Abstract
The tissue and organs (muscle, brain, liver, and gill) of four species of freshwater fish from Lake Baiyangdian were analyzed for hexachlorocyclohexanes (HCHs) and dichloro-diphenyl-trichloroethanes (DDTs). The distribution characteristics were analyzed for HCHs and DDTs in various tissue and organs, which determined the health risks for humans. The research results showed that the wet weight content of all HCHs (∑HCHs) ranged from 0.05 ∼ 14.53 ng g−1, with a mean of 3.47 ng g−1. The wet weight content of all DDTs (∑DDTs) ranged from ND to 8.51 ng g−1, with a mean of 2.41 ng g−1. For the various species of fish, the residual level of ∑HCHs was relatively higher in chub and grass carp and lowest in snakehead. The residual level of ∑DDTs was the highest in snakehead and did not exhibit a significant variance in the other three species. For the various tissues and organs, the contents of HCHs and DDTs were both highest in the fish liver, second highest in the fish gill, and lowest in the fish brain and muscle. Among the four types of isomers, the residual level of γ-HCH was relatively higher, while the residual level of α-HCH was the lowest. The content of p,p′-DDE was significantly greater to other forms of DDT and its isomer. The residual levels of HCHs and DDTs in fish were both below the national standard. However, the carcinogenic risk from the HCHs in parts of the tissue and organs of four fish species in Lake Baiyangdian exceeded the screen value threshold set by USEPA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As typical persistent organic pollutants (POPs), which are the most widely used organochlorine pesticides in the world, hexachlorocyclohexane (HCH) and dichloro-diphenyl-trichloroethane (DDT) have received considerable attention due to their widespread effect and damage to ecosystems and human health (Willett et al. 1998; Jones and Voogt 1999; Almeida-Gonzalez et al. 2012; Wang et al. 2013). Dichloro-diphenyl-trichloroethane, α-HCH, β-HCH, and γ-HCH (lindan) were listed at the Stockholm Convention on Persistent Organic Pollutants in 2001 and in 2009. In China, the agricultural application of technical HCH and DDT has been officially banned since 1983, and the production of lindan (99 % γ-HCH) ceased in 2000 (Gong et al. 2007). However, HCH and DDT are still frequently detected in the environment (Feng et al. 1998; Doong et al. 2002; Li et al. 2007; Tao et al. 2005, 2007, 2008; Yang et al. 2010, 2012). Various freshwater bodies such as ponds, rivers, lakes, reservoirs, and estuaries widely suffered from DDT and HCH pesticide pollution (Wang et al. 2012, 2013; Tao et al. 2007; Janiot et al. 1994; Dua et al. 1996; Yamashita et al. 2000; Maskaoui et al. 2005; Zhou et al. 2008; Sun et al. 2012). Fish play a very important role in aquatic ecosystems and in human food (Xu et al. 2011). They are frequently used to study POPs contaminations and associated health risks, as well as to predict the bioconcentration and bioaccumulation factor (Barron 1990; Haruhiko et al. 2003; Dong et al. 2006; Lanfranchi et al. 2006; Cheung et al. 2007; Dennis 2007; Guo et al. 2008; Xu et al. 2011). Many studies on the occurrence of DDTs and HCHs in different fish species were reported during the last decades (Kalyoncu et al. 2009; Sarkar et al. 2008; Luo 2011; Szlinder-Richert et al. 2008; Li et al. 2008; Sun et al. 2005; Dou and Zhao 1996; Zhang et al. 2012). However, the distributions of DDTs and HCHs in different fish tissue are still not clearly documented (Guo et al. 2008). The distributions of DDTs and HCHs in other tissues, such as fish brain, liver, and gill tissue, have not been fully investigated. The distributions of OCPs such as DDTs and HCHs in fish tissue other than muscle could provide more clues about their bioaccumulation and metabolism in fish. Residual DDTs and HCHs in edible tissues such as fish brain could also provide more information on the risk levels of DDTs and HCHs to human health through fish consumption (Xu et al. 2011).
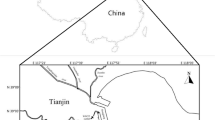
Lake Baiyangdian, the largest freshwater lake in North China and an important base of fish production in China (Fig. 1), played an important role in maintaining the ecological balance of North China and provided domestic, agricultural, and industrial water sources for the lake catchment area (Xu et al. 2011). The Haihe Plain, where Lake Baiyangdian is located, was an important center for the production of DDT and HCH pesticides and was one of the primary agricultural areas where large quantities of DDT and HCH were applied (Tao et al. 2005). Severe contamination of agricultural soils and surface water from DDT and HCH in this area was demonstrated in places near Lake Baiyangdian, such as Beijing (Li et al. 2008; Zhu et al. 2009) and Tianjin (Gong et al. 2007; Tao et al. 2005, 2006). Various studies on the distributions of DDT and HCH in the water and sediment in Lake Baiyangdian were conducted during the last two decades (Wang et al. 2013; Dou and Zhao 1996; Hu et al. 2010). The distributions and bioaccumulation of DDTs and HCHs in the muscles of carnivorous, omnivorous, and herbivorous fish in Lake Baiyangdian were studied in 1996 (Dou and Zhao 1996). However, little information is available on the distributions of HCH and DDT in the fish tissues other than muscle and on the associated health risks to the residents through the consumption of the fishes (Dou and Zhao 1996).
