Abstract
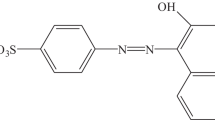
Transition-metal is known to catalyze peroxymonosulfate (PMS) decomposition to produce sulfate radicals. Here we report reactions between PMS and chloride, without a need of transition metals, also can be used to degrade organic dye pollutant (Rhodamine B, (RhB)). Some important operating parameters, such as dosages of PMS and Cl−, pH of solution, temperature, ionic strength, and several common cations, were systematically investigated. Almost complete decoloration of RhB was achieved within 5 min ([PMS] = 0.5 mM, [Cl−] = 120 mM, and pH 3.0), and RhB bleaching rate increased with the increased dosages of both PMS and chloride ion, following the pseudo-first-order kinetic model. However, the total organic carbon (TOC) removal results demonstrated that the decoloration of RhB was due to the destruction of chromophore rather than complete degradation. RhB decoloration could be significantly accelerated due to the high ionic strength. Increasing of the reaction temperature from 273 K to 333 K was beneficial to the RhB degradation, and the activation energy was determined to be 32.996 kJ/mol. Bleaching rate of RhB with the examined cations increased with the order of NH4 + < Na+ < K+ < Al3+ < Ca2+ < Mg2+. Some major degradation products of RhB were identified by GC-MS. The present study may have active technical implications for the treatment of dyestuff wastewater in practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large amount of inorganic salts (e.g., NaCl and Na2SO4) are used or produced during the dyeing process, and therefore their effects should be necessarily considered when dye wastewater is treated (Paprocki et al. 2010; Ramjaun et al. 2011; Salman et al. 2006). The previous investigations indicated that chloride ions had a detrimental effect on the treatment performance of advanced oxidation processes (AOPs; Muthukumar and Selvakumar 2004; Yuan et al. 2011, 2012a, b; Lachheb et al. 2002; AlHamedi et al. 2009; Ghodbane and Hamdaoui 2010; Dong et al. 2007; Kiwi et al. 2000; Muruganandham and Swaminathan 2006; Anipsitakis et al. 2006; Sun et al. 2009). The widely accepted mechanism is based on the quenching effect of chloride on ·OH radical that is capable of bleaching dyes, that is, Cl− scavenges ·OH radical (E 0 = 2.8 V) to generate less reactive chlorine radicals (E 0 Cl•/Cl− = 2.47 V), thus significantly decelerating the rate of dye degradation.
Even such adverse effect of chloride on AOPs wastewater treatment has been verified in Fe(II)/H2O2, UV/TiO2, O3, and UV/H2O2, the underlying mechanism on chloride interference with AOPs is not comprehensively understood. Our recent work indicated a dual role of chloride on Co(II)/PMS system where the sulfate radical was a dominant reactive oxidant (Yuan et al. 2011; Wang et al. 2011b). Similar to Fenton reaction, lower concentration of Cl− (0.05–10 mM) actually decreased the bleaching rate of Acid Orange 7; but higher concentration of Cl− (>50 mM) unexpectedly accelerated the rate of dye degradation. Despite rapid decolorization, dye was not eventually mineralized. Instead, some refractory chlorinated compounds were detected in this Co(II)/PMS/Cl− system. This result was of great significance for applying such Co(II)/PMS technology to treat dyes wastewater. However, the reaction pathways involving chloride in dye degradation have not been clearly resolved. We found the direct reaction between PMS and Cl− should be responsible for the enhanced decolorization under high chloride concentration (Eqs. 1, 2, 3, and 4; Narender et al. 2002; Yuan et al. 2011).
It is worthy to note that lots of influencing factors in real dye wastewater may potentially affect the kinetics of these reactions (Eqs. 1, 2, 3, and 4). Dye wastewater effluents are characterized as broad pH value, large temperature fluctuations, as well as abundance of other co-existing inorganic salt such as NaBr, NaF, and Na2SO4. Frank et al. found that the high temperature would accelerate the reactions between H2O2 and HSO3 −, whereas the ionic strength had a dual effect. When the ion strength was less than 1 M, the reaction rate constants would ascend with the increase of ion strength, while there was an inhibitory effect on the reaction rate when ionic strength was above 1 M (Frank et al. 1999). However, the effects of these dynamic parameters in dye wastewater on the stoichiometric reaction between PMS and Cl− have not been examined, despite its importance in evaluating the specific contribution of this reaction to overall Co(II)/PMS treatment efficacy.
