Abstract
A set of periurban calcareous agricultural Mediterranean soils was spiked with a mixture of Cd, Cu, Pb and Zn at two levels within the limit values proposed by current European legislation, incubated for up to 12 months, and subjected to various one-step extraction procedures to estimate mobile (neutral salts) and potentially mobile metal fractions (complexing and acidic extraction methods). The results obtained were used to study metal extractability patterns according to the soil characteristics. The analytical data were coupled with mineralogical investigations and speciation modelling using the Visual Minteq model. The formation of soluble metal-complexes in the complexing extracts (predicted by the Visual Minteq calculations) led to the highest extraction efficiency with complexing extractants. Metal extractability patterns were related to both content and composition of carbonate, organic matter, Fe oxide and clay fractions. Potentially mobile metal fractions were mainly affected by the finest soil fractions (recalcitrant organic matter, active lime and clay minerals). In the case of Pb, scarce correlations between extractable Pb and soil constituents were obtained which was attributed to high Pb retention due to the formation of 4PbCO3·3PbO (corroborated by X-ray diffraction). In summary, the high metal proportion extracted with complexing agents highlighted the high but finite capacity to store potentially mobilizable metals and the possible vulnerability of these soils against environmental impact from metal accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil degradation is a serious problem in Europe, mainly driven or exacerbated by human activity. The number of potentially contaminated sites in the European Union-25 is estimated at approximately 3.5 million, with soil pollution from diffuse sources being recognized as one of the major soil threats by the European Union Soil Thematic Strategy (European Commission 2006). This is especially true in metropolitan areas subjected to risks from pollution due to a strong industrial development and spreading urbanization, leading to an increase of “urban elements” in soils (Ba, Cd, Co, Cu, Mg, Pb, Sb, Ti and Zn) (de Miguel et al. 1997). This scenario is typical of periurban agricultural soils which are affected and likely to become more so in the future unless sustainability criteria are applied (Iram et al. 2012; Peris et al. 2007). Based on these premises, there is a potential risk of metal contamination of groundwater and of accumulation in food crops which is directly related to the mobility of metals in soils.
The mobility of metals can be greatly decreased in calcareous Mediterranean soils where low rainfall and high evapotranspiration lead to metal accumulation in the first few centimetres of the soil. This metal accumulation capacity, enhanced by the high metal retention provided by carbonates, can however be affected by other capacity-controlling properties such as texture, content and type of organic matter, soil pH–redox conditions and the content of oxides of Fe, Al and Mn. Metals accumulated and retained could be potentially mobilizable by organism activity (Sayyad et al. 2010) and/or by changes in the conditions in the environment (Bolan et al. 2003). The high but finite capacity to store potentially mobilizable metals suggests that these soils should be considered to be the most vulnerable (Batjes 2000). This high vulnerability which allows metals to move from one environmental echelon to another, combined with the complexity of the interaction between metals and the constituents of calcareous soils, highlights the importance of the study of the soil properties affecting the mobility (and thus the availability) of metals in these soils.
It is generally accepted that to assess the mobility and availability of metals in soils it is not sufficient merely to analyse the total metal content in soils, nor is this a useful tool for determining potential risks, as soils are dynamic systems. A very delicate equilibrium exists among the metal pseudo-total content (inactive and inert), the metal mobile fraction (effective soluble, very active, bioavailable) and the metal mobilizable/potentially mobile fraction (potentially bioavailable, leachable and partly active) (Gupta et al. 1996). Using chemical-extracting media of different strengths, it is possible to differentiate between these fractions. The combination of indirect methods (analytical data from metal extractions and speciation modelling of the soil extracting solution) with direct methods (soil mineralogical investigations) is a useful tool for elucidating soil properties and constituents as well as the chemical processes that affect metal extractability, mobility and availability (Ettler et al. 2007; Pérez-Esteban et al. 2013; Sipos et al. 2008).
The objective of the study was to investigate the mobile and potentially mobile fractions of Cd, Cu, Pb and Zn using a set of extraction methods of different strengths, speciation modelling using Visual Minteq Model, and mineralogical investigations in several metal-spiked periurban calcareous agricultural soils representative of the Mediterranean area. Since the role of soil constituents in metal desorption processes is not well defined for these soils, we placed particular emphasis on the soil constituents and properties affecting metal extractability patterns.
Materials and methods
Study area, soil characteristics and sampling
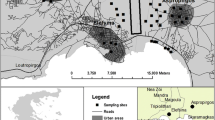
The study area is part of a periurban axis that combines agricultural activity and the main residential and industrial uses in the Madrid region; it is exposed to atmospheric deposition from transportation and industrial activities. The soil samples come from different plots in the “El Encín” Agricultural Research Station (Alcalá de Henares, Madrid, Spain), at an altitude of 588 m, located on the Henares River on quaternary sediments (IGME 1990). These alluvial sediments have led to an ancient calcaric Fluvisol (Moreno Merino 1998) which presents Anthric characteristics today (FAO 2006) mainly as a result of agricultural use. The average annual temperature is 13 °C, average annual rainfall is 401 mm year−1 and potential evaporation is about 760 mm year−1. The site is typical of a Mediterranean pluviseasonal-oceanic bioclimate on an upper meso-Mediterranean low dry bioclimatic belt (Rivas-Martínez and Rivas-Saenz 2009).