The primary objectives of this study are: (1) to investigate the residual levels, tissue distributions, and compositions of DDT and its metabolites (DDXs) as well as HCH and its isomers (HCHs) in the four fish species taken from the Lake Baiyangdian; (2) to analyze the effects of the lipid contents as well as the octanol–water partition coefficient (Kow) of DDXs and HCHs on the residues and distributions of DDXs and HCHs in the fish; and (3) to assess the associated health risks of DDXs and HCHs of residents through fish consumption.
Materials and methods
Sample collection
In October 2007, four species of commonly consumed freshwater fish, including 15 individuals of crucian carp (Carassius auratus) and 10 individuals for the each species of snakehead fish (Channa argus), grass carp (Ctenopharyngodon idellus), and silver fish (Hypophthalmichthysmolitrix) were collected from Lake Baiyangdian (Fig. 1), which is located in Anxin county within the Hebei province. Four fish tissues including the brain, liver, gill, and a muscle mixture from both the dorsal and chest were sampled. To eliminate individual diversity, specific tissues from three to five individuals of the same fish species were combined into one sample. All of the fish samples were freeze-dried after weighing, and then preserved in the dryer prior to analysis. The general physiological information of the fish is shown in Table 1.
Sample extraction and cleanup
The freeze-dried fish tissue samples, which weighed approximately 3 g, were first ground with anhydrous sodium sulfate. The samples were then Soxhlet extracted with 100 ml of mixed solvent of dichloromethane and n-hexane (v/v, 1:1) for 24 h at 50 °C. The extracted mixed solvent was then transformed into an n-hexane solvent and concentrated into 3 mL. The next step was the liquid–liquid extraction followed by Haruhiko’s procedure (Haruhiko et al. 2003), in which the extract was subjected to n-hexane and saturated with acetonitrile to remove lipids. Furthermore, lipid content within the tissues and organs was measured using the quality-subtraction method (Haruhiko et al. 2003). A silica gel column was used for the sample cleanup. The cleanup column was eluted with 25 ml of n-hexane followed by 50 ml of a 3:2 mixture of n-hexane and dichloromethane at a rate of 2 ml/min. The eluate collected from the silica column during cleanup was concentrated into 1 ml using the vacuum rotary evaporator. The samples were sealed in vials and stored at −4 °C prior to analysis.
Sample analysis and quality control
The measurements of HCHs and DDTs were carried out with a Hewlett Packard 6890 Gas Chromatography system equipped with a 63Ni electron capture detector (μECD). High-purity helium was used as carrier gas. Samples of 1 μl were injected using the splitless mode. The temperatures of the injection port and ion source were maintained at 220 and 280 °C, respectively. The oven temperature was programmed at 50 °C, raised to 150 °C at 10 °C/min, then increased to 240 °C at 3 °C/min and finally held at 240 °C for 15 min. The quantification of OCPs was carried out with an internal calibration procedure.
All samples were extracted and analyzed three times. Both the method blank and procedure blank were finished together with the sample disposal. The method recovery and method detected limits were conducted prior to the sample analysis. Therefore, data lower than the detected limits were counted as 0. Table 2 shows the recovery rates and detection limits for HCHs and DDTs.
Data processing
The data were then processed with Microsoft Excel 2007 and SPSS 13.0. One-way analysis of variance (ANOVA) was completed to detect differences in data among the fish tissues and species. The relationship between the data was determined using Pearson’s sample correlation, and when the value of p was less than 0.05, the linear regression was regarded as significant. The software used was SPSS 13.0.
Results and discussion
Residuals and distribution of HCHs and DDTs in the fish
Residues of HCHs and DDTs in the fish
The contents of ∑HCHs of four species of common freshwater fish (crucian carp, snakehead fish, grass carp, and silver fish) in Lake Baiyangdian ranged from 0.05 to 14.53 ng g−1, with a mean of 3.47 ng g−1. The content range of ∑DDTs was ND ∼ 8.51 ng g−1 (ww), with a mean of 2.41 ng g−1. Table 3 shows the contents of HCHs and DDTs in fish samples. According to the results, the residual level of γ-HCH was relatively high, while the residual level of α-HCH was relatively low. The content of p,p′-DDE was much higher than DDT and other DDT metabolites.
