after arsenite and/or silicate treatments were used for enzymatic activity analysis. About 0.3 g roots or leaves was homogenized in 3 mL of sodium phosphate buffer (50 mM; pH 7.8) that includes 0.2 mM EDTA and 0.2 % (w/v) PVP at 4 °C. The homogenate was centrifuged at 12,000×g for 20 min at 4 °C. And the obtained supernatant was used for analyzing the activities of antioxidant enzymes.
The activity of SOD was assayed by the method of Giannopotitis and Ries (1977). Briefly, 3 mL nitro blue tetrazolium (NBT) reaction mixture was added to 50 μL of the supernatant. The mixture was placed below a light source (4,000-lx fluorescent lamp) at 25 °C for 30 min, and the absorbance at 560 nm was recorded. Accordingly, one unit of the enzyme activity was measured as the amount of proteins required to inhibit 50 % initial reduction of NBT under light.
APX activity was determined by estimating the rate of ascorbate (ASC) oxidation (Nakano and Asada 1981). A total volume of 2 mL reaction mixture containing 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbate, 1.0 mM H2O2, and 100 μL enzyme extract was prepared. The absorbance of the mixture at 290 nm was recorded, and the enzyme activity was assessed accordingly.
The measurement of CAT activity was according to the method described in Cakmak and Marschner (1992). The reaction mixture contains 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 10 mM H2O2, and 100 μL enzyme extract to a final volume of 2 mL. The decrease in absorbance was recorded at 240 nm for 30 s, which was used to measure the initial rate of disappearance of H2O2.
Determination of POD activity was based on the Pütter (1974) method by measuring the change of absorption at 470 nm due to guaiacol oxidation. The reaction mixture (2 mL) comprising 25 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA, 10 mM H2O2, 0.05 % (w/v) guaiacol, and 100 μL enzyme extract was prepared to assay the enzyme activity.
Statistical analysis
All the data were presented as the mean value ± SD (standard deviation) of three or four replicates. Data were compared and tested by the least significant difference (LSD) test method implemented in the ANOVA analysis to examine the significant difference among different treatments at the 0.05 level (DPS v7.05).
Results
Comparison of rice cultivars for As accumulation and translocation
In total, five rice cultivars were hydroponically cultured under arsenic stress for 6 days. In the solution, the arsenic was provided as the form of As(III) at 25 μM. From Fig. 1, it was observed that the wild rice accumulated significantly higher concentration of arsenic (595.04 ± 75.53; Fig. 1a), while its As TF value (0.112 ± 0.011) was markedly lower than Minghui 86 (0.211 ± 0.021) but higher than 93-11 (0.088 ± 0.009) and similar with the other varieties (Nipponbare, 0.112 ± 0.011; and Zhonghua 11, 0.118 ± 0.012; Fig. 1b). For the cultivated rice, great variation was observed in As concentration among cultivars (Fig. 1a). The highest and lowest level of As concentration was found in 93-11 (461.16 ± 47.27) and Zhonghua 11 (336.82 ± 32.84), respectively, whereas the cultivars Nipponbare and Minghui 86 showed moderate accumulation of As (413.46 ± 38.24 and 368.05 ± 36.81, respectively). In addition, the ability of translocation of As from roots to shoots also differed significantly among genotypes (Fig. 1b). The indica rice Minghui 86 had the highest TF value, followed by Zhonghua 11, Nipponbare, and 93-11. Taken together, 93-11 was selected for the following additional analyses.
Specific arsenic uptake (SAU; a) and As translocation factor (TF; b) of five rice genotypes. Arsenic concentration of rice plants (21 dap), treated for 6 days with 25 μM arsenite. All the values are reported as the mean ± SD. Different letters indicate significant difference among rice genotypes (LSD, p ≤ 0.05)
Effect of silicate supply on photosynthesis
We found that the net photosynthetic rate (P n) was slightly inhibited but not significant after 3 days of arsenite exposure. However, P n was significantly enhanced by addition of external Si (1.0 mM), as compared to the control. With increasing treatment time (6 days), P n was extensively inhibited under arsenite stress, which was however significantly recovered by Si supplementation (1.0 mM; Fig. 2a).
