Abstract
The levels of hexachlorocyclohexane (HCH) and dichloro-diphenyl-trichloroethane (DDT) in the water, suspended particulate matter (SPM), and sediments from Lake Small Baiyangdian were measured by gas chromatograph with a 63Ni microelectron capture detector. The residual levels of the total HCHs in the water, SPM, and sediments were 1.59 ± 2.24 ng L−1, 25.42 ± 1.72 ng g−1 dw (dry weight), and 0.86 ± 1.44 ng g−1 dw, respectively. DDTs were not detected in the water samples. The concentrations of total DDTs were 158.79 ± 1.67 ng g−1 dw in SPM and 0.46 ± 1.97 ng g−1 dw in the sediments. Compared to other areas in China and abroad, the levels of residual HCH and DDT were relatively low in the water and sediments, but they were moderate to high in the SPM. Organic carbon partition coefficient values for HCH in this study were higher than previously published values and may reflect new input in this area. The residual HCHs in this area could be derived from a mixture of technical HCH and lindane because ongoing lindane use may be occurring. DDT in the majority of the study area was primarily attributed to historical discharge, but some regions may be receiving new input. The ecological risks of γ-HCH in the water were very low according to species sensitivity distribution models. The concentrations of HCH and DDT in the sediments from the study area did not exceed the sediment quality guidelines, which indicate little risk for benthic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hexachlorocyclohexane (HCH) and dichloro-diphenyl-trichloroethane (DDT), which are the most widely used organochlorine pesticides in the world, are persistent organic pollutants and have received considerable attention due to their global transport and damage to ecosystems and human health (Willett et al. 1998; Jones and De Voogt 1999). DDT was first listed in the Stockholm Convention on Persistent Organic Pollutants in 2001 followed by α-HCH, β-HCH, and γ-HCH (lindane) in 2009 (SCPOPs 2009). Although the agricultural application of technical HCH and DDT has been officially banned in China since 1983, and the production of lindane (99 % γ-HCH) ceased in 2000 (Gong et al. 2007), HCH and DDT are extensively detected in the environment (Li et al. 2007; Tao et al. 2005, 2007, 2008). The freshwater ecosystem is one of the major sinks for contaminants (Feng et al. 1998; Doong et al. 2002). DDT and HCH pesticide residues are widely distributed in freshwater bodies, such as ponds, rivers, lakes, reservoirs, and estuaries (Janiot et al. 1994; Dua et al. 1996; Yamashita et al. 2000; Maskaoui et al. 2005; Tao et al. 2007; Zhou et al. 2008). Therefore, it is important to investigate the residual levels, distributions, and potential ecological risks of HCH and DDT in water–sediment systems.
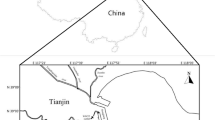
Lake Baiyangdian, which is located in the triangular region defined by three large cities, Beijing, Tianjin, and Baoding (Fig. 1), is the largest freshwater lake in north China and is regarded as the “Pearl of North China”. Lake Baiyangdian plays an important role in maintaining the ecological balance of north China and in providing domestic, agricultural, and industrial water sources for the lake catchment. The Haihe Plain, where Lake Baiyangdian is located, was an important center for the production of DDT and HCH pesticides, and was one of the primary agricultural areas where large quantities of DDT and HCH were applied (Tao et al. 2005). Severe contamination of agricultural soils and surface water by DDT and HCH in this area has been demonstrated in places near Lake Baiyangdian, such as Beijing (Zhang et al. 2005; Zhu et al. 2005; Li et al. 2006) and Tianjin (Gong et al. 2004; Tao et al. 2005, 2006; Wang et al. 2006). Studies on DDT and HCH contaminants in Lake Baiyangdian sediments have been conducted during the last two decades (Dou and Zhao 1998; Hu et al. 2010). However, little information is available on the residual HCH and DDT in water and suspended particulate matter (SPM) in Lake Baiyangdian.