According to the experimental results in the research, the content of HCHs and DDTs in four species of common freshwater fish was relatively lower in comparison to other studies, which is shown in Fig. 2. In one aspect, results of the residual levels of HCHs and DDTs in this research were comparable within an order of magnitude but were lower than the results of fish in adjacent lakes, such as the Guanting reservoir (Sun et al. 2005) and Gaobeidian Lake (Li et al. 2008). In another aspect, the measurements of the results of this research which were conducted in 2008 were significantly lower than the experimental results found in 1996 (Dou and Zhao 1996). Using this information, it can be estimated that the pollutants of HCHs and DDTs are partially alleviated in the Lake Baiyangdian region.
Distribution of HCHs and DDTs in various fish species and organs
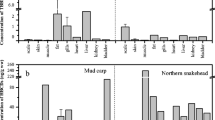
The distribution of HCHs and DDTS of four fish species in Lake Baiyangdian is shown in Fig. 3. The overall content of the HCHs pollutant was relatively high in grass carp and silver fish and was the lowest in snakehead fish. The overall content of DDTs was the highest in snakehead fish, and similar average residual levels were found in the other three fish species. The content of HCHs varies significantly (p = 0.02 < 0.05) in contrast to the minor differences in the content of ∑DDTs (p = 0.778 > 0.05) in all four fish species.
Other studies on the concentration of HCHs and DDTs in various fish species with distinct feeding habits have found higher residual levels of organochlorine pesticides in carnivorous fish and lower levels in omnivorous fish and vegetarian fish (Dou and Zhao 1996). However, these differences are not detected in this study. The wet weight content of both HCHs and DDTs in the snakehead fish, which is a carnivorous fish, is relatively low.
The distribution of HCHs and DDTs in various fish organs and tissues can be seen in Figs. 4 and 5. The residual levels of α-HCH, β-HCH, γ-HCH, and δ-HCH in fish brain, gill, liver, and muscle were consistent with the content of ∑HCHs. The residual level of HCHs was the highest in the fish liver, followed by the fish gill, and lowest in both the fish brain and muscle (Fig. 4). Similar residual tendencies of p,p′-DDE, o,p′-DDD, p,p′-DDD, and p,p′DDT were found in the fish brain, gill, liver, and muscle, which is consistent with the content of ∑DDTs. The highest values of these chemicals were found in the liver, followed by the gill, and the lowest values were found in the fish brain and muscle. The residual levels of o,p′-DDE and o,p′-DDT in the fish brain and other organs and tissues are relatively approximate to each other (Fig. 5). The results of the ANOVA analysis show that the contents of HCHs and DDTs among various tissues and organs have significant differences (p < 0.05). This study was in line with Guo et al. (2008).
The residual level of pollutants in a living body is linked with the biological functions of various tissues and organs. For example, the research results have shown that the concentration of organic pollutants in the liver is the highest among the various tissues and organs, which relates to the detoxification function of the liver (Wan et al. 2006). An ideal illustration for this is the liver block phenomenon, which describes that pollutants in the living body will integrate with related proteins to form a compound. This compound, which mainly consists of various cytochromes of P450, will subsequently be transferred into the liver and cause the accumulation and concentration of pollutants there. For example, Wan and his fellow researchers found that the concentration of PCDD/Fs in the herring gull was higher in the liver than in the fat and muscle (Ballabh et al. 2004). The low concentration of pollutants in fish is thought to be related to the blood–brain barrier, which consists of a layer of endothelial cells and widely exists in living creatures (Ballabh et al. 2004). The main biological function of this blood–brain barrier is to resist various pathogens and poisonous substances. The selective entry of molecules in the brain lies in its structural characteristics of being both highly complex and highly ordered. This can ensure an accurate identification for outgoing substances in its biological, chemical, and physical properties, as well as its spatial structure (Roney et al. 2005).
Effects of lipid and Kow on the residues of DDTs and HCHs in the fish
DDTs and HCHs are typical hydrophobic lipophilic organic pollutants. Their residues and distribution in organisms are affected not only by their physicochemical properties (e.g., Kow) but also by the lipid contents in organisms (Swackhamer et al. 1988; Zhou et al. 2008). The effects of Kow and lipid content on the distributions of HCHs and DDTs in the fishes from Lake Baiyangdian were presented in Tables 4 and 5.