Effects of exogenous silicate supplementation on the net photosynthetic rate (a) and stomatal limitation (b). The 21-day-old 93-11 seedlings were treated with 25 μM arsenite along with or without 1.0 mM sodium silicate for 3 days and 6 days, respectively. Data are represented as the mean of three replicates ±SD. Different letters indicate significant difference among different treatments (LSD, p ≤ 0.05)
On the other hand, the stomatal limitation (L s) value was calculated and compared. It was observed from Fig. 2b that L s value increased significantly under As after 3 days of treatment, but returned to the normal level when 1.0 mM Si was added (Fig. 2b). However, no significant differences in L s values were observed among treatments at 6 days (Fig. 2b).
Effects of silicate supply on root elongation and As accumulation
Compared with the control, excess arsenite exposure (50 μM) significantly inhibited root growth. Addition of 1.0 mM Si slightly promoted the root elongation. However, increasing external Si concentration (1.873 mM) significantly recovered the root growth to the control level (Fig. 3).
Effect of external silicate supplementation on the root elongation of 93-11 seedlings (21 days old) under 50 μM arsenite stress for 6 days. The extent of root elongation was estimated by subtracting the root length after As(III) treatment with that of before treatment. Data are reported as the average root elongation length ± SD. Different letters indicate significant difference in comparison with the control (LSD, p ≤ 0.05)
Under excess arsenite stress (50 μM), the rice plants accumulated on average 662.33 ± 12.50 and 95.67 ± 10.50 mg kg−1 As in roots and shoots, respectively (Table 2). Higher level of external Si concentration (1.873 mM) could significantly decrease As content in roots (424.33 ± 25.03) and total As uptake, while it increased As concentration in shoots (121.00 ± 19.00) of rice seedlings, compared to the As exposure alone (Table 2). However, addition of 1.0 mM Si unexpectedly significantly increased roots (805.00 ± 45.00) and shoots (124.67 ± 5.03) As concentrations and total As uptake in 93-11 seedlings (Table 2 and Fig. 4a). In addition, it was observed that the specific As uptake (SAU) and translocation factor (TF) of 93-11 seedlings markedly increased along with the increase in external arsenite concentrations from 25 μM to 50 μM (Figs. 1 and 4b). Notably, Si concentration of 1.873 mM significantly enhanced the seedlings’ ability for As translocation from roots to shoots, although the total As uptake decreased extensively (Fig. 4b).
Specific As uptake (SAU; a) and As translocation factor (TF; b) of 93-11 seedlings. The rice plants were treated with 50 μM arsenite for 6 days along with addition of exogenous 1.0 mM or 1.873 mM sodium silicate. All values are the mean of four replicates (±SD). Different letters indicate significantly different values among treatments (LSD, p ≤ 0.05)
Effect of silicate supply on lipid peroxidation
The MDA content that is a biomarker for lipid peroxidation was determined to estimate the extent of oxidative damage to lipids. A significant increase in MDA content was observed after 6 days of excess arsenite treatment (Fig. 5), indicative of severe As-induced oxidative stress in roots. Expectedly, the MDA content significantly decreased after the supplementation with both 1.0 mM and 1.873 mM concentration of external silicate, inferring the beneficial effect of Si on the amelioration of oxidative stress under As(III) stress.
Effect of arsenite and silicate interaction on the MDA content in the roots of 93-11 seedlings. Arsenic concentration of rice plants (21 dap), treated for 6 days with 50 μM arsenite. Data are reported as the mean ± SD. Different letters indicate significant difference among different treatments (n = 3; LSD, p ≤ 0.05)
Effects of silicate supply on antioxidant enzymes
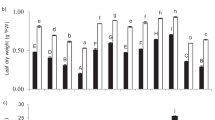
The effects of Si supplementation on SOD, APX, CAT, and POD activities under excess As(III) exposure were examined. It was observed that the SOD activity was strongly suppressed after treatment with 50 μM concentration of arsenite for 3 and 6 days, and this phenomenon became more severe after the addition of external silicate in roots (Fig. 6a). In leaves, strong inhibition of SOD activities were also observed after 3 and 6 days of the As(III) treatments, while Si supply with the concentration of 1.0 mM significantly ameliorated the suppression of SOD activities caused by excess As (Fig. 6b).