Lake Baiyangdian is composed of 143 small lakes and ponds, among which Lake Small Baiyangdian is the largest, with a total area of 13.3 km2. This study of DDT and HCH in Lake Small Baiyangdian had four primary objectives: (1) to investigate the residual levels and compositions of DDT and HCH pesticides in water, SPM, and sediments; (2) to elucidate the partitioning of DDT and HCH in water, SPM, and sediments; (3) to identify potential sources of DDT and HCH; and (4) to assess the ecological risks of DDT and HCH in water and sediments.
Materials and methods
Reagents and materials
Analytical grade n-hexane and dichloromethane (DCM) (Beijing Reagent Company, Beijing, China) were purified by distillation. An organochlorine pesticide standard mixture stock was prepared by diluting a commercial mixed standard (AccuStandard Inc., New Haven, CT, USA) with n-hexane, and the working standard solution was prepared by diluting the standard stock in n-hexane. 2,4,5,6-Tetrachloro-m-xylene (TCMX) and pentachloronitrobenzene (PCNB) (AccuStandard) were used as a surrogate standard and an internal standard, respectively. A solid-phase extraction (SPE) device and C18 SPE cartridges (ENVI-18, 6 mL, 500 mg) were purchased from Supelco Co. (Bellefonte, Pennsylvania, USA), and the methanol (pesticide grade) that was used for the activation of the SPE columns was purchased from Tedia Co., Inc. Granular anhydrous sodium sulfate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was heated at 650 °C in a furnace for 6 h and then stored in a sealed desiccator until use. All glassware was cleaned in an ultrasonic cleaner (KQ-500B; Kunshan Ultrasonic Instrument, Kunshan, China) and heated at 400 °C for 6 h.
Sample collection and pretreatment
Six surface water samples were collected from Lake Small Baiyangdian on October 8th, 2007. The distribution of sampling sites is shown in Fig. 1. Twenty liters of water was collected from each sampling site. After shaking and mixing, a 1-L aliquot of each collected water sample was filtered through a 0.45-μm glass fiber filter (ashed at 450 °C for 4 h) using a peristaltic pump (80EL005; Millipore Co., USA) and a filter plate with a diameter of 142 mm to separate SPM. Before filtration, 100 ng of TCMX was added to the water as a surrogate standard. The separated SPM samples were freeze-dried and stored in aluminum foil in desiccators to maintain a constant weight until extraction. Filtered water samples were collected using pre-cleaned dark bottles and were stored at 4 °C until analysis. All of the containers were washed with deionized water, acetone, and the previous river sample in sequence to eliminate artifacts.
Surface sediment samples were collected using a grab sampler. After centrifugation (>3,000 rpm; Centrifuge TDL-5, China) and freeze drying (EYELA-FDU-830, Japan), all sediment samples were ground with a mortar and pestle into a granular powder, sieved through a 70-mesh sieve, and stored in opaque glass jars until analysis.
Sample extraction and cleanup
The pretreated water samples were passed through SPE cartridges with an octadecylsilane C18 filler. The cartridges were first cleaned using 10 mL (five times, 2 mL per wash) of DCM, activated by 6 mL of methyl alcohol (three times, 2 mL per wash), and washed using 10 mL of ultrapure water. During the activation and washing steps, the liquid level was tangent to the filler to maintain C18 wetness. After enrichment of the target compound, 10 mL of DCM (two passes with 3 mL; one pass with 4 mL) was used to elute the C18 SPE cartridge that was connected to an anhydrous sodium sulfate (5 g) cartridge. The extracts were concentrated to approximately 1 mL in an eggplant-shaped flask using a vacuum rotary evaporator (R-201; Shanghai Shen Sheng Technology Co., Ltd., Shanghai, China) at a temperature below 38 °C. PCNB was added to the flask as an internal standard. The samples were sealed in vials and stored at −20 °C until analysis.