A set of ten agricultural soil samples (M1, M2, M3, M4, M5, L1, L2, VL1, VL2 and VL3), differing in their carbonate content, amount of organic matter and textural class, was selected according to information from previous studies (de Santiago-Martín et al. 2013; Lafuente et al. 2008). Sampling was done at randomly selected points. To avoid potential bias, 30–40 kg was taken from each sampling point (0–30 cm) and homogenized. Soil samples were air-dried and passed through a 2-mm sieve.
Experimental design
The environmental impact from metal accumulation in periurban agricultural areas was simulated by the addition of a multi-elemental salt solution of metals to the soil samples. Cd, Cu, Pb and Zn were selected owing to their different speciation, mobility and extractability in soils. Three containers (40 cm wide × 59 cm long × 21 cm high) of 10 kg each were set for each sample: one un-spiked sample with the addition of distilled water, and the other two spiked at two different concentration levels using nitrate salts of metals in aqueous solution: low level (Tt1) (3 mg kg−1 of Cd + 140 mg kg−1 of Cu + 300 mg kg−1 of Pb + 300 mg kg−1 of Zn) or high level (Tt2) (20 mg kg−1 of Cd + 875 mg kg−1 of Cu + 600 mg kg−1 of Pb + 2000 mg kg−1 of Zn) within the limits proposed by current European legislation (Directive 86/278/EEC). Each soil sample and the corresponding solution was mixed and left to equilibrate for a period of 12 months at room temperature without cover or drainage. During this equilibration period, the soils were air-dried, mixed and rewetted with distilled water in cycles of about 2 weeks, in order to favour metal redistribution processes into the soil matrix. The appropriate amount of water was added to bring the soil samples to the each estimated field capacity (Jalali and Khanlari 2008). Distribution processes play a key role in determining the extractability and availability of metals in soils (McLaughlin 2001).
At the end of the equilibration period (12 months), duplicates were randomly removed from each un-spiked and metal-spiked soil sample for extraction of the metals by means of one-step extraction methods. Different extracting solutions have been used to assess mobile and potentially mobile fractions of metals, both in equilibrium with inactive and inert fractions (Gupta et al. 1996). The mobile fraction was estimated with 0.01 M CaCl2, 1 M MgCl2, 0.1 M NaNO3 and 1 M NH4NO3 methods, and the potentially mobile fraction was estimated with 5 mM DTPA, 0.05 M EDTA, 0.5 M HNO3, 0.11 M HAc, 10 mM LMWOA (a mixture of low molecular weight organic acids) and 1 M NH4Ac methods. The analytical data were coupled with mineralogical investigations (Sipos et al. 2008), measurement of the pH in extracts and speciation modelling using the Visual Minteq model (Ettler et al. 2007; Pérez-Esteban et al. 2013). In order to evaluate the soil sorption capacity, a sorption test was conducted prior to the incubation experiment and the distribution coefficients were calculated.
Analytical methods
All chemicals were obtained from analytic grade reagents of Merck (Germany). All glassware used was pre-washed with an aqueous solution of HNO3 0.1 % for 24 h and rinsed with de-ionized type I water.
Soil physicochemical parameters
According to ISRIC (2002) methods, the following parameters were determined: soil pH in a 1:2.5 soil to water ratio, equivalent CaCO3 (ECC) according to the acid neutralization method, total organic C by the Walkley–Black wet oxidation procedure, particle-size distribution by Robinson’s pipette method, cation exchange capacity (CEC) by the ammonium acetate method, and crystalline and amorphous Fe and Mn oxide contents by dithionite–citrate extraction, in addition to acid oxalate extraction. The active equivalent CaCO3 finely divided or “active lime” (AL) was determined with NH4-oxalate as described by Drouineau (1942). We used the two-step acid hydrolysis procedure with H2SO4 to determine the recalcitrant pool of organic matter (RP) (Rovira and Vallejo 2000). Total Ca, Mg, Fe, Cd, Cu, Pb, and Zn contents of the soil samples were determined after wet digestion (150 °C, 6 h) with a mixture of nitro-perchloric-hydrofluoric acids under high-pressure conditions using PHAXE 2000 reactors (SISS 1985). Fe, Mn, Ca, Mg, Cd, Cu, Pb and Zn concentration in the corresponding extracts was quantified by atomic absorption spectroscopy, AAS (Analytikjena NovAA 300). All samples were extracted and analysed in duplicate. Quantification limits in milligrams per litre were: Ca = 1, Mg = 0.05, Fe = 0.5, Mn = 0.2, Cd = 0.2, Cu = 0.2, Pb = 0.5 and Zn = 0.1.
Soil mineralogical analyses
Mineral composition of soil samples was examined by X-ray diffraction (XRD) using an EQ 0434520 31 02 (X'Pert MPD) diffractometer with Cu Kα radiation operated at 45 kV and 40 mA. All XRD patterns were recorded with a dwell time of 1 s and 0.04° 2θ step. Un-spiked and metal spiked (Tt1 and Tt2 levels) soil samples (<2 mm) were examined on randomly oriented powders. Separation of the clay fraction of un-spiked soils (<2 μm) was performed by sedimentation in aqueous suspension previous NH4OH dispersion and posterior H2O2 treatment. The clay fraction in air-dried samples after saturation with Mg was calcinated at 550 °C for 2 h and after an ethylene glycol solvation. Abundance of soil minerals in both fine earth and clay fractions was assessed semi-quantitatively (Bish 1994).