Effects of exogenous silicate supplementation (1.0 or 1.873 mM) on the antioxidant enzymes SOD, APX, CAT, and POD activity in the roots (a, c, e, g) and leaves (b, d, f, h) of 93-11 21-day-old seedlings grown in nutrient solution containing 0 or 50 μM arsenite for 3 and 6 days. Data are the mean ± SD of four replicates. Different letters indicate significant difference among different treatments (LSD, p ≤ 0.05)
The APX activities were severely inhibited after 3 and 6 days of As(III) treatments, but were considerably recovered in roots when 1.0 mM Si was added. Moreover, addition of 1.873 mM Si elevated the enzyme activity, but did not reach a significant difference level (Fig. 6c). In leaves, excess As(III) alone as well as As(III) combination with Si supplementation slightly increased the APX activity at different times (3 and 6 days) of arsenite challenge. This phenomenon was more obvious by the addition of 1.0 mM Si, especially at 6 days of treatment (Fig. 6d).
The CAT activity was strongly inhibited in roots when 1.873 mM Si was added at 6 days of treatment (Fig. 6e). Besides that, a slight but not significant decrease and increase in CAT activities under As and/or by Si addition were observed at all other treatment time courses (Fig. 6e). The CAT activities were not changed extensively among different treatments in leaves as well, although the sole addition of 1.0 mM Si at 3 days considerably decreased, while the treatment of As(III) combination with 1.873 mM Si slightly increased the enzyme activity at 6 days, respectively (Fig. 6f).
Excess As significantly decreased the POD activity but was slightly promoted when Si was added in roots. However, this recovery of the enzyme activity by Si supplementation was not significant in comparison with control (Fig. 6g). A contrary change of the enzyme activity was observed in leaves (Fig. 6h). Si supply significantly increased and decreased the POD activity at 3 days and 6 days of treatment, respectively.
Discussion
Hydroponic culture was widely adopted to investigate the plant–environment interactions, although this method has several limitations compared with the field experiments. In hydroponic experiments, the compositions of nutrient solution may have potential impacts on the As uptake and As content. Compared to the concentration of 100 to 600 μM endogenous silicon in most soils (Epstein 1999), using either the Hewitt (Tripathi et al. 2013) or the half-strength Kimura solution in this study, the rice plants were grown under Si depletion, and thus the silicon transporters were likely up-regulated causing an increase in As(III) uptake (Seyfferth and Fendorf 2012). On the other hand, soil parameters including the mobile As species and content, concentration of poorly crystalline iron-(hydr)oxides, plant-available P and Si, soil texture, as well as the pH and redox potential of the soil solution may affect As accumulation in rice (Bogdan and Schenk 2009; Williams et al. 2011; Moreno-Jiménez et al. 2012). In soil solution of aerobic soils, As is strongly adsorbed to iron-, aluminum-, and manganese-(hydr)oxides. Under flooding conditions, the redox potential of the soil solution decreased, leading to the dissolution of As into the pore water, where the predominant species is As(III) followed by As(V) and smaller amounts of methylated As species (Xu et al. 2008). Interestingly, in paddy fields, iron plaque would have formed on the rice roots by micro-aeration and oxidation of Fe(II) contained in soil water, which plays a barrier role for As uptake by rice plants (Garnier et al. 2010). In this study, only one specific As species, As(III), was added to the nutrient solution in which the pH value was at 5.5. Under such conditions, the redox potential in the solution should be relatively higher, thereby maintaining the As as the predominant species As(III). In addition, under hydroponic culture, no iron plaque formation is expected to have occurred due to NaFe(III)–EDTA being used in nutrient solution. Thus, effects of iron plaque on the inhibition of As uptake by rice roots in nutrient solution should be unlikely. However, compared with the field trials, the experimental conditions are under better control using hydroponics that can avoid the effects of soil biogeochemical processes, such as Fe plaque formation, redox cycling as well as the effect of microbes, and that may be beneficial for studying only the impact of silicate addition on arsenite uptake/toxicity.