The freeze-dried filters with SPM were processed using a Soxhlet extractor in a water bath with anhydrous sodium sulfate using 200 mL of dichloromethane for 48 h. Two grams of activated Cu was added to the extract bottles to remove elemental sulfur. The extracts were concentrated to approximately 2–3 mL using a rotary evaporator; next, 10 mL of hexane was added, and the samples were concentrated again to approximately 1 mL. The 1-mL extract was purified using a glass column packed with 12 cm SiO2 (top) and 6 cm Al2O3 (bottom). The initial extracts that were eluted with 15 mL hexane were discarded. The eluents containing HCHs were collected by eluting with 70 mL of a hexane–dichloromethane solution (2:5, v/v), concentrated to more than 1 mL by rotary evaporation and to exactly 1.0 mL under a gentle stream of purified N2.
Sediment samples (10 g) with activated Cu (2 g) were extracted by the Dionex Accelerated Solvent Extraction technique (ASE300 system) using hexane/dichloromethane (1:1, v/v) at 125 °C for 5 min under 1,500 psi. The static extraction lasted 10 min and was cycled twice. The extraction pool was 34 mL, and the eluent was 60 % (V). Nitrogen was purged for 60 s. The extracts (~60 mL) were concentrated to approximately 10 mL using a rotary evaporator and were sulfonated with concentrated sulfuric acid three times (10 mL, 6 mL, 6 mL) in separatory funnels until the lower concentrated sulfuric acid layer clarified. The organic phase was collected and concentrated to 2 mL by rotary evaporation after eluting twice with 20 mL of 4 % sodium sulfate. The organic phase was purified using a glass column that was loaded in series with 1 cm of anhydrous sodium sulfate, 1 g activated copper powder, 1 cm of anhydrous sodium sulfate, 6 g activated Florisil, and 1 cm of anhydrous sodium sulfate. The final column elution procedure was the same as the elution procedure for SPM.
Sample analysis and quality assurance
The samples were analyzed using an Agilent 6890 gas chromatograph equipped with a 63Ni microelectronic capture detector and an HP-5 column (30 m × 0.32 mm inside diameter, 0.25 μm film thickness). The samples were injected using an autosampler at 50 °C in splitless mode with a venting time of 0.75 min. The oven temperature was programmed to increase from 50 °C to 150 °C at 10 °C min−1, then increase to 240 °C at 3 °C min−1, and finally maintain 240 °C for 15 min. Nitrogen was used as both the carrier (1 mL min−1) and makeup gas (60 mL min−1). The injector and detector temperatures were 220 °C and 280 °C, respectively.
A mixed working standard was used for calibration. The average recoveries of α-HCH, β-HCH, γ-HCH, and δ-HCH in water samples by this method were 101 %, 91 %, 110 %, and 87 %, respectively; average recoveries of o,p′-DDT, p,p′-DDT, o,p′-DDD, p,p′-DDD, o,p′-DDE, and p,p′-DDE were 40 %, 75 %, 78 %, 80 %, 41 %, and 74 %, respectively. The recovery of these compounds in SPM and sediment samples ranged from 73 % to 97 %. The detection limits for the water samples were 0.5 ng L−1 for p,p′-DDT and o,p′-DDE; 0.3 ng L−1 for p,p′-DDD; 0.1 ng L−1 for p,p′-DDE; 0.05 ng L−1 for α-HCH, γ-HCH, δ-HCH, and o,p′-DDD; and 0.01 ng L−1 for β-HCH and o,p′-DDT. Two procedural blank samples were analyzed to check for solvent and glassware contamination. All samples were extracted and analyzed in duplicate.
Other analyses
A small amount of filtered water was used to detect dissolved organic carbon using a total organic carbon (TOC) analyzer (TOC-5000A; Shimadzu Corp., Japan). Dried SPM was divided into two parts to determine the total carbon and inorganic carbon using a TOC solid sampler (SSM-5000A) to obtain TOC levels. TOC levels in pre-treated sediments were detected using the same method as that used for the SPM samples.
Results and discussion
Residual levels of HCH and DDT
The levels of residual HCH and DDT in the water, SPM, and sediments from Lake Small Baiyangdian and comparisons with other studies are presented in Tables 1 and 2, respectively.