Table 4 shows that there were significant positive correlations between the lipid contents and the log-transformed contents of γ-HCH, ΣHCHs, as well as o, p′-DDE and o, p′-DDT in the fishes; and that the correlation coefficients (R = 0.46 and 0.43) and the significance levels (P < 0.01) between the lipid contents and the log-transformed contents of γ-HCH, ΣHCHs were higher than these between the lipid contents and the log-transformed contents of o, p′-DDE and o, p′-DDT (R = 0.37 and 0.33, P = 0.02 and 0.03). There was a weak linear relationship between the lipid contents and the log-transformed ∑HCHs contents in the fishes (R 2 = 0.185); however, there was no a linear relationship between the lipid contents and the log-transformed ∑DDTs contents in the fishes. The previous studies also showed that there were different relationships between the lipid contents and the residues of HCHs and DDTs in fishes. For instance, the good linear relationships were found between the lipid contents and the residues of HCHs and DDTs in the fishes from Zhujiang River and Dayawan Bay, China with the R 2 values of 0.41–0.66 (Guo et al. 2008). However, the significant relationships were found only between the lipid contents and the residues of p,p′-DDE and p,p′-DDD in the fishes from the freshwater lakes in Turkey (Erdogrul et al. 2005).
It can be seen from Table 5 that there were different relationships between the Kow values and the contents of HCHs and DDTs in the same tissues of different fish species, and in the different tissues of the same fish species. There were significant correlations between log-transformed Kow values and the contents of HCHs in the brain, gill, and muscle of snakehead fish; however, there were no correlations between log-transformed Kow values and the contents of HCHs in the four tissues of other three fish species. There were significant correlations between log-transformed Kow values and the residues of DDTs in the muscle of four fish species, and in the brain of three fish species except for silver carp, as well as in the liver of grass carp and crucian carp. However, there were no correlations between log-transformed Kow values and the residues of DDTs in the gill of four fish species, as well as in the brain and liver of silver carp. This is perhaps because the contents of pollutants in the gills with a filter function are more susceptible to be affected by the contents of pollutants in water and suspended solids, and are less affected by the physicochemical properties (e.g., Kow) of pollutants. However, for the accumulation and metabolic organs of organisms, such as brain, muscle, and liver, their pollutants’ contents may be more affected by the physicochemical properties (e.g., Kow) of pollutants.
The constitution of HCHs and DDTs in fish
Figures 6 and 7 show the percent composition of HCH isomers and DDT compounds in four fish species and in different tissues and organs. According to Fig. 6, we can see that γ-HCH comprised the highest proportion in crucian carp, grass carp, and silver carp, ranging from 44 to 83 %. The contents of β-HCH and δ-HCH were relatively lower and α-HCH was the lowest, at only 2.4–5 %. In snakehead fish, β-HCH was the primary content at 35.8 %; δ-HCH followed comprising 29.8 %; γ-HCH was lower at 27.3 %, and α-HCH was the lowest at 7.1 %. The proportions of four types of isomers of HCHs in the fish brain, gill, liver, and muscle manifest similar structures as γ-HCH > β-HCH ∼ δ-HCH > α-HCH. Various research results for the residual levels of HCHs in freshwater fish have shown that the residual levels of γ-HCH and β-HCH are relatively high; however, δ-HCH are lower and α-HCH are the lowest in China (Li et al. 2007; Guo et al. 2008; Li et al. 2008). Yet, as γ-HCH is a key ingredient of organochlorine pesticide named Lindan, the relatively high residual level of γ-HCH in the environment normally indicates that the organochlorine pesticide Lindan is still in use. Meanwhile, α-HCH and γ-HCH could transform into β-HCH during the long metabolic process. Although the water solubility of β-HCH is the lowest among the various types of isomers of HCHs, it is easier to be concentrated inside a living body (John 2006).
According to Fig. 7, DDE was the dominant residual composition of DDTs in crucian carp, snakehead fish, grass carp, and silver carp, which was between 60 and 80 %. The proportion of DDT compounds was ordered as follows: p,p′-DDE > o,p′-DDT > o,p′-DDE ∼ o,p′-DDD > p,p′-DDT ∼ p,p′-DDD. DDE was also the main composition of residual DDTs in the fish brain, gill, liver, and muscle. The proportion was between 50 and 85 %. The proportion of DDT compounds in the fish gill, liver, and muscle was ordered as follows: p,p′-DDE > o,p′-DDD > o,p′-DDT > o,p′-DDE ∼ p,p′-DDT ∼ p,p′-DDD. For the fish brain, the order becomes o,p′-DDT > o,p′-DDE ∼ p,p′-DDE > p,p′-DDD > o,p′-DDD ∼ p,p′-DDT. Various results on the residual levels of DDTs in freshwater fish have shown that p,p′-DDE is largely a dominant component of residual DDTs at 30–80 %. In contrast, DDT displays a certain extent of residual levels in fish (Zhou et al. 2008; Guo et al. 2008; Li et al. 2008; Qiu et al. 2005). In the living body, DDT could transform into DDE and DDD through a metabolic process, which then easily remain and concentrate inside the living body (Oyuna 2003). The transformation of DDT into DDD and DDE with time depends on the right conditions (oxygen source, bacteria or enzyme, etc.) and the length of time. Therefore, the proportion between DDT and its metabolite will alter through time, with a decreasing percentage of DDT and an increasing percentage of DDE and DDD. As DDE is the most stable metabolite of DDT, it takes the biggest portion of DDT in the living body (Sun et al. 2005). This conclusion is also verified and confirmed in this study by the research results that DDE is the dominant component of residual DDT in all four fish species.