Soil, plant roots, and microbes may be involved in the reduction–oxidation cycle of arsenate–arsenite in soils (Zhao et al. 2009). In aerated nutrient solution, arsenate is rapidly converted to arsenite by the roots of rice (Xu et al. 2007), whereas in aerobic soils, arsenite is oxidized rapidly to arsenate either by arsenite-oxidizing microbes (Macur et al. 2004) or by reactions with Mn(IV) oxides (Oscarson et al. 1981). On the other hand, although arsenite was determined as the predominant As species in submerged soil solutions, whether As(III) may be locally oxidized to As(V) in the root rhizosphere remains to be further clarified (Bogdan and Schenk 2008). As reported, due to the diffusion of some oxygen into the rhizosphere (Armstrong 1967), the rice root has developed an oxidized root zone (Flessa and Fischer 1992). However, in strongly reduced soil, the oxidization zone in rhizosphere is typically confined to within 1 mm of rice roots (Flessa and Fischer 1992). In addition, under anaerobic conditions, rice roots develop a barrier by suberization of the exodermis and lignification of sclerenchyma to counteract the diffusion of oxygen from the root to rhizosphere (Fleck et al. 2011). Therefore, it can be inferred that O2 loss into the rhizosphere alone is unlikely to oxidize a bulk of arsenite to arsenate under hydroponic culture or flooded conditions (Moore et al. 2011; Roberts et al. 2011). Notably, Guo et al. (2009) reported that in nutrient solution, 10–20 % of the added arsenite would be oxidized to arsenate in the absence of rice seedlings. We found that the components in the nutrient solution of Guo et al. (2009) are inconsistent with that of ours. Even if a similar case occurred in this study, arsenite is still the prevalent As species in the nutrient solution. In combination with the rapid reduction of arsenate in roots, the major arsenic toxicity to rice has been caused by As(III) uptake (Verbruggen et al. 2009).
Due to anthropogenic activities, 52,000–112,000 tons of arsenic was annually released to soil (Nriagu and Pacyna 1988). In most soils, the arsenic concentration varies normally between 0.2 and 40 mg kg−1 (Moreno-Jiménez et al. 2012). But in some districts of the following countries—Bangladesh, India, China, Chile, and USA, significant As contamination has occurred (Liao et al. 2005; Patel et al. 2005). For example, Patel et al. (2005) reported 9–390 mg kg−1 total As in the soil samples from a range of moderately and highly contaminated soils in the central India. In Chenzhou, Hunan province of China, the local agricultural soils near industrial districts were heavily contaminated, with the highest As concentration of 1,217 mg kg−1 found (Liao et al. 2005). Considering the high As contamination in many soil sites in China, 50 μM arsenite was adopted in this study to examine whether external Si supplementation has a significant role on alleviation of heavy arsenic toxicity to rice plants, although this concentration is much higher than those found normally in soil solution (Moreno-Jiménez et al. 2012).
Although a number of studies revealed the significant variance in As uptake and concentration between rice cultivars (Dwivedi et al. 2010; Norton et al. 2010; Rai et al. 2011; Dave et al. 2012), researches regarding the effect of arsenite on Chinese cultivated and wild rice are relatively limited. In this study, using a hydroponic cultivation strategy with the supply of As in the form of arsenite, we found that both the ability of total As uptake and translocation differed greatly among the four widely cultivated rice in China. Thus, selection of rice cultivars is indeed an effective measure that is used to reduce the accumulation of As in rice (Li et al. 2009). Especially, the observation that the Chinese wild rice accumulated the highest As in comparison with the cultivated rice suggests that wild rice should be cautiously used as gene resources for breeding rice cultivars with low As concentration.