Four HCH isomers were detectable in all three media at six sampling sites (Table 1). Concentrations of total HCH ranged from 0.53 to 6.81 ng L−1 (mean 1.59 ng L−1) for water, 0.05 to 104.35 ng g−1 dw (mean 25.42 ng g−1 dw) for SPM, and 0.15 to 3.11 ng g−1 dw (mean 0.86 ng g−1 dw) for sediments. The levels of DDT and its metabolites (DDD and DDE) in the water and o,p′-DDT and o,p′-DDE in the SPM were below the detection limits. The mean concentrations of p,p′-DDT, p,p′-DDE, o,p′-DDD, and p,p′-DDD in SPM were 6.49, 15.99, 90.04, and 39.68 ng g−1 dw, respectively. The total DDT contents in sediments ranged between 0.03 ng g−1 dw and 1.41 ng g−1 dw with a mean value of 0.46 ng g−1 dw. The highest concentrations of both HCH and DDT were found in the SPM.
Table 2 shows that the HCH concentrations in Lake Small Baiyangdian water were comparable to the Huaihe River and the Wuhan section of the Yangtze River. The levels of residual HCH were one or two orders of magnitude lower than those in the Nanjing section of the Yangtze River, Pearl River, and Qiantang River, but higher than those in Lake Baikal in Russia. The levels of HCH and DDT in the SPM were relatively high, and both were one or two orders of magnitude higher than the levels of the Nanjing and Wuhan sections of the Yangtze River. However, the DDT concentrations were lower than those in six different rivers in Tianjin. In the sediments from Lake Small Baiyangdian, the HCH levels were similar to those of the Bahlui River of Romania and the Ulsan Bay of Korea and were slightly higher than those of Lake Baikal. However, the HCH levels were considerably lower than those in the Qiantang River and Haihe River. The DDT concentrations were generally one or two orders of magnitude higher than those that have been reported for the other areas mentioned here. Relative to other studies of Lake Baiyangdian, the results of this study were comparable to data that were acquired in the 1990s but were lower than the results of another survey that was performed during 2007 and 2008. These differences may be due to variations in sampling areas and indicate that pollution in Lake Small Baiyangdian was less severe than in other areas in Lake Baiyangdian.
Composition of HCH and DDT
The compositional profile of HCH isomers in the water, SPM, and sediments is shown in Fig. 2a. γ-HCH and δ-HCH were the predominant isomers in water and accounted for 40.5 % and 24.4 % of total HCH, respectively. Analyses of the SPM showed similar results with δ-HCH and γ-HCH and together accounted for more than 68 % of total HCH. In the sediments, δ-HCH accounted for the most abundant fraction at 47.7 % of total HCH, followed by β-HCH (22.3 %), γ-HCH (15.6 %), and α-HCH (13.7 %).
Figure 2b illustrates that in SPM, DDD contributed to more than 85 % of the total DDT: 59.2 % was derived from o,p′-DDD and 26.1 % was derived from p,p′-DDD. Low levels of p,p′-DDE and p,p′-DDD were also observed. A study of SPM from Deep Bay, South China by Qiu et al. (2009) showed similar results. In sediments, the proportions of individual DDT isomers were almost the same with the exception of p,p′-DDT (31.7 %). These proportions were consistent with studies of DDT in sediments from the Qiantang River and Bohai Sea in which p,p′-DDT was the predominant DDT compound (Zhou et al. 2008; Hu et al. 2010).
Partitioning of HCH and DDT in water, SPM, and sediments
To obtain a better understanding of the distributions of HCH and DDT between water, SPM, and sediments, the potential influence of certain parameters on the partitioning of these compounds requires further discussion. Unlike each isomer’s proportion in one phase, which should be decided by the relative contents of all HCH or DDT isomers, an isomer’s K oc value relates only to its own concentrations in different media. The partitioning of each isomer between water and SPM or sediments was assessed qualitatively using the organic carbon partition coefficient K oc, which can be expressed as,
where K d represents the adsorption coefficient and f oc is the organic carbon fraction (%) in sediments or SPM. K d is defined as the ratio between the solid-phase or particulate-phase concentration (C s, ng kg−1) and aqueous-phase concentration (C w, ng L−1) when dynamic sorption and desorption have reached chemical equilibrium.