Therefore, the aim of this work was to examine the influences of PMS concentration, Cl− concentration, pH, temperature, ionic strength, and cations on the kinetics of dye degradation in PMS/Cl− system. Furthermore, the major reaction products were identified by GC-MS. These results may provide valuable insight to dyeing wastewater treatment in practice.
Materials and methods
Materials
Rhodamine B (RhB; Acros Organics), a commonly used nonbiodegradable dye was selected as model dye pollutant. Oxone® ([2KHSO5·KHSO4·K2SO4] salt, 95 %) was supplied by Sigma-Aldrich. NaCl, NaOH, Na2SO4, NaNO2, NH4Cl, KCl, MgCl2, CaCl2, and AlCl3 were of reagent grade and used without further purification. All sample solutions were prepared using deionized water from Barnstead UltraPure instrument. Stock solutions of all chemicals were prepared freshly. Prior to each experiment, certain aliquots were transferred to the reactor vessel to obtain the specific concentrations.
Experimental procedures
All oxidation reactions were performed in 50 mL conical flask by mixing appropriate concentrations of RhB, NaCl, and Oxone® without adjusting pH at ambient temperature, except for considering the effects of pH and temperature. Samples were withdrawn and measured immediately. All UV–vis absorption spectra were carried out in time scan mode using a Hitachi U-2900 spectrophotometer. The variation of absorbance at 552 nm was applied to evaluate the extent of RhB degradation. The pH of solution was adjusted by addition of NaOH. A Tekmar Dohrmann Apllo 9000 TOC analyzer was used to measure the TOC of the solutions which were quenched by sodium nitrite. The experiments were conducted in duplicate, and the relative error was less than 2 %.
RhB degradation products were identified by GC/MS analysis. Five hundred milliliters of completely decolorized 0.005 mM RhB samples was pretreated by solid-phase extraction (SPE) method using CNWBOND LC-C18 SPE tube. The gas chromatograph (Agilent 7890A) was equipped with HB-5 MS capillary column (30 m × 320 μm × 0.5 μm film thickness), which was interfaced directly to the mass spectrometer (5975A inert XL MSD with Triple-Axis Detector). The GC column was operated in a temperature programmed mode with an initial temperature of 40 °C held for 4 min, ramp first to 80 °C with a 10 °C/min rate which was held for 2 min, then to 280 °C with 10 °C/min rate, and held at that temperature for 10 min. Electron impact (EI) mode at 70 eV was used and the spectra were obtained in a scan range of 10–400 m/z. The product analysis was consulted from the NIST08 mass spectral library database.
Results and discussion
Effect of PMS concentration
The RhB degradation rates under different PMS concentrations (i.e., 0.1, 0.2, 0.3, and 0.5 mM) were determined to investigate the impact of PMS. As seen in Fig. S1 (Supplementary Information), RhB degraded faster at the higher concentration of PMS. When the concentration of PMS varied from 0.1 to 0.5 mM, the decoloration rate of RhB within 5 min were 21.6 %, 57.1 %, 84.2 %, and 95.7 %, respectively. A series of linear functions were obtained through fitting −ln(C/C0) versus reaction time, which was shown in Fig. 1a. RhB degradation followed the pseudo-first-order kinetic model regarding to the concentration of PMS shown below (Huang et al. 2002):
Where C is the concentration of RhB at the time t; C0 is the initial concentration of RhB; k is the observed reaction rate constant, which could be obtained from the slopes of the lines in the plots of −ln(C/C0) versus reaction time. In this study, the reaction rate constants were 6.28 × 10−4 s−1, 1.33 × 10−3 s−1, 2.37 × 10−3 s−1, and 5.48 × 10−3 s−1 corresponding to 0.1, 0.2, 0.3, and 0.5 mM PMS, respectively (R 2 > 0.9). The higher efficiency of RhB degradation at higher PMS dosage might result from the more production of HSO5 − which could react with chloride yielding more active chloride species.