One-step extraction methods of metals
The procedures to determine extractable metals are described as follows: 0.01 mol L−1 CaCl2 solution, 1:5 w/v for 2 h (Van Ranst et al. 1999); 1 mol L−1 MgCl2 solution, 1:8 w/v for 1 h (Tessier et al. 1979); 0.1 mol L−1 NaNO3 solution, 1:2.5 w/v for 2 h (Gupta and Aten 1993); 1 mol L−1 NH4NO3 solution, 1:2.5 w/v for 2 h (DIN 1995; Legislation Germany); 0.005 mol L−1 diethylene triamine pentaacetic acid (DTPA) solution in 0.01 mol L−1 CaCl2 solution and 0.01 mol L−1 triethanolamine (TEA), 1:2 w/v for 2 h (Lindsay and Norwell 1978); 0.05 mol L−1 ethylene diamine tetra-acetic acid (EDTA) solution, 1:10 w/v for 1 h (Quevauviller et al. 1996); 0.5 mol L-1 HNO3 solution, 1:5 w/v for 30 min (Van Ranst et al. 1999); 0.11 mol L−1 CH3COOH solution (HAc), 1:40 w/v for 16 h (Rauret et al. 1999); 0.01 mol L−1 low molecular weight organic acids (LMWOA) solution consisting of acetic, lactic, citric, malic and formic acids with a molar ratio of 4:2:1:1:1, 1:10 w/v for 16 h (Feng et al. 2005); and 1 mol L−1 CH3COONH4 solution (NH4Ac) buffered by HAc at pH 7, 1:30 w/v for 4 h in column (Van Ranst et al. 1999). In all cases, except for NH4Ac extractions, the samples and the extraction solution were shaken in a vibrator agitator (Vibromatic, Selecta) at 400 opm. The supernatant of each extraction was centrifuged at 3,500 rpm for 15 min and then filtered (low ash filters, 5–7 μm). Dilutions were made with the corresponding extraction solution. Cd, Cu, Pb and Zn concentration in the extracts was quantified by AAS.
The computer program Visual Minteq v.3.0 (Gustafsson 2011) was used to predict the metal speciation in the extracts of three representative soil samples (M1, L1 and VL1 soils). We used the database that comes by default in the Visual Minteq program. The measured values of pH, metal concentration in extracts and the concentration of the extractant applied were used as the input data. Cd, Cu, Pb, and Zn were simultaneously entered into the code, and the calculations were performed for each individual extract.
Sorption capacity of metals
The metallic solution and each soil sample (1:2 w/v) were mechanically shaken for 24 h, and the supernatant of each extraction was centrifuged (3,500 rpm, 15 min) and then filtered. Concentration of Cd, Cu, Pb and Zn in the supernatant was quantified by AAS. The distribution coefficients (K d) of the metals were calculated (Lafuente et al. 2008) using the equation: K d = (M sorbed)/(M solution), where M sorbed is the amount of sorbed metal per unit weight of soil (milligrammes per kilogramme) and M solution is the amount of metal in solution per unit volume of liquid (milligrammes per litre).
Statistical analysis
Significance of differences of the means (n = 10) of the relative metal extractability was investigated by means of one-way ANOVA using a post hoc test (Tukey). The homogeneity of variances was verified by Levene test. Correlation coefficients were calculated for metal-spiked (Tt1 and Tt2 level) soil samples (n = 10, for each contamination level) to relate both the Kd values and the amount of metals extracted with the different methods employed to the soil physicochemical parameters (Pearson’s correlation test) and the mineralogical composition (Spearman’s correlation test). Since mineralogical properties are semiquantitative variables, we used a nonparametric correlation test, the Spearman’s test. Analyses were conducted using SPSS (Statistical Package for the Social Sciences) v.17 (SPSS, Inc.) software.
Results and discussion
Soil characteristics
Soil physicochemical parameters
The main results of the physicochemical analyses from un-spiked soils are shown in Table 1. All soil samples showed pH values above 8. The ECC content ranged from moderate (M1, M2, M3, M4 and M5 soils) to low (L1 and L2 soils) and very low (VL1, VL2 and VL3 soils). Differences were observed in organic matter (OM) content and particle-size distribution within each group of soil samples. Thus, the OM content ranged from low to high, in all cases showing a high proportion of RP. The textural classes of soils ranged from sandy-loam to sandy-clay loam, with fine sand being the most abundant size fraction. Crystalline Fe oxide content was low (∼10 g kg−1). In general, the total Ca and Mg content varied in line with the carbonate fraction. The total Cu, Pb and Zn content was similar to that obtained by other authors for agricultural soils in the Mediterranean area (Jiménez Ballesta et al. 2010; Micó et al. 2006; Peris et al. 2007) and in no case exceeded the levels established by the European Union (Directive 86/278/EEC). The total Cd content was lower than the quantification limit.