Risks to human health from DDTs and HCHs in the fish
Various international organizations have subsequently established a series of standards and instructions to estimate the risks from organochlorine pesticides in fish to human health. Thus far, evaluative methods such as the Acceptable Daily Intake (ADI) indicator comparison, the Maximum Residue Limit (MRL) evaluation, and the Potency Equivalent Concentration (PEC) study are broadly applied (Li et al. 2008). All of these methods clearly exhibit both pros and cons. For example, the ADI indicator comparison method is easily calculated but is generally only suitable for evaluating toxic effects and does not account for the time issue of exposure. The MRL evaluation method categorizes the toxic effects of pollutants in the short-, mid-, and long terms well. However, it is still highly inadequate in its consideration of the indicators for heredity, heteromorphosis, and carcinogenic risk. Yet, the U.S. Environmental Protection Agency (USEPA) method is highly effective and only focuses on the carcinogenic risk of pollutants in humans. For the sake of comprehensive estimation, this paper used all three methods in a comparative manner to evaluate the risks to human health from residual HCHs and DDTs in common freshwater fish of the Lake Baiyangdian region.
ADI is a concept proposed by the World Health Organization (WHO) for evaluating the risks of human health from pesticides and food additives. ADI refers to a calculated daily amount of the maximum intake of certain types of chemicals before any risk develops over an entire life span. Health risks from HCHs and DDTs will be estimated by comparing the ADI and estimated daily intake (EDI). The equation for the calculation of EDI is:
In the Eq. (1), we adopt data from the USEPA (142.2 g⋅d−1) for the daily intake of fish; Average body weight is calculated as approximately 70 kg. The calculation result is shown in Table 6, in which we can see that the actual daily intake of HCHs and DDTs from fish in this research is far lower than the WHO standard (8,000 ng kg−1 day−1 for γ-HCH, 20,000 ng kg−1 day−1 for p,p′-DDT).
The Agency for Toxic Substances and Disease Registry (ATSDR) considered the relationship between the quantity of intake pollutants and the exposure time in estimating risks to human health from persistent organic pollutants. In this research, the No Observed Adverse Effect Level and Uncertainty Factor (UF) were employed to determine the MRL (Li et al. 2008). The MRL was categorized into a short-term toxic exposure (1 to 14 days), mid-term toxic exposure (14 to 364 days), and long-term toxic exposure (above 365 days). Table 7 shows the estimated results from the ATSDR on the MRL of residual HCHs and DDTs. According to a comparison between the estimated daily intake of HCHs and DDTs from four fish species in the Lake Baiyangdian region and MRL value, we can see that risks from mid-term exposure of γ-HCH are relatively high (EDI value is 4.49 ng kg−1 day−1,MRL value is 10 ng kg−1 day−1); however, the daily intake of the other types of pollutants is much lower than the MRL value for low-risk standards. Because the MRL data we acquired are primarily from short-term and mid-term toxic exposure, this estimation could reflect a very low but urgent short-term toxic risk for human health from fish in the Lake Baiyangdian region.
Using the guidelines of USEPA (USEPA 2000, 2004), screen value (SV) and PEC were used in this study to assess the human health risks of HCHs and DDTs through the consumption of fish from the Lake Baiyangdian region. The SV is defined as the concentration of chemicals in edible tissue that are a potential public health concern. The SV indicator is calculated according to the following formula (USEPA 2000):
where SV is the screening value (micrograms per gram) that is used as a threshold value against the tissue residue level of contamination in similar tissue collected from the environment; RL is the maximum acceptable risk level (dimensionless, used 10−5); SF is the oral slope factor(micrograms per gram per day); the SF value of γ-HCH is adopted for the carcinogenic risk of HCHs; the SF value of DDT is 0.34 (micrograms per gram per day); BW is the body weight (kilograms), and an average of 70 kg is used for the calculations; CR refers to the consumption rate (grams per day) and is substituted by the USEPA standard value for the average intake rate of fish, which is 142.2 g day−1; When the carcinogenic risk is 10−5, the SV threshold of HCHs is 3.78 ng g−1(ww) and the SV threshold of DDTs is 14.4 ng g−1 (ww). The wet weight contents of HCHs and DDTs in various tissues and organs acquired from this research are implemented as the data for estimating the carcinogenic risk of eating fish from the Lake Baiyangdian region.