Effect of Cl− concentration
The influence of Cl− on RhB degradation was performed with various Cl− concentrations, keeping all other reactants concentrations and conditions constant. The rate constant k increased exponentially with the chloride concentrations from 0 to 40 mM. This might be attributed to the more active chloride species which generated from the additional Cl−. It should be noted that RhB could not be effectively degraded by PMS alone, which was shown in the inset of Fig. 1b. As Cl− was added, the rate of the reaction would increase. According to the linearity of plots of −ln(C/C0) versus reaction time (R 2 > 0.9), the reactions followed pseudo-first-order kinetic model with respect to Cl−.
According to our previous reports (Wang et al. 2011b; Yuan et al. 2011), the chloride ions should be directly oxidized through two-electron transfer in PMS/Cl− system, thereby producing reactive chlorine species (Cl2/HClO). Therefore, the rates of dye bleaching by PMS/Cl− system were positively correlated with chloride concentrations. However, a dual effect of chloride on dye decolorization in Co(II)/PMS/Cl− system was observed by Yuan et al. (2011). An inhibitory effect of Cl− on dye degradation dominated at low Cl− lever (<10 mM). This was due to that the sulfate ion radical generated via a one-electron process was scavenged by the less reactive chlorine radicals. At higher dosage of Cl− (>50 mM), direct reaction between PMS and Cl− governed the overall degradation reactions. Hence, an accelerating effect on dye bleaching was found. The present data further verified our previous conclusion obtained in Co(II)/PMS/Cl− system (Wang et al. 2011b; Yuan et al. 2011).
Effect of pH
The dyeing wastewater generally has broadly fluctuating pH value varying from 2 to 12 (Lu et al. 2009). Therefore, it is necessary to study the dyeing wastewater degradation at various pHs. Four experiments of RhB degradation with PMS/Cl− system were carried out at initial pH 3.0, 4.4, 5.3, and 6.2, respectively. Owing to the H+ from PMS, the pH of solution was 3 without being adjusted. With the increase of pH, the efficiency of RhB bleaching decreased, as shown in Fig. 2a, indicating the favorable impact of acidic conditions on reaction rate. This might be due to the pH dependence of active chlorine speciation. Cl2 and HOCl were the main chlorine species in water at pH 3. With pH increased, chlorine in water would hydrolyze completely into hydrogen chloride and hypochlorous acid. HOCl, as a weak acid, dissociates to ClO−, which has a lower oxidation potential (E0 = 0.94 V) than that of HOCl (E0 = 1.49 V; Krishna et al. 2010; Gerritsen and Margerum 1990; Wang et al. 2011a). Thus, the higher pH could lead to less HOCl and more ClO−, resulting in weaker activity and poorer bleaching effects (Deborde and Gunten 2008; Huang et al. 1997).
Effect of temperature
The influence of temperature was taken into consideration, as there was a large range of temperature fluctuation in dye effluent. The temperature dependence was illustrated in Fig. 2b. Increasing temperature had a positive effect on RhB degradation. The reaction rate constants were 3.84 × 10−4 s−1, 8.09 × 10−4 s−1, 1.49 × 10−3 s−1, 3.55 × 10−3 s−1, and 5.47 × 10−3 s−1 at 273 K, 293 K, 313 K, 323 K, and 333 K, respectively (R 2 > 0.9). There was nearly no degradation at 273 K, while RhB was completely bleached in about 5 min at 333 K, implying in practical application the treatment of dye wastewater was more efficient in summer than in winter. Furthermore, the apparent activation energy of RhB decomposition by PMS/Cl− reagent was computed, according to the apparent reaction rate constant (k) at different temperatures, which followed Arrhenius equation (Chen and Zhu 2007):
where A is the Arrhenius pre-exponential constant; R is the universal gas constant (8.314 J mol−1 K−1); T is the absolute temperatures; Ea is the apparent activation energy (kJ/mol), which is 32.996 kJ/mol according to the slope of the plot.