Mineralogical composition
Examinations by XRD of the un-spiked soil samples indicated high contents of quartz and phyllosilicates in varying proportions (Table 2). The content in feldspars was always very low. Calcite was the predominant mineral in the carbonate group. Dolomite was not detected in L2, VL1 and VL3 soil samples. The analysis of XRD of the metal-spiked soils showed the presence of lead oxide carbonate (4PbCO3·3PbO; JCPDS No. 00-017-0732), an intermediate oxycarbonate confirmed by the reflections at 0.364, 0.286, 0.267, 0.215 and 0.165 nm in samples originally characterized as containing calcite and dolomite in their composition (mainly M1, M2 and M3 soils at Tt2 level). Figure 1 shows the XRD patterns from un-spiked and metal-spiked (at Tt2 level) samples of M1 soil. Table 2 shows the mineralogical composition of the clay fraction of un-spiked soils. The most common phyllosilicate was illite, identified as a trioctahedral mineral based on the intensities of the 001/002 basal reflections (Douglas 1985). The minerals 1:1 of the kaolinite group displayed lower percentages. Vermiculite was occasionally present (M1 and VL1 soils). With regard to non-phyllosilicate minerals, it is worth noting the presence of quartz, calcite and dolomite in varying proportions.
Metal sorption capacity
The sorption test showed that the metal sorption capacity of the selected soils was very high, ranging from 94 to 100 % for Cu, Pb and Zn (data not shown), in agreement with the results obtained previously in the study area (de Santiago-Martín et al. 2013). The Cd sorption capacity, at about 64–92 %, was lower than for other metals, and the lowest percentage was found in VL3 soil, with the highest coarse sand (CS) content. These results indicate a priori that these soils would be able to buffer an impact from metal contamination. Nevertheless, high metal extraction percentages were obtained with the one-step extraction methods used, as discussed below. The distribution coefficients (K d) calculated highlighted that Pb was in all cases the most retained metal (Online resource 1), Cd was the metal with the lowest K d value, and Cu and Zn presented intermediate values. In order to elucidate what soil constituents affect metal sorption capacity, a Pearson’s correlation analysis was performed. Table 3 shows that negative and significant correlations were obtained between sand fractions and K d values in all cases, pointing out the key role of the finest mineral fractions in affecting metal sorption patterns.
Extraction efficiency and metal speciation in extracts
Values of Cd, Cu, Pb and Zn concentration obtained in single chemical extractions in the un-spiked and metal-spiked soil samples are shown in Fig. 2, as a percentage of total metal. The absolute values of the extractable metals, expressed in milligrammes per kilogramme, are shown in Online resource 2. It was observed that the highest proportion of metals extracted in metal-spiked soil samples was in all cases with complexing and acidic extractants, and the lowest was with neutral salts. The high metal proportion extracted with complexing agents indicates a high potential metal mobility in these soils.
Relative Cd, Cu, Pb and Zn extractability (%) with DTPA, EDTA, HNO3, HAc, LMWOA, NH4Ac, CaCl2, MgCl2, NaNO3, and NH4NO3-methods in un-spiked (white rectangle) and metal-spiked soil samples at Tt1 (grey rectangle) and Tt2 levels (black rectangle). The boxplots show the lower quartile, the median and the upper quartile, with whiskers extending to the most extreme data point (n = 10). Different letters indicate significant differences between extraction methods at p < 0.05 after one-way ANOVA. Extractable Cd was lower than the quantification limit in un-spiked soil samples
The Visual Minteq thermodynamic calculations were useful in determining the metal speciation in the extracts, which depends on the pH and chemical composition of the solutions (Online resource 3). The pH values of the extracts are shown in Table 4. In the case of acids and chelating agents, the pH values of soil extracts were lower in VL1 soil (with a lower ECC content) than in M1 and L1 soils.
The high proportion of metal extractable with EDTA (60–82 %) was attributed to its high chelation stability constant (Meers et al. 2007). Similarly, the pH range of our soils favoured the formation of soluble complexes with metals in the EDTA and DTPA extracts (up to 100 % of Me-EDTA2− and Me-DTPA3−). Nevertheless, it should be taken into account that the very high percentage of negatively charged complexes could lead to lower metal extraction in soils with a higher OM content, due to re-adsorption processes in the organic matter (Ettler et al. 2007; Peters 1999). In HAc extractions, the speciation was dominated by Me-Acetate+, Me-(Acetate)2 and Me2+. The highest proportion of Me-(Acetate)2 was in M1 soil, and the highest rate of free metal ion was found in VL1 soil, which was attributed to the different pH value of the soil extracts (Table 4). The speciation was more complex with LMWOA extractions. Metals were present as Me-Citrate- (up to 97 %) due to the higher chelation stability constant of citric acid than of the other organic acids in the mixture employed. The lower stability constants of the citrate complexes with Cd and Pb than with Cu and Zn (Barton and Abadía 2006) resulted in a lower Cd and Pb extraction percentage at Tt2 level. Moreover, at this level, we observed the appearance of other Cd and Pb species in minor proportions (free metal ion, Me-Malate (aq), Me-Lactate+ and Me-Acetate+) due to different complexing abilities in response to pH (Qin et al. 2004). In NH4Ac extracts, acetic complexes were the dominant species of Cd, Pb and Zn, which were mainly present as Me-(Acetate)3 −, Me-(Acetate)2 (aq) and Me-Acetate+. The dominant species in the case of Cu were amine complexes.