As reported, photosynthetic inhibitions mainly include stomatal and non-stomatal limitations. In this study, the decrease of P n along with the increase of L s suggests that the major determinant for the photosynthetic inhibition might be due to extensive stomatal closure when treated with As(III) for 3 days. However, the severe inhibition of photosynthesis by arsenite at 6 days of treatment should be caused by other non-stomatal limitations, e.g., the inhibition of pigment synthesis (Jain and Gadre 1997). Azizur Rahman et al. (2007) reported that both the content of chlorophylls a and b in rice leaves at the flowering stage decreased along with the increase in soil arsenic concentrations for all tested varieties in Bangladesh. Accordingly, under arsenite challenge, stomatal limitation could have played a greater role in the early stage, while non-stomatal limitation (biochemical limitation) was predominant in the later stage of As(III) treatment in 93-11 seedlings.
The solubility and mineralogy of silicon amendments have great impacts on the amelioration effect of Si supplementation on arsenic toxicity. In the previous hydroponic and field trial experiments, addition of external Si, in the forms of K2SiO3·nH2O and Si gel, respectively, decreased significantly the total As concentration in rice plants (Guo et al. 2005, 2007; Li et al. 2009; Seyfferth and Fendorf 2012; Tripathi et al. 2013). By contrast, the supplementation of diatomaceous earth, another biogenic Si form and having low dissolution in pore water, unexpectedly promoted As uptake and accumulation in the aerial parts of rice plants (Seyfferth and Fendorf 2012). Li et al. (2009) discussed that the inhibitory effect of silicate fertilizer on As concentration would be through a competitive replacement of As(III) or As(V) adsorbed on Fe oxides/hydroxides by silicic acid under the field trail condition. It is worthy to note that Si amendments may have opposing impacts on As uptake by rice. The higher the solubility of Si amendments, the stronger the inhibition ability of Si on As uptake and translocation (Seyfferth and Fendorf 2012).
At pH <8, arsenite is predominantly present as a neutral molecule As(OH)3 due to its high pK a (9.22; Moore et al. 2011), which enters rice root cells and subsequently transports to xylem through Lsi1 and Lsi2, respectively (Ma et al. 2008). The two genes are localized in the distal and proximal side of the exodermis and endodermis cells, respectively (Ma et al. 2006, 2007), where the Casparian strips are formed that disallow solutes to pass freely through the endodermis into the stele in mature parts of the roots (Moore et al. 2011). Under hydroponic culture, the decrease in As(V) uptake was not due to the direct competition of Si for the As (P) influx transporter on root cell membranes (Guo et al. 2007), in contrast to the external Si-mediated reduction in As(III) uptake by rice (Guo et al. 2009; Tripathi et al. 2013), because arsenite and silicic acid compete with the same Si transporters during uptake and xylem unloading (Ma et al. 2008). In younger portion of the roots where the Casparian strip had not formed, the Si transporters are less expressed in this region (Yamaji and Ma 2007). Besides, continuous high-concentration silicic acid supply could suppress the expression of Lsi1 and Lsi2 in the rice cv. Oochikara (Ma et al. 2006, 2007), and thereby possibly further affects the extent of arsenite uptake and accumulation in rice (Hoffmann and Schenk 2011; Moore et al. 2011). We found that under 50 μM As(III) stress, addition of 1.873 mM external silicate decreased the As content in roots and total As uptake, but increased the As accumulation in shoots of 93-11 seedlings. Norton et al. (2010) identified a positive correlation between shoot Si and shoot As that is contrary to that observed by Bogdan and Schenk (2008), and they explained this phenomenon as the result of genetic differences between the cultivars used. In accordance with these observations, Seyfferth and Fendorf (2012) found that there exists differences in As and Si interactions and As toxicity between indica and japonica varieties. Accordingly, the inconsistency observed between the results of Guo et al. (2005) and ours could be appropriately interpreted by the differences in the selected rice cultivars (Weiyou 77 vs. 93-11), tested As species and concentrations (0.5 and 1.0 mg L−1 arsenate vs. 50 μM arsenite), as well as stress exposure time (15 vs. 6 days). On the other hand, the enhanced As accumulation by 1.0 mM Si supplementation might infer that lower concentration of external Si supply may have induced the expression of Si transporters Lsi1 and Lsi2 under excessive arsenite burden, and thereby promotes the As uptake and translocation. Alternatively, the effect of an extensive As availability in the solution greatly outweighed the inhibition of arsenite uptake by 1.0 mM Si. After the addition of higher dose external Si (1.873 mM), however, Si and As(III) compete strongly for uptake (Ma et al. 2008; Guo et al. 2009), and subsequently decreased the total As content in roots. Notwithstanding, the significant increase of As translocation from roots to shoots after 1.873 mM Si supplementation may not be simply explained by the regulation of expression of Lsi2 (Li et al. 2009), but may involve other transporters given that, except for Lsi1 and Lsi2, other NIP subfamily proteins can mediate the permeability of As(III) in rice (Bienert et al. 2008; Ali et al. 2009; Mitani-Ueno et al. 2011).