Effect of ionic strength
There are various kinds of ions with different concentrations in dyeing wastewater which contribute to the ionic strength (Guillard et al. 2003). The ionic strength was one of influencing factors for reaction rate demonstrated by Debye–Hückel–Bronsted–Davies (DHBD) semiempirical theory (Wang and Margerum 1994; Yu et al. 2004) (Eq. 7):
where k is the observed rate constant; k 0 is the rate constant at infinite dilution; A is the Debye–Huckel constant (A = 0.509 at 298 K); Z A and Z B are charges for species A and B, respectively; I is the total ionic strength, and b is an empirical parameter (b ≈ 0.25).
In our study, the ionic strength of the reaction medium was adjusted by Na2SO4, whose concentrations ranged from 0.018 to 1.8 M. Fig. 3 shows that the experimental points fit well to the DHBD theory at relatively low ionic strengths from 0 to 1.0 M, which was similar to the previous studies (Beckwith et al. 1996; Wang and Margerum 1994), while the dependence of reaction rate constants on high ionic strength was shown in the inset of Fig. 3. At higher ionic strength, an empirical relationship between the rate constant and the ionic strength had the following form:
where b′ and c are experimentally determined coefficients (Adam and Gordon 1999). The increases in k with increasing I could be ascribed to a positive salt effect (Church 1995).
Effect of cations
The amount of ions may cause different effects on dye degradation, in addition to their influences on ionic strength. Most previous investigations focused on the anions effect, while the effect of cations on dye degradation was ignored (Muthukumar and Selvakumar 2004; Hu et al. 2003; Sohrabi and Ghavami 2008). In the present study, six kinds of chloride salts (i.e., Na+, K+, Mg2+, Ca2+, Al3+, NH4 +) were chosen to investigate the impact of cations, keeping chloride concentration at 40 mM for each sample, as illustrated in Fig. 4. Different cations had various impacts on RhB decoloration rate and their effects could be ranked with an increased order of NH4 + < Na+ < K+ < Al3+ < Ca2+ < Mg2+. The maximum change (89 %) in degradation was observed in the case of Mg2+ within 3 min, whereas only 45 % of RhB was decolored in the case of NH4 +. For NH4 +, it would hydrolyze into neutral amine which might react with HOCl forming chloramines (Eqs. 9, 10, and 11), which were much weaker oxidants than HOCl/OCl− (Deborde and Gunten 2008; Pinkston and Sedlak 2004). The increasing rate with the addition of K+ might be related to its weaker interactions with Cl− than Na+ (Millero et al. 1995). The accelerated bleaching rates with the addition of Mg2+, Ca2+, and Al3+ could be attributed to the dihydrolysis between them and OCl−, which would produce more HOCl to bleach RhB. However, the detailed mechanism is still need to be further clarified.
TOC analysis
TOC removal efficiency was set as a general index to evaluate dye mineralization in PMS/Cl− system. Although the RhB was bleached quickly within a few minutes, the removal of TOC was much more slowly than the RhB decoloration. Only 5.34 % of RhB could be mineralized without the addition of NaCl after 120 min, and 14.9 % of TOC was removed in the presence of 120 mM Cl− under the similar conditions. The lower mineralization and higher decolorization of treated dye in PMS/Cl− system indicated that the bleaching of RhB was due to the destruction of chromophore rather than complete degradation.
Intermediates identification
GC-MS method was performed in order to further investigate the major degradation intermediates of RhB in PMS/Cl− system. The major intermediates could be divided into two groups: the aromatic compounds with different substituent groups and the resultants with relatively lower molecular weights, which were shown in Table 1. The molecular structures and MS spectra of all the identified intermediates were shown in Fig. S2–S8 (Supplementary Information). From all the identified products we concluded that the oxidation process of RhB could be described by a series of consecutive decomposition reactions. RhB was firstly degraded to aromatic compounds, and then oxidized to ring opening products and organic acids. However, chlorinated products such as 4-chlorophthalic acid, 1,2-dichlorobenzene proposed by other investigator (Yuan et al. 2011) were not detected in our research. This might be due to the different pathways of selected dyes towards chemical oxidation and chlorination.