Very high amounts of metals were extracted with the HNO3 method (Fig. 2). In general, free metal ion was the predominant species despite the large difference in the pH of the extractant solution from the various soils (Table 4). This could indicate that the main processes affecting metal desorption with the HNO3 extraction method could be either the dissolution of some of the soil components, or the metal displacement by excess H+ from the exchangeable complex (Vidal et al. 2004).
Different patterns were obtained in the case of neutral salt extractions. Metal speciation in CaCl2 and NaNO3 extracts was dominated by the free ionic form, except for Cu and Pb in NaNO3 extracts which were in a hydroxocomplex form at pH 7.8–8.1. Moreover, it should be noted that a similar metal percentage was extracted in un-spiked and in metal-spiked soils, suggesting that metal redistribution within the contact time of this study (12 months) could reduce their concentration in exchangeable positions (McLaughlin 2001). Metals in MgCl2 extracts were mainly in the form of chlorocomplexes. In the case of NH4NO3 extracts, amine complexes were the dominant species of Cd, Cu and Zn, while Pb formed mainly nitric complexes. It should be noted that the calculated ionic strengths for MgCl2 and NH4NO3 extract exceeded 1 mol L−1, so the results of this calculation must be considered with caution. The low metal extraction percentage with neutral salts indicates that sorption to exchange positions is not the main metal retention mechanism in these soils but the specific adsorption. Moreover, it is worth noting that competition mechanisms can be established between the alkaline-earth cations, major cations in these soils and the cations of the extractants. In contrast, high amount of metals were extracted with MgCl2 which was attributed to its higher salt concentration, the combined effect of complexation by chloride and metal displacement from the exchangeable complex by Mg2+ (Meers et al. 2007), and to slight carbonate dissolution (Gleyzes et al. 2002).
Metal extractability patterns
Metal extractability patterns in metal-spiked soils were studied according to the soil physicochemical characteristics (Tables 5, 6, 7, 8), and by mineralogical investigations. In this regard, it was observed in general that soil properties and components affecting metal extractability patterns depend on the metal being studied, as discussed below. The extractions with EDTA are an exception, showing a general extractability pattern for all metals. In all cases, significant negative correlations were observed between EDTA-extractable metals and OM content, mainly with the recalcitrant fraction, showing that re-adsorption processes could occur in organic material.
Cadmium extractability patterns
The key role of the soil carbonate fraction in Cd extractability was evident from the correlation coefficients calculated. Cadmium extractability patterns, estimated with MgCl2, NH4NO3, DTPA and LMWOA methods, showed significant and negative correlations with all the soil constituents measured in relation to the carbonate fraction (ECC, AL, total Ca–Mg contents and the relative proportion of calcite and dolomite) (Table 5). This indicates that the soil carbonate fraction govern both mobile and potentially mobile fractions of Cd in these soils. Due to the contact time of the incubation experiment (12 months), it would produce a slow diffusion of soluble Cd species into the crystal defects and pores of the lime, resulting in stable bonds (Buekers et al. 2007), and hence decreasing Cd extractability. The positive and significant correlations obtained between acidic extractants and ECC and AL contents could corroborate this hypothesis, since these extractants total or partially dissolve the carbonate, thereby releasing Cd into the solution (Vidal et al. 2004). This pattern has been observed by other authors when studying Cd desorption by fibrous minerals, i.e. palygorskite and sepiolite group (Shirvani et al. 2007), and Mn oxides (Zaman et al. 2009). Despite the mineralogical study showing no evidence of formation of Cd-carbonate or oxide, probably due to the fact that the Cd concentrations used were below the quantification limit of the XRD equipment (<2 %), surface precipitation phenomena can occur.
On the other hand, negative and significant correlations at Tt2 level were obtained between DTPA-extractable Cd and the crystalline Fe oxide and OM contents, and between EDTA-extractable Cd and RP content. The formation of stable organomineral associations, favoured by the high affinity of Cd for high molecular weight organic acids, can explain these patterns (Prokop et al. 2003). In view of these results, it could be concluded that potentially mobile Cd, estimated with EDTA and DTPA, is affected by crystalline Fe oxides and OM. Nevertheless, re-adsorption processes of negatively charged Me-DTPA and EDTA complexes in the organic matter should be taken into account (Ettler et al. 2007).
At Tt2 level, significant and positive correlations were obtained between MgCl2-extractable Cd and clay content and CEC, showing that exchangeable processes also play a relevant role at this contamination level. The significant and negative correlations between HAc-extractable Cd and clay content and CEC may be an artefact of carbonate, as the soils with a lower proportion of clay generally present higher carbonate contents in this study.
Copper extractability patterns
There were significant and positive correlations between NH4Ac and NH4NO3-extractable Cu and ECC, AL and total Ca contents at Tt1 level (Table 6), showing that carbonate fraction affects Cu extractability patterns with these extraction methods. Since the mineralogical study showed no evidence of crystallized Cu species, sorption processes, others that precipitation ones, are affecting these patterns, probably adsorption ones. In this scenario, ammonium ions could displace the Cu adsorbed from carbonates. The highest correlation coefficients (p < 0.01) obtained for AL indicated that the finest carbonate fraction could play a greater role in affecting Cu extractability.