According to our estimation, the carcinogenic risk of HCHs in the tissue and organs of four fish species from the Lake Baiyangdian region partially exceeds the SV threshold. In Fig. 8, the contents of HCHs in the various tissues and organs of snakehead fish and crucian carp are below the SV threshold; the contents of HCHs in the various tissues and organs of grass carp approach or exceed the SV threshold, and the carcinogenic risk range reaches 8.40 × 10−6 ∼ 2.04 × 10−5. The content of HCHs in the liver and muscle of silver fish is above the SV threshold, and the carcinogenic risks are 3.03 × 10−5 and 1.52 × 10−5, respectively. For all four fish species, the content of HCHs in the fish brain is below the SV threshold, ranging from 3.51 × 10−6 to 8.40 × 10−6, while the content in fish liver is much higher (7.90 × 10−5 ∼ 3.03 × 10−5) and has a significantly greater carcinogenic risk.
The carcinogenic risks of DDTs in tissues and organs of four fish species from the Lake Baiyangdian region are all below the SV threshold of 10−5. In Fig. 9, for all four fish species, the content of DDTs in the fish brain and muscle is even lower, ranging from 3.13 × 10−7 to 1.28 × 10−6, while the content in both the gill and liver is relatively higher (2.65 × 10−7 ∼ 5.34 × 10−6).
According to the national standard of limiting pollutants in food, the content of HCHs and DDTs should be controlled at a level below 0.1 and 0.5 mg kg−1, respectively. The measured values of the concentrations of HCHs and DDTs in this research are both below this national standard.
In order to better protect human health, the following suggestions for the fish consumptions are proposed according to the results from risk assessment: (1) the liver and gill should be removed, especially for grass carp and silver carp; (2) It is best not to eat the muscle of the silver carp with high health risk; and (3) Try to eat less the brain of grass carp and crucian carp since the risks in these two fish species approach the maximum acceptable risk level of HCHs in fish suggested by USEPA.
Conclusion
The average wet weight contents of HCHs and DDTs in the fish from Lake Baiyangdian (3.47 and 2.41 ng g−1) were lower compared with the results from other researches. The higher residues of ∑HCHs were found in the silver carp and grass carp, while the residual level of ∑DDTs was the highest in the snakehead fish. The highest contents of HCHs and DDTs were found in the fish liver, followed by the fish gill and by the brain and muscle of the fish. The high level residue of γ-HCH might indicate a probable recent usage of lindane in the Lake Baiyangdian region. The residual levels of HCHs and DDTs in the fish were both below the Chinese national standard. However, the carcinogenic risk of HCHs in the muscle of the silver carp from Lake Baiyangdian exceeded the screen value (SV) threshold of 10−5. So, it is not safe for the people to eat the muscle of the silver carp from Lake Baiyangdian. For the liver and gill of the grass carp and silver carp from Lake Baiyangdian, although their carcinogenic risk of HCHs exceeded the SV threshold of 10−5, they are safe to human health since they are not edible tissues.
References
Almeida-Gonzalez M, Luzardo OP, Zumbado M, Rodriguez-Hernandez A, Ruiz-Suarez N, Sangil M, Camacho M, Henriquez-Hernandez LA, Boada LD (2012) Levels of organochlorine contaminants in organic and conventional cheeses and their impact on the health of consumers: an independent study in the Canary Islands (Spain). Food Chem Toxicol 50(12):4325–4332
Agency for Toxic Substances and Disease Registry (ATSDR) (1996) Minimal risk levels (MRLs) for hazardous substance. ATSDR, Washington, DC, pp 1–347
Ballabh P, Braun A, Nedergaard M (2004) The blood–brain barrier: an overview structure, regulation, and clinical implications. Neurobiol Dis 16(1):1–13
Barron MG (1990) Bioconcentration. Will water-borne organic chemicals accumulate in aquatic animals? Environ Sci Technol 24(11):1612–1618
Cheung KC, Leung HM, Kong KY (2007) Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere 66(3):460–468
Dennis TL (2007) Perspective on ecotoxicology of PAHs to fish. Hum Ecol Risk Assess 13(2):302–0316