Conclusion
Effects of system parameters on dye degradation by PMS/Cl− system were investigated in this study. Positive effects of PMS and chloride concentrations on dye bleaching kinetics were observed, which were fitted for the pseudo-first-order. The lower pH could accelerate the decoloration of dye under acid condition. The temperature effect implied a favorable performance of PMS/Cl− system at higher temperature, and the apparent activation energy for RhB degradation in PMS/Cl− system was determined to be 32.996 kJ/mol. The quantity and kinds of ions in dye effluence might cause ionic strength and cations impacts on dye decomposition. The results showed that higher ionic strength had positive effect on RhB degradation. The presence of cations had significant effects on RhB decoloration with an increasing sequence of NH4 + < Na+ < K+ < Al3+ < Ca2+ < Mg2+. In addition, there was little TOC removal in PMS/Cl− system. However, RhB could be degraded to aromatic compounds with different substituent groups and the resultants with relatively lower molecular weights, identified by GC-MS. Since most of these intermediates are biodegradable, this kind of chloride-induced PMS oxidative degradation of dye can be used to pre-treat saline dye wastewater to enhance its biodegradability, as well as reduce its toxicity.
References
Adam LC, Gordon G (1999) Hypochlorite ion decomposition: effects of temperature, ionic strength, and chloride ion. Inorg Chem 38:1299–1304
AlHamedi FH, Rauf MA, Ashraf SS (2009) Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 239:159–166
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ Sci Technol 40:1000–1007
Beckwith RC, Wang TX, Margerum DW (1996) Equilibrium and kinetics of bromine hydrolysis. Inorg Chem 35:995–1000
Chen J, Zhu L (2007) Heterogeneous UV-Fenton catalytic degradation of dyestuff in water with hydroxyl-Fe pillared bentonite. Catal Today 126:463–470
Church JA (1995) Kinetics of uncatalyzed oxygen evolution from sodium hypochlorite. Ind Eng Chem Res 34:4235–4237
Deborde M, Gunten UV (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-kinetics and mechanisms: a critical review. Water Res 42:13–51
Dong Y, Chen J, Li C, Zhu H (2007) Decoloration of three azo dyes in water by photocatalysis of Fe(III)-oxalate complexes/H2O2 in the presence of inorganic salts. Dyes Pigm 73:261–268
Frank M, Horst E, Klaus JW (1999) Kinetics of the oxidation of hydrogen sulfite by hydrogen peroxide in aqueous solution: ionic strength effects and temperature dependence. Atmos Environ 33:4413–4419
Gerritsen CM, Margerum DW (1990) Non-metal redox kinetics: hypochlorite and hypochlorous acid reactions with cyanide. Inorg Chem 29:2757–2762
Ghodbane H, Hamdaoui O (2010) Decolorization of antraquinonic dye, C.I. Acid Blue 25, in aqueous solution by direct UV irradiation, UV/H2O2 and UV/Fe(II) processes. J Chem Eng 160:226–231
Guillard C, Lachheb H, Houas A, Mohamed K, Elaloui E, Herrmann JM (2003) Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2. J Photochem Photobiol A: Chem 158:27–36
Hu C, Yu JC, Hao Z, Wong PK (2003) Effect of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl Catal B: Environ 46:35–47
Huang J, Li W, Ren N, Ma F (1997) Disinfection effect of chlorine dioxide on bacteria in water. Water Res 31:607–613
Huang KC, Hoag GE, Chheda P, Woody BA, Dobbs GM (2002) Kinetics and mechanism of oxidation of tetrachloroethylene with permanganate. Chemosphere 46:815–825
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the OH-Radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl−). Environ Sci Technol 34:2162–2168
Krishna BM, Murthy UN, Kumar MB, Lokesh KS (2010) Electrochemical pretreatment of distillery wastewater using aluminum electrode. J Appl Electrochem 40:663–673
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C, Herrmann JM (2002) Photocatalytic degradation of various types of dye (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl Catal B: Environ 39:75–90