On the other hand, significant and negative correlations were obtained between DTPA, MgCl2 and NaNO3-extractable Cu and the clay content and CEC (Tt1 level) and between HAc-Cu and the crystalline Fe oxide content (whatever the level). This indicated that even though AL should affect Cu extractability patterns, other soil constituents present in the finest soil fraction <2 μm, such as Fe oxides and phyllosilicates, were implied. The enhanced adsorption capacity of illite minerals in the presence of Fe (Sipos et al. 2008), together with the high ability of Cu2+ to displace the Fe2+, can explain these patterns. Nevertheless, the significant and positive correlations observed between EDTA-extractable Cu and CEC at Tt2 level show that other sorption mechanisms, such as exchangeable processes, also can take place (Sayen and Guillon 2009).
Similarly, significant and negative correlations were also obtained between HAc-Cu and OM content at Tt1 level, and between EDTA-Cu and RP at Tt2 level. The higher Cu electronegativity, the predominance of positively charged Cu hydroxocomplexes (characteristics of the pH range studied) and the degree of humification of the soil samples (C/N ∼ 11) favour the formation of stable associations between Cu and humic substance. These results indicate that the potential mobility of Cu may be governed by both inorganic and organic fractions through organomineral associations (Besnard et al. 2001), probably humate–Fe oxide associations (Sayen and Guillon 2009; Sipos et al. 2008) at both levels of contamination.
These results therefore highlight that the combined action of the finest organic and inorganic fractions govern the Cu extractability patterns in these calcareous agricultural soils.
Lead extractability patterns
Few correlations were obtained for extractable Pb (Table 7). At level Tt1, significant and positive correlations were obtained between acidic extractants and the ECC and AL contents, suggesting the total or partial dissolution of the carbonate fraction (Vidal et al. 2004). Nevertheless, significant correlations were not obtained at Tt2 level, despite the formation of lead oxide carbonate observed (Fig. 2). The lack of correlations with soil physicochemical parameters at this level led us to study the relationship with soil mineralogical composition. The significant and positive correlations obtained between the HAc-extractable Pb and the relative proportion of dolomite (<2 mm) at both levels suggest that the Pb extractability pattern with acidic extraction methods is mainly affected by the dolomite phase. However, extractable Pb with other extraction methods with lower strength (DTPA, LMWOA, MgCl2, NH4NO3 and NaNO3) showed significant and negative correlations with the total Mg content. Rangel-Porras et al. (2010), studying the mechanism for retention of Pb ions in presence of calcite, observed the nucleation of micro-precipitates of Pb on the calcite surfaces. In our case, the pH range of our soils, combined with the incubation time of our experiment (12 months), favours these precipitation processes on the surfaces of Mg-rich clay minerals, such as dolomite, explaining the negative correlations obtained. These results therefore suggest that the potential mobility of Pb depends to some degree on the turnover of inorganic C. Nevertheless, the intensities of reflections obtained from XRD were not high, showing the low crystallinity degree. Hence, precipitation mechanisms in carbonate fraction cannot be regarded as the only process affecting Pb retention in these soils. As previously reported, adsorption and complexing processes could play a key role in affecting Pb retention (Bradl 2004).
The predominant species in the pH range in the study, Pb(OH)+, form stable bonds with OM and clay, probably through organomineral associations regulating the retention of Pb. However, the slow Pb desorption from organic matter, in contrast to its rapid initial sorption (Strawn and Sparks 2000), explain the scarce correlations obtained with clay and OM. This sorption mechanism may not affect Pb extractability patterns or its potential mobility as high sorption does not necessarily lead to high bond strength at the same time. On the other hand, NH4NO3-extractable Pb correlated positively and significantly with clay content and CEC at Tt1 level, suggesting that a fraction of Pb could be in exchange positions at this level.
Zinc extractability patterns
The Pearson’s correlation coefficients calculated showed that particle-size distribution was the main soil characteristic affecting Zn extractability patterns, and no significant correlations were obtained with other soil physicochemical parameters, except the general case of EDTA and OM (Table 8). Thus, significant and negative correlations were obtained between extractable Zn with almost all extractants and clay and silt contents, suggesting that the soil fractions with a higher adsorption capacity are primarily responsible for governing both mobile and potentially mobile Zn fractions. From the mineralogical study, we obtained positive correlations between the relative proportion of kaolinite, illite and quartz (<2 μm), and HNO3 and EDTA-extractable Zn. This indicates that the presence of these minerals, with a low specific surface area, favours a higher Zn extractability. The significant and negative correlations obtained between extractable Zn with several extractants and the total Mg content, mainly at Tt2 level, indicate that lower Zn extractability may be linked to the presence of Mg-rich minerals (Lafuente et al. 2008), probably due to the similarity of ionic radius between Zn2+ and Mg2+. Similarly, significant and negative correlations were obtained between EDTA-extractable Zn and the dolomite relative proportion (<2 μm) at the Tt2 level. Therefore, from these results, it can be deduced that the Zn extractability patterns are affected by Mg-rich minerals, probably dolomite, and it is expected that Mg ions were released into the soil solution by displacement of Zn ions. In previous works (data not shown), we observed an increase in the content of Mg in soil solution in the metal-spiked soils at Tt2 level. These results therefore highlight the fact that the clay content alone cannot explain the Zn-extractability patterns, since soil mineral composition plays a significant role in controlling its extractability.