Dong J, Luan TG, Zou SC (2006) Residues and risk assessment of DDTs and PAHs in sediments and fish from pearl river delta area. Ecol Environ 15(4):693–696 (in Chinese)
Doong RA, Peng CK, Sun YC, Liao PL (2002) Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River estuary, Taiwan. Mar Pollut Bull 45(1–12):246–253
Dou W, Zhao ZX (1996) A study on bioaccumulation of BHC and DDT in fish muscles of different food structure in Baiyangdian Lake food web. Adv Environ Sci 4(6):41–43 (In Chinese)
Dua VK, Kumari R, Sharma VP (1996) HCH and DDT contamination of rural ponds of India. Bull Environ Contam Toxicol 57(4):568–574
Erdogrul O, Covaci A, Schepens P (2005) Levels of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in fish species from Kahramanmaras, Turkey. Environ Int 31(5):703–711
Feng H, Cochran JK, Lwiza H, Brownawell BJ, Hirschberg DJ (1998) Distribution of heavy metal and PCB contaminants in the sediments of an urban estuary: the Hudson River. Mar Environ Res\ 45(1):69–88
Gong XY, Qi SH, Wang YX, Julia EB, Lv CL (2007) Historical contamination and sources of organochlorine pesticides in sediment cores from Quanzhou Bay, Southeast China. Mar Pollut Bull 54(9):1434–1440
Guo LL, Qiu YW, Zhang G (2008) Levels and bioaccumulation of organochlorine pesticides (OCPs) and polybrominated diphenyl ethers (PBDEs) in fishes from the Pearl River estuary and Daya Bay, South China. Environ Pollut 152(3):604–611
Haruhiko N, Yasufumi S, Takashi M (2003) Bioaccumulation and toxic potencies of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in tidal flat and coastal ecosystems of the Ariake Sea. Japan Environ Sci Technol 37(16):3513–3521
Hu GC, Luo XJ, Li FC, Dai JY, Guo JY, Chen SJ, Hong C, Mai BX, Xu MQ (2010) Organochlorine compounds and polycyclic aromatic hydrocarbons in surface sediment from Baiyangdian Lake, North China: concentrations, sources profiles and potential risk. J Environ Sci 22(2):176–183
Janiot LJ, Sericano JL, Roses OE (1994) Chlorinated pesticide occurrence in the Uruguay River (Argentina-Uruguay). Water Air Soil Pollut 76(3–4):323–331
John B (2006) DDT and human health. Sci Total Environ 355(1–3):78–89
Jones KC, Voogt P (1999) Persistent organic pollutants (POPs): state of the science. Environ Pollut 100(1–3):209–221
Kalyoncu L, Agca I, Aktumsek A (2009) Some organochlorine pesticide residues in fish species in Konya, Turkey. Chemosphere 74(7):885–889
Lanfranchi AL, Menone ML, Miglioranza KSB, Janiot LJ, Aizpu’n JE, Moreno VJ (2006) Striped weakfish (Cynoscion guatucupa): a biomonitor of organochlorine pesticides in estuarine and near-coastal zones. Mar Pollut Bull 52(1):74–80
Li J, Zhang G, Guo LL, Xu WH, Li XD, Lee CSL, Ding AJ, Wang T (2007) Organochlorine pesticides in the atmosphere of Guangzhou and Hong Kong: regional sources and long-range atmospheric transport. Atmos Environ 41(18):3889–3903
Li XM, Gan YP, Yang XP (2008) Human health risk of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in edible fish from Huairou Reservoir and Gaobeidian Lake in Beijing, China. Food Chem 109(2):348–354
Luo J (2011) Occurrence of organochlorine pesticides and polychlorinated biphenyls in six fish species from Baihua Lake, China. Adv Mater Res 347–353(2012):2073–2077
Maskaoui K, Zhou JL, Zheng TL, Hong H, Yu Z (2005) Organochlorine micropollutants in the Jiulong River Estuary and Western Xiamen Sea, China. Mar Pollut Bull 51(8–12):950–959
Oyuna VT, Valeriy BB, Lidwig W (2003) Pollution of the lake Baikal Basin: organochlorine pesticides. Chemisty Sustain Dev 11:349–352
Qiu X, Zhu T, Yao B (2005) Contribution of dicofol to the current DDT pollution in China. Environ Sci Technol 39(12):4385–4390
Roney C, Kulkarni P, Arora V (2005) Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease. J Control Release 108(2–3):193–214
Sarkar SK, Bhattacharya BD, Bhattacharya A, Chatterjee M, Alam A, Satpathy KK, Jonathan MP (2008) Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ Int 34(7):1062–1071
Sun JH, Feng JL, Liu Q, Li QL (2012) Distribution and sources of organochlorine pesticides (OCPs) in sediments from upper reach of Huaihe River, East China. J Hazard Mater 184(1–3):141–146
Sun YZ, Wang XT, Li XH (2005) Distribution of persistent organochlorine pesticides in tissue/organ of silver carp (Hypophthalmichthys molitrix) from Guanting Reservoir, China. J Environ Sci 17(5):722–726