Lu XJ, Yang B, Chen JH, Sun R (2009) Treatment of wastewater containing azo dye reactive brilliant red X-3B using sequential ozonation and upflow biological aerated filter process. J Hazard Mater 161:241–245
Millero FJ, Gonzalez-Davila M, Santana-Casiano JM (1995) Reduction of Fe(III) with sulfite in natural waters. J Geophys Res 100:7235–7244
Muruganandham M, Swaminathan M (2006) TiO2–UV photocatalytic oxidation of Reactive Yellow 14: effect of operational parameters. J Hazard Mater 135:78–86
Muthukumar M, Selvakumar N (2004) Studies on the effect of inorganic salts on decolouration of acid dye effluents by ozonation. Dyes Pigm 62:221–228
Narender N, Srinivasu P, Kulkarni SJ, Raghavan KV (2002) High efficient, para-selective oxychlorination of aromatic compounds using potassium chloride and oxone®. Synth Commun 32:279–286
Paprocki A, dos Santos HS, Hammerschitt ME, Pires M, Azevedo CMN (2010) Ozonation of azo dye acid black 1 under the suppression effect by chloride ion. J Braz Chem Soc 21:452–460
Pinkston KE, Sedlak DL (2004) Transformation of aromatic ether- and amine-containing pharmaceuticals during chlorine disinfection. Environ Sci Technol 38:4019–4025
Ramjaun SN, Yuan RX, Wang ZH, Liu JS (2011) Degradation of reactive dyes by contact glow discharge electrolysis in the presence of Cl− ions: kinetics and AOX formation. Electrochim Acta 58:364–371
Salman Ashraf S, Rauf MA, Alhadrami S (2006) Degradation of methyl Red using Fenton’s reagent and the effect of various salts. Dyes Pigm 69:74–78
Sohrabi MR, Ghavami M (2008) Photocatalytic degradation of direct red 23 dye using UV/TiO2: effect of operational parameters. J Hazard Mater 153:1235–1239
Sun SP, Li CJ, Sun JH, Shi SH, Fan MH, Zhou Q (2009) Decolorization of an azo dye orange G in aqueous solution by Fenton oxidation process: effect of system parameters and kinetic study. J Hazard Mater 161:1052–1057
Wang P, He YL, Huang CH (2011a) Reactions of tetracycline antibiotics with chlorine dioxide and free chlorine. Water Res 45:1838–1846
Wang TX, Margerum DW (1994) Kinetics of reversible chlorine hydrolysis: temperature dependence and general-acid/base-assisted mechanisms. Inorg Chem 33:1050–1055
Wang ZH, Yuan RX, Guo YG, Xu L, Liu JS (2011b) Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent: kinetic analysis. J Hazard Mater 190:1083–1087
Yu XY, Bao ZC, Barker JR (2004) Free radical reactions involving Cl•, Cl2 −•, and SO4 −• in the 248 nm photolysis of aqueous solutions containing S2O8 2- and Cl− J Phys Chem A 108:295–308
Yuan RX, Ramjaun SN, Wang ZH, Liu JS (2011) Effects of chloride ion on degradation of acid orange 7 by sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds. J Hazard Mater 196:173–179
Yuan RX, Ramjaun SN, Wang ZH, Liu JS (2012a) Concentration profiles of chlorine radicals and their significances in · OH-induced dye degradation: kinetic modeling and reaction pathways. Chem Eng J 209:38–45
Yuan RX, Ramjaun SN, Wang ZH, Liu JS (2012b) Photocatalytic degradation and chlorination of azo dye in saline wastewater: kinetics and AOX formation. Chem Eng J 192:171–178
Acknowledgments
The authors would like to acknowledge the financial support from the Fundamental Research funds for Central Universities Central (12D11317) and State Key Laboratory of Pollution Control and Resource Reuse Foundation (No. CRRF11023). This work was partially supported by National Science Foundation of China (Nos. 21007009, 41273108) and “Chen Guang” project (10CG34).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1106 kb)
Rights and permissions
About this article
Cite this article
Lou, XY., Guo, YG., Xiao, DX. et al. Rapid dye degradation with reactive oxidants generated by chloride-induced peroxymonosulfate activation. Environ Sci Pollut Res 20, 6317–6323 (2013). https://doi.org/10.1007/s11356-013-1678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1678-x