As shown in Fig. 3a, the calculated logK oc values for the sediment–water system in our study were generally greater than those in previous publications (IPCS 2001; New York State Department of Environmental Conservation 2004). The mean logK oc values for α-, β-, γ-, and δ-HCH were 4.22, 5.36, 4.75, and 5.56, respectively. A similar situation was observed for the SPM–water system. The mean logK oc values for α-, β-, γ-, and δ-HCH in the SPM–water system were 4.20, 4.83, 4.67, and 4.99, respectively (Fig. 3b). One possible explanation for these differences is that previously published logK′oc values were calculated based on soil–water partitioning. Li et al. (1995) noted that one difference between sediments and soil is the extent of water saturation. With higher polarity, the dissolution of organics in water leads to a lower polarity of organic matter in sediments than in soil. Because organic matter polarity has a substantial effect on organic carbon partitioning of non-ionic pesticides in sediments and soil, a lower polarity relates directly to a higher K oc value (Li et al. 1995). Furthermore, logK′oc was measured in a laboratory after dynamic sorption and desorption reached chemical equilibrium conditions, which cannot be guaranteed in the field. From the observed logK oc values, it is likely that the partitioning of HCHs was not in equilibrium between the solid and aqueous phase in Lake Small Baiyangdian, and therefore, a portion of HCHs may partition into the aqueous phase (Tan et al. 2009).
Identification of HCH sources
Technical HCH consists of 60–70 % α-HCH, 5–12 % β-HCH, 10–15 % γ-HCH, and a minor proportion of δ-HCH with an α-/γ-HCH ratio of approximately 4–7. For lindane, which is primarily γ-HCH, the α-/γ-HCH ratio is less than 0.1. Because of their high vapor pressures, α-HCH and γ-HCH are the main isomers in the gaseous phase and could be transported over long distances in air. Therefore, the α-/γ-HCH ratio in the gaseous phase is greater than the ratio for industrial sources of HCH, e.g., α-/γ-HCH >7, which can be used to identify an atmospheric source of HCH (Iwata et al. 1993, 1995).
The α-/γ-HCH ratios (Fig. 4) in Lake Small Baiyangdian ranged from 0.23 to 0.73 for water, 0 to 1.38 for SPM, and 1.41 to 2.55 for sediments, which suggested a mixture of technical HCH and lindane sources. Ongoing lindane use may have occurred as indicated by the low α-/γ-HCH ratios in water. The α-/γ-HCH ratios for sediments were relatively high compared to those in SPM and water. One possible explanation is that water and SPM are more sensitive to recent in the surrounding areas. After their application to soil, pesticides readily leach into water through rain and runoff, which has a direct influence on HCH concentrations in water. For SPM, the contribution from contaminated soil particles washed by rain and runoff cannot be undervalued (Gomez-Gutierrez et al. 2006). Sediments typically accumulate over many years and are mainly indicative of historical contamination conditions.
Identification of DDT sources
DDT sources can be identified on the basis of their composition and metabolic products. Technical DDT that is produced for manufacturing contains 15 % o,p′-DDT and 85 % p,p′-DDT, but 1 kg of dicofol contains approximately 11 % o,p′-DDT and less than 2 % p,p′-DDT. Therefore, the o,p′-/p,p′-DDT ratios for technical DDT and dicofol are approximately 0.2 and 7, respectively. When o,p′-/p,p′-DDT is ≥7, dicofol can be assumed to be the main DDT source; an o,p′-/p,p′-DDT ratio of approximately 0.2 indicates a technical DDT source. The relative proportions of DDT metabolic products are indicative of whether DDT was derived from new inputs or historical use. DDT (o,p′-DDT and p,p′-DDT) can be degraded to DDD (o,p′-DDD and p,p′-DDD) and DDE (o,p′-DDE and p,p′-DDE). If the (DDE + DDD)/ΣDDT (sum of DDE, DDD, DDT) ratio is more than 0.5, DDT is present due to historical input and vice versa (Hitch and Day 1992).