Swackhamer DL, Pearson RF, Schottler SP (1988) Toxaphene in the Great Lakes. Chemosphere 37(9–12):2545–2561
Szlinder-Richert J, Barska I, Mazerski J (2008) Organochlorine pesticides in fish from the southern Baltic Sea: levels, bioaccumulation features and temporal trends during the 1995–2006 period. Mar Pollut Bull 56(5):927–940
Tao S, Li BG, He XC, Liu WX, Shi Z (2007) Spatial and temporal variations and possible sources of dichlorodiphenyltrichloroethane (DDT) and its metabolites in rivers in Tianjin, China. Chemosphere 68(1):10–16
Tao S, Liu WX, Li Y, Yang Y, Zuo Q, Li BG, Cao J (2008) Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environ Sci Technol 42(22):8395–8400
Tao S, Xu FL, Wang XJ, Liu WX, Gong ZM, Fang JY, Zhu LZ, Luo YM (2005) Organochlorine pesticides in agricultural soil and vegetables from Tianjin, China. Environ Sci Technol 39(8):2494–2499
Tao S, Yang Y, Cao HY, Liu WX, Coveney RM, Xu FL, Cao J, Li BG, Wang XJ, Hu JY, Fang JY (2006) Modeling the dynamic changes in concentrations of γ-hexachlorocyclohexane (γ-HCH) in Tianjin region from 1953 to 2020. Environ Pollut 139(1):183–193
U.S. Environmental Protection Agency (USEPA) (2000). Guidance for assessing chemical contaminant data for use in fish advisories, volume 2: Risk assessment and fish consumption limits, 3rd ed. US EPA, Office of Water, Office of Science and Technology.
U.S. Environmental Protection Agency (USEPA) (2004). National recommended water quality criteria (4304T). US EPA, Office of Water, Office of Science and Technology.
Wan Y, Hu JY, An W (2006) Congener-specific tissue distribution and hepatic sequestration of PCDD/Fs in wild herring gulls from Bohai Bay, north China: comparison to coplanar PCBs. Environ Sci Technol 40(5):1462–1468
Wang XQ, Xu J, Guo CS (2012) Distribution and sources of organochlorine pesticides in Taihu Lake, China. Bull Environ Contam Toxicol 89(6):1235–1239
Wang Y, Wu WJ, He W, Qin N, He QS, Xu FL (2013) Residues and ecological risks of organochlorine pesticides in Lake Baiyangdian, North China. Environ Monit Assess 185(1):917–929
Willett KL, Ulrich EM, Hites RA (1998) Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environ Sci Technol 32(15):2197–2207
Xu FL, Wu WJ, Wang JJ, Qin N, Wang Y, He QS, He W, Tao S (2011) Residual levels and health risk of polycyclic aromatic hydrocarbons in freshwater fishes from Lake Small Bai-Yang-Dian, Northern China. Ecol Model 222(2):275–286
Yamashita N, Urushigawa Y, Masunaga S, Walash MI, Miyazaki A (2000) Organochlorine pesticides in water, sediment and fish from the Nile River and Manzala Lake in Egypt. Int J Environ Anal Chem 77(4):289–303
Yang LY, Xia XH, Liu SD, Bu QW (2010) Distribution and sources of DDTs in urban soils with six types of land use in Beijing, China. J Hazard Mater 174(1–3):100–107
Yang LY, Xia XH, Hu LJ (2012) Distribution and health risk assessment of HCHs in urban soils of Beijing, China. Environ Monit Assess 184(4):2377–2387
Zhang J, Liu F, Chen RB, Feng T, Dong SJ, Shen HQ (2012) Levels of polychlorinated biphenyls and organochlorine pesticides in edible shellfish from Xiamen (China) and estimation of human dietary intake. Food Chem Toxicol 50(12):4285–4291
Zhou RB, Zhu LZ, Chen YY (2008) Concentrations and characteristics of organochlorine pesticides in aquatic biota from Qiantang River in China. Environ Pollut 151(1):190–199
Zhu Y, Wu WJ, Wang JJ (2009) The distributions, sources and ecological risks of polycyclic aromatic hydrocarbons in water-sediment system in Lake Baiyangdian. Lake Sci 21(5):44–53 (in Chinese)
Acknowledgments
This research is financed by the National Science Fund for Distinguished Young Scholars (no. 40725004) and the National Natural Science Foundation of China (nos. 40671165, 41030529, and 41271462).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wu, WJ., Qin, N., Zhu, Y. et al. The residual levels and health risks of hexachlorocyclohexanes (HCHs) and dichloro-diphenyl-trichloroethanes (DDTs) in the fish from Lake Baiyangdian, North China. Environ Sci Pollut Res 20, 5950–5962 (2013). https://doi.org/10.1007/s11356-013-1607-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1607-z