Arsenic exposure greatly induced the generation of free radicals and reactive oxygen species (ROS), which further results in the oxidative damage of various biomolecules, e.g., lipids and proteins (Shri et al. 2009). Therefore, increasing the plants’ antioxidant capacity is a feasible and effective approach to improve their tolerance to arsenic stress (Srivastava et al. 2009). In cell, SOD, APX, CAT, and POD comprised one of the major defense systems against oxidative stress (Shri et al. 2009). Choudhury et al. (2011) found that with the treatments of various concentrations of arsenate, the activity of the enzymes SOD and CAT was significantly induced and inhibited in the rice cv. MTU 1010, respectively. Due to the low CAT activities, the elevated level of H2O2 cannot be efficiently scavenged, leading to generation of toxicity to rice plants (Choudhury et al. 2011). Using two genotypes with contrasting tolerance to arsenic (Triguna and IET-4786), Tripathi et al. (2013) established that Si supplementation enhanced the activity of antioxidant enzymes including SOD, APX, and GR in both genotypes; however, the extent of amelioration of As-induced oxidative stress in tolerant rice (Triguna) was much stronger. In 93-11 seedlings, the activities of SOD, APX, and POD in roots as well as the SOD in leaves were significantly suppressed when exposed to 50 μM dose of arsenite; the inhibition, however, could be partially or totally alleviated by Si addition, with the exception of the SOD activity in roots (Fig. 6). As known, SOD played an important role in dismutation of the superoxide radicals to hydrogen peroxide (H2O2; Bowler et al. 1992). The significant inhibition of the SOD activity was suggestive of more severe oxidative stress in roots, which might be due to the inactivation of enzyme directly by As or ROS. In cytosol and chloroplasts, APX catalyzes the conversion of H2O2 into water by the ascorbate–glutathione (ASC–GSH) cycle (Noctor and Foyer 1998). The enzyme activity was significantly alleviated by Si supply in roots and leaves (Fig. 6). However, another peroxide-degrading enzyme, CAT, showed no significant change in its activity, which might be due to the less availability of H2O2 in peroxisomes due to its efficient breakdown in ASC–GSH cycle (Dwivedi et al. 2010). These observations may suggest the differential mechanisms of As responses in roots and leaves in the rice cv. 93-11. Notably, the decreases of SOD and APX activities under arsenite challenge in the present study were not consistent with the results of Shri et al. (2009), where the activities of the two enzymes were strongly induced. The reasons underlying this inconsistency were very complex and should be mainly attributed to the treated As concentration, treatment time, and genotypes, etc. In general, addition of external silicate extensively improved the rice plant’s tolerance to arsenite, probably through protecting the photosynthetic machinery, improving the membrane permeability, and enhancing the plant’s antioxidant capacity, etc.