In this study, DDT was undetectable in water, and therefore, only the sources of DDT in SPM and sediments were analyzed. Usually, sediment resuspension and terrigenous source are two essential sources for SPM (Gomez-Gutierrez et al. 2006). The significant differences in compositions between the SPM and sediments in Lake Small Baiyangdian suggested that DDT in SPM more likely came from soil remobilization during agricultural activities than the sediments. Moreover, the overwhelming predominance of o,p′-DDD in the SPM (60 %) suggested that DDT should have been degraded in anaerobic soil for some time and that dicofol had primarily contributed to the soil contamination. For sediments, the o,p′-/p,p′-DDT ratios were between 0.2 and 0.9 (Fig. 5), which indicated that technical DDT was the main source of DDT. Assuming that there were only two dominant types of DDT sources (technical DDT and dicofol) in the environment, the contributions of technical DDT and dicofol were 85.6 % and 14.4 %, respectively, according to the formula proposed by Liu et al. (2009).
However, o,p′-DDT is metabolized more quickly than p,p′-DDT (Martijn et al. 1993) in the environment, and this effect may also result in observed o,p′-/p,p′-DDT ratios that are lower than those of the product formulas. However, the ratios of (DDE + DDD)/ΣDDT were all above 0.5 except for the values from sampling sites 2 and 3; these ratios suggested that DDTs in the majority of the study area may be attributed to historical discharge, but that some regions may be receiving new input.
Ecological risks of HCH and DDT in water
A species sensitivity distribution (SSD) model was applied to evaluate the ecological risks of HCH and DDT to different species. An SSD describes the cumulative distribution of eco-toxicity and exposure data (Posthuma et al. 2002; Wang et al. 2009). Toxicity data were collected from the EPA ECOTOX database (http://cfpub.epa.gov/ecotox/) using search criteria that included the type of freshwater body, LC50 endpoints, exposure duration of less than 10 days, and laboratory tests. Vertebrate and invertebrate species were considered in the distribution. Fish were the representative vertebrates, and invertebrates included crustaceans, insects, and spiders. The BurrliOZ tool, which was designed by Australia’s Commonwealth Scientific and Industrial Research Organization (CSIRO) (CSIRO 2008), was employed to calculate the relevant SSD parameters (Table 3). The potentially affected fraction (PAF) is the cumulative probabilities on the SSD curve corresponding to a given concentration, which is a measure of the percentages of species being affected under this concentration accounting for all species. PAF can reflect the actual toxic risk probability of pollutants on species. By calculating the PAF values of different species, the relative damage can also be obtained, providing an in-depth understanding of the ecological risks of Lake Small Baiyangdian. In this study, PAF was obtained using the BurrIII (Eq. 2), ReWeibull (Eq. 3), or Repareto (Eq. 4) equations, where x is the pollutant concentration (μg L−1) and b, c, and k are the parameters fitted by the BurrliOZ tool using toxicity data.
In the present study, the DDT contents in water were below detection limits, and there was no sufficient toxicity data to construct SSD curves for α-, β-, and δ-HCH. Only the ecological risks of γ-HCH in terms of the PAF could be evaluated using the SSD model. Table 4 shows that the ecological risks of γ-HCH were very low with PAF values of less than 10−7. The ecological risk probability of γ-HCH for all of the species varied from 2.11 × 10−13 to 1.479 × 10−11. The ecological risks of γ-HCH for vertebrates (e.g., fish; 3.368 × 10−237–4.638 × 10−115) were much lower than those for invertebrates (e.g., insects and spiders; 1.342 × 10−9–1.283 × 10−8). The sensitivity of species to γ-HCH was ranked as follows: insects and spiders > crustaceans > fish.