Conclusions
Despite carbonate fraction typically conferring a high metal sorption capacity, this fraction alone cannot explain the metal extractability patterns in the metal-spiked soils studied, under the experimental conditions used. Instead, the content and composition of some soil fractions (mainly organic matter, Fe oxides, and clay) have been shown to govern metal extractability patterns, highlighting the need to consider the intrinsic soil characteristics of each polluted site. In particular, the finest organic and inorganic soil fractions played the main role in affecting the both mobile and potentially mobile fractions of metals. In this respect, the combination of indirect and direct methods employed in this study has proved to be an essential tool for investigating soil constituents affecting (potentially) mobile fractions of Cd, Cu, Pb and Zn in these calcareous agricultural soils. The high metal extraction percentage, mainly with chelating agents, highlights the potential vulnerability of these soils against an environmental impact from metal accumulation in periurban agricultural areas. Results are also useful for remediation purposes since the application of chelating agents to agricultural soils becomes to be a generalized practice.
References
Barton L, Abadía J (2006) Iron nutrition in plants and rhizospheric microorganisms. Springer, Dordrecht
Batjes NH (2000) Soil vulnerability to diffuse pollution in Central and Eastern Europe SOVEUR Project (Version 1.0). FAO and ISRIC
Besnard E, Chenu C, Robert M (2001) Influence of organic amendments on copper distribution among particle-size and density fractions in Champagne vineyard soils. Environ Pollut 112:329–337
Bish DL (1994) Quantitative x-ray diffraction analysis of soils. In: Amonette JE, Zelazny LW (eds) Quantitative methods in soil mineralogy. SSSA, Madison, pp 267–295
Bolan NS, Adriano DC, Curtin D (2003) Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv Agron 78:215–278
Bradl HB (2004) Adsorption of heavy metal ions of soils and soils constituents. J Colloid Interf Sci 277:1–18
Buekers J, Van Laer L, Amery F, Van Buggenhout S, Maes A, Smolders E (2007) Role of soil constituents in fixation of soluble Zn, Cu, Ni and Cd added to soils. Eur J Soil Sci 58:1514–1524
de Miguel E, Llamas JF, Chacon E, Berg T, Larssen S, Røyset O, Vadset M (1997) Origin and patterns of distribution of trace elements in street dust: unleaded petrol and urban lead. Atmos Environ 31(17):2733–2740
de Santiago-Martín A, Valverde-Asenjo I, Quintana JR, González-Huecas C, Lafuente AL (2013) Soil properties affecting metal extractability patterns in periurban calcareous agricultural soils in the Mediterranean area. Int J Environ Res, in press
DIN (Deutsches Institut für Normung, Bodenbeschaffenheit) (1995) Extraktion von Spurenelemente mit Ammonium-nitratlösung, Vornorm DINV 19730. DIN Boden-Chemische Bodenuntersuchungs-verfahren, Berlin
Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture
Douglas LA (1985) Criteria for vermiculitic and chloritic family classes in soil taxonomy. In: Kittrick JA (ed) Mineral classification of soils, vol 16. Soil Science Society of America, Madison
Drouineau G (1942) Dosage rapide du calcaire actif de sols. Ann Agron 12:441–450
Ettler V, Mihaljevič M, Šebek O, Grygar T (2007) Assessment of single extractions for the determination of mobile forms of metals in highly polluted soils and sediments—analytical and thermodynamic approaches. Anal Chim Acta 602(1):131–140
European Commission (2006) Thematic strategy for soil protection. COM (2006) 231, Brussels, Belgium
FAO (2006) World reference base for soil resources. A framework for international classification, correlation and communication. FAO, Roma
Feng M, Shan X, Zhang S, Wen B (2005) A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2, and NaNO3 extraction methods for prediction of bioavailability of metals in soil to barley. Environ Pollut 137:231–240
Gleyzes C, Tellier S, Astruc M (2002) Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trends Anal Chem 21(6+7):451–467
Gupta SK, Aten C (1993) Comparison and evaluation of extraction media and their suitability in a simple model to predict the biological relevance of heavy metal concentrations in contaminated soils. Int J Environ Anal Chem 51:25–46
Gupta SK, Vollmer MK, Krebs R (1996) The importance of mobile, mobilisable and pseudo total heavy metal fractions in soil for three-level risk assessment and risk management. Sci Total Environ 178:11–20
Gustafsson JP (2011) Visual MINTEQ, v.3.0. Department of Land and Water Resources Engineering , Royal Institute of Technology, Stockholm http://www2.lwr.kth.se/English/OurSoftware/Vminteq/index.htm
IGME (Instituto Geológico y Minero de España) (1990) Mapa geológico de España nº 535, vol 1. Escala, Madrid, p 50.000, Algete)
Iram S, Ahmad I, Akhtar S (2012) Distribution of heavy metals in peri-urban agricultural areas soils. J Chem Soc Pak 34(4):861–869
ISRIC (2002) Procedures for soil analysis, 3rd edn. International Soil Reference and Information Center, Wageningen
Jalali M, Khanlari ZV (2008) Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 143:26–40