References
Ali W, Isayenkov SV, Zhao FJ, Maathuis FJM (2009) Arsenite transport in plants. Cell Mol Life Sci 66:2329–2339
Armstrong W (1967) The use of polarography in the assay of oxygen diffusing from roots in anaerobic media. Physiol Plantarum 20:540–553
Azizur Rahman M, Hasegawa H, Mahfuzur Rahman M, Nazrul Islam M, Majid Miah MA, Tasmen A (2007) Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere 67:1072–1079
Berry JA, Downton WJS (1982) Environmental regulation of photosynthesis. In: Govindjee S (ed) Photosynthesis, IIth edn. Academic, New York, pp 263–343
Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6:26
Bogdan K, Schenk MK (2008) Arsenic in rice (Oryza sativa L.) related to dynamics of arsenic and silicic acid in paddy soils. Environ Sci Technol 42:7885–7890
Bogdan K, Schenk MK (2009) Evaluation of soil characteristics potentially affecting arsenic concentration in paddy rice (Oryza sativa L.). Environ Pollut 157:2617–2621
Bowler CM, Van Montagu M, Inzé D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-East Asia. Environ Int 35:647–654
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Choudhury B, Chowdhury S, Biswas AK (2011) Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J Plant Interact 6:15–24
Dave R, Tripathi RD, Dwivedi S, Tripathi P, Dixit G, Sharma YK, Trivedi PK, Corpas FJ, Barroso JB, Chakrabarty D (2012) Arsenate and arsenite exposure modulate antioxidants and amino acids in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes. J Hazard Mater doi: j.jhazmat.2012.06.049
Dwivedi S, Tripathi RD, Srivastava S, Singh R, Kumar A, Tripathi P, Dave R, Rai UN, Chakrabarty D, Trivedi PK, Tuli R, Adhikari B, Bag MK (2010) Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown on arsenic-contaminated soils of West Bengal. Protoplasma 245:113–124
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot 62:2001–2011
Flessa H, Fischer WR (1992) Plant-induced changes in the redox potentials of rice rhizospheres. Plant Soil 143:55–60
Garnier JM, Travassac F, Lenoble V, Rose J, Zheng Y, Hossain MS, Chowdhury SH, Biswas AK, Ahmed KM, Cheng Z, van Geen A (2010) Temporal variations in arsenic uptake by rice plants in Bangladesh: the role of iron plaque in paddy fields irrigated with groundwater. Sci Total Environ 408:4185–4193
Giannopotitis CN, Ries SK (1977) Superoxide dismutase in higher plants. Plant Physiol 59:309–314
Gottardi S, Iacuzzo F, Tomasi N, Cortella G, Manzocco L, Pinton R, Römheld V, Mimmo T, Scampicchio M, Dalla Costa L, Cesco S (2012) Beneficial effects of silicon on hydroponically grown corn salad (Valerianella locusta (L.) Laterr) plants. Plant Physiol Biochem 56:14–23
Guo W, Hou YL, Wang SG, Zhu YG (2005) Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil 272:173–181
Guo W, Zhang J, Teng M, Wang LH (2009) Arsenic uptake is suppressed in a rice mutant defective in silicon uptake. J Plant Nutr Soil Sci 172:867–874
Guo W, Zhu YG, Liang YC, Liu WJ, Chen Z (2006) Effect of application of silicon on arsenic uptake by rice seedlings in soil. Environ Sci 27:1393–1397
Guo W, Zhu YG, Liu WJ, Liang YC, Geng CN, Wang SG (2007) Is the effect of silicon on rice uptake of arsenate (AsV) related to internal silicon concentrations, iron plaque and phosphate nutrition? Environ Pollut 148:251–257
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoffmann H, Schenk MK (2011) Arsenite toxicity and uptake rate of rice (Oryza sativa L.) in vivo. Environ Pollut 159:2398–2404
Jain M, Gadre RP (1997) Effect of As on chlorophyll and protein contents and enzymic activities in greening maize tissues. Water Air Soil Pollut 93:109–115
Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783
Liao XY, Chen TB, Xie H, Liu YR (2005) Soil As contamination and its risk assessment in areas near the industrial districts of Chenzhou City, Southern China. Environ Int 31:791–798
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448:209–213
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Macur RE, Jackson CR, Botero LM, McDermott TR, Inskeep WP (2004) Bacterial populations associated with the oxidation and reduction of arsenic in an unsaturated soil. Environ Sci Technol 38:104–111