SSD curve represents the cumulative probabilities of species being affected; more species were affected when the concentration went higher. Usually, the concentration corresponding to 5 % cumulative probability in an SSD curve (HC5) was used to evaluate ecological risk. HC5 represents the hazardous concentration for 5 % of species being adversely affected in an ecosystem. A lower HC5 value indicates species were harmed at a lower pollutant concentration, suggesting a greater ecological risk. In the present study, the HC5 values of γ-HCH for vertebrates, invertebrates, fish, crustaceans, and insects and spiders were 13.937, 3.715, 13.101, 4.271, and 3.217 μg L−1, respectively. These results implied that the ecological risks of γ-HCH were higher for invertebrates than for vertebrates. The ecological risks of γ-HCH to insects and spiders were the highest followed by the risks to crustaceans and fish. This ranking was the same as the rankings that were based on the PAF evaluation.
Ecological risks of HCH and DDT in sediments
There is no uniform standard for risk assessment in sediments. In the present study, the ecological risks of HCH and DDT in sediments were compared to published numerical sediment quality guidelines (SQGs) (Hu et al. 2010; MacDonald et al. 2000; Qiu et al. 2009; Tang et al. 2007; Long et al. 1995). However, these published SQG values may not accurately represent the actual ecological risks. In the absence of other criteria, SQGs can be used as a reference to indicate relative potential risks. Table 5 presents several HCH and DDT SQG values that were collected from the literature, including threshold-effect concentrations (TECs) and probable-effect concentrations (PECs). The residual levels of HCHs and DDTs in sediments that were measured here were much lower than these guidelines, which suggest that there was little ecological risk from HCH and DDT in the sediments in Lake Small Baiyangdian.
Toxicity tests are widely used in the derivation of SQGs, and toxicity unit (TU) is an important approach to identify potentially toxicity to organisms brought by pollutants (Hoke et al. 1997; Pedersen et al. 1998; McCready et al. 2006). TU was calculated as the observed pollutant concentration divided by the LC50, both on an organic carbon (oc) normalized basis. Usually, following the protocols of the U.S. Environmental Protection Agency, 7–10-day-old Hyalella azteca and 10-day-old larvae of Chironomus tentans are the testing species (Weston et al. 2004). In this study, due to laboratory limitations, no sediment toxicity testing was conducted on sediment samples collected in Small Baiyangdian. However, sediment LC50 values for both species were collected from related publication and TU values at each sampling sites were calculated for reference, both listed in Table 6. According to Weston et al. (2004), 0.5 TU was chosen to be the threshold above which there were great probabilities that the pollutant made potential contribution to the mortality of testing species. For both the cases of H. azteca and C. tentans, the TU values of γ-HCH and DDT isomers were much lower than 0.5, indicating that pollutants were unlikely to account for potential toxicity in Small Baiyangdian.
Conclusions
The present study investigated the extent of HCH and DDT contamination in the water, SPM, and sediments of Lake Small Baiyangdian. HCH and DDT were found throughout his area. Compared to other areas in China and abroad, residual levels in the water and sediments were relatively low, but those in the SPM were higher. The HCH K oc values that were calculated in this study were higher than those provided in the literature possibly due to new input in this area. The origin of HCH in the study area was a mixture of technical HCH and lindane, and new input of lindane may have occurred. The DDT in sediments may be present due to the historical application of technical DDT. The potential risks posed by γ-HCH in water were small. The concentrations of HCH and DDT in Lake Small Baiyangdian did not exceed the guideline values and, therefore, were of little concern.
References
CSIRO (Australia's Commonwealth Scientific and Industrial Research Organisation). (2008). A flexible approach to species protection. http://www.cmis.csiro.au/envir/burrlioz/.
Doong, R. A., Peng, C. K., Sun, Y. C., & Liao, P. L. (2002). Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River estuary, Taiwan. Marine Pollution Bulletin, 45, 246–253.
Dou, W., & Zhao