Jiménez Ballesta R, Conde Bueno P, Martín Rubí JA, García Giménez R (2010) Pedo-geochemical baseline content levels and soil quality reference values of trace elements in soils from the Mediterranean (Castilla La Mancha, Spain). Eur J Geosci 2(4):441–454
Lafuente AL, González C, Quintana JR, Vázquez A, Romero A (2008) Mobility of heavy metals in poorly developed carbonate soils in the Mediterranean region. Geoderma 145:238–244
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
McLaughlin MJ (2001) Aging of metals in soils changes bioavailability. Fact Sheet Environ Risk Assess 4:1–6
Meers E, Du Laing G, Unamuno V, Ruttens A, Vangronsveld J, Tack FMG, Verloo MG (2007) Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma 141:247–259
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Moreno Merino L (1998) Estudio de la influencia del suelo sobre la composición de las aguas subterráneas a través de la solución del suelo. Modelo en Fluvisoles calcáricos. Dissertation, Universidad Complutense de Madrid
Pérez-Esteban J, Escolástico C, Moliner A, Masaguer A (2013) Chemical speciation and mobilization of copper and zinc in naturally contaminated mine soils with citric and tartaric acids. Chemosphere 90:276–283
Peris M, Micó C, Recatalá L, Sánchez R, Sánchez J (2007) Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci Total Environ 378:42–48
Peters RW (1999) Chelant extraction of heavy metals from contaminated soils. J Hazard Mater 66:151–210
Prokop Z, Cupr P, Zlevorova-Zlamalikova V, Komarek J, Dusek L, Holoubek I (2003) Mobility, bioavailability, and toxic effects of cadmium in soil samples. Environ Res 91:119–126
Qin F, Shan XQ, Wei B (2004) Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils. Chemosphere 57:253–263
Quevauviller P, Lachica M, Barahona E, Rauret G, Ure A, Gómez A, Muntau H (1996) Interlaboratory comparison of EDTA and DTPA procedures prior to certification of extractable trace elements in calcareous soil. Sci Total Environ 178:127–132
Rangel-Porras G, García-Magno JB, González-Muñoz MP (2010) Lead and cadmium immobilization on calcitic limestone materials. Desalination 262:1–10
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Queqauviller P (1999) Improvement of the BCR three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Rivas-Martínez S, Rivas-Saenz S (2009) Worldwide bioclimatic classification system. Centro de Investigaciones Fitosociológicas, España. http//:www.ucm.es/info/cif
Rovira P, Vallejo VR (2000) Evaluating thermal and acid hydrolysis methods as indicators of soil organic matter quality. Commun Soil Sci Plant Anal 31:81–100
Sayen S, Guillon E (2009) Aging effect on the copper sorption on a vineyard soil: Column studies and SEM–EDS analysis. J Colloid Interface Sci 331:47–54
Sayyad G, Afyuni M, Mousavi SF, Abbaspour KC, Richards BK, Schulin R (2010) Transport of Cd, Cu, Pb and Zn in a calcareous soil under wheat and safflower cultivation - A column study. Geoderma 154:311–320
Shirvani M, Shariatmadari H, Kalbasi M (2007) Kinetics of cadmium desorption from fibrous silicate clay minerals: influence of organic ligands and aging. Appl Clay Sci 37:175–184
Sipos P, Németh T, Kovács Kis V, Mohai I (2008) Sorption of copper, zinc and lead on soil mineral phases. Chemosphere 73:461–469
SISS (1985) Metodi Normalizzati di Analisi del Suolo. Edagricole, Bologna
Strawn D, Sparks DL (2000) Effects of soil organic matter on the kinetics and mechanisms of Pb(II) sorption and desorption in soil. Soil Sci Soc Am J 64:144–156
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Van Ranst E, Verloo M, Demeyer A, Pauwels JM (1999) Manual for the soil chemistry and fertility laboratory. Faculty Agricultural and Applied Biological Sciences, Ghent University, pp 243
Vidal J, Pérez-Sirvent C, Martínez-Sánchez MJ, Navarro MC (2004) Origin and behaviour of heavy metals in agricultural Calcaric Fluvisols in semiarid conditions. Geoderma 121:257–270
Zaman MI, Mustafa S, Khan S, Xing B (2009) Heavy metal desorption kinetic as affected by of anions complexation onto manganese dioxide surfaces. Chemosphere 77:747–755
Acknowledgments
This work was supported by the Universidad Complutense de Madrid and the Madrid Autonomous Region through Grant GR58/08, Research Team 950605 and Network CARESOIL, Ref. P2009/AMB-1648. We especially wish to thank Miguel Juanco Ortenbach (Centro Superior de Investigaciones Científicas, CSIC) and Julián Velázquez Cano (Universidad Complutense de Madrid, UCM) for their helpful advice on mineralogical analysis, Ms Pru Brooke-Turner for the revision of the English version of the manuscript and the comments and suggestions of the editor and the anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Céline Guéguen
Rights and permissions
About this article
Cite this article
de Santiago-Martín, A., Valverde-Asenjo, I., Quintana, J.R. et al. Metal extractability patterns to evaluate (potentially) mobile fractions in periurban calcareous agricultural soils in the Mediterranean area—analytical and mineralogical approaches. Environ Sci Pollut Res 20, 6392–6405 (2013). https://doi.org/10.1007/s11356-013-1684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1684-z