Meharg AA (2004) Arsenic in rice—understanding a new disaster for South-East Asia. Trends Plant Sci 9:415–417
Mitani-Ueno N, Yamaji N, Zhao FJ, Ma JF (2011) The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot 62:4391–4398
Mondal D, Polya DA (2008) Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl Geochem 23:2987–2998
Moore KL, Schröder M, Wu Z, Martin BGH, Hawes CR, McGrath SP, Hawkesford MJ, Ma JF, Zhao FJ, Grovenor CRM (2011) High-resolution secondary ion mass spectrometry reveals the contrasting subcellular distribution of arsenic and silicon in rice roots. Plant Physiol 156:913–924
Moreno-Jiménez E, Esteban E, Peñalosa JM (2012) The fate of arsenic in soil–plant systems. Rev Environ Contam Toxicol 215:1–37
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Norton GJ, Islam MR, Duan G, Lei M, Zhu Y, Deacon CM, Moran AC, Islam S, Zhao FJ, Stroud JL, McGrath SP, Feldmann J, Price AH, Meharg AA (2010) Arsenic shoot–grain relationships in field grown rice cultivars. Environ Sci Technol 44:1471–1477
Nriagu JO, Pacyna JM (1988) Quantitative assessment of world-wide contamination of air, water and soils by trace metals. Nature 333:134–139
Oscarson DW, Huang PM, Defosse C, Herbillon A (1981) Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature 291:50–51
Patel KS, Shrivas K, Brandt R, Jakubowski N, Corns W, Hoffmann P (2005) Arsenic contamination in water, soil, sediment and rice of central India. Environ Geochem Health 27:131–145
Pütter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic, New York, pp 685–690
Rai A, Tripathi P, Dwivedi S, Dubey S, Shri M, Kumar S, Tripathi PK, Dave R, Kumar A, Singh R, Adhikari B, Bag M, Tripathi RD, Trivedi PK, Chakrabarty D, Tuli R (2011) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:986–995
Roberts LC, Hug SJ, Voegelin A, Dittmar J, Kretzschmar R, Wehrli B, Saha GC, Badruzzaman ABM, Ali MA (2011) Arsenic dynamics in porewater of an intermittently irrigated paddy fields in Bangladesh. Environ Sci Technol 45:971–976
Savant NK, Snyder GH, Datnoff LE (1997) Silicon management and sustainable rice production. Adv Agron 58:151–199
Seyfferth AL, Fendorf S (2012) Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ Sci Technol 46:13176–13183
Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J (2005) Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66:1551–1559
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P (2009) Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour Technol 100:1115–1121
Su YH, McGrath SP, Zhao FJ (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328:27–34
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Goutam D, Shri M, Trivedi PK, Chakrabarty D (2013) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Williams PN, Zhang H, Davison W, Meharg AA, Hossain M, Norton GJ, Brammer H, Islam MR (2011) Organic matter–solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environ Sci Technol 45:6080–6087
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176:590–599
Yamaji N, Ma JF (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol 143:1306–1313
Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF (2010a) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol 186:392–399
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao FJ, McGrath SP, Meharg AA (2010b) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Ann Rev Plant Biol 61:535–559
Acknowledgments
The authors thank the anonymous reviewers for their valuable and constructive suggestions and for careful editing of the manuscript. This work was financially supported by grants from the Zhejiang Provincial Natural Science Foundation of China (Y3090152), National Natural Science Foundation of China (31000170), and the intramural fund from Zhejiang A & F University (2010FR060) to Q. Liu.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Haichao Hu and Junting Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, H., Zhang, J., Wang, H. et al. Effect of silicate supplementation on the alleviation of arsenite toxicity in 93-11 (Oryza sativa L. indica). Environ Sci Pollut Res 20, 8579–8589 (2013). https://doi.org/10.1007/s11356-013-1811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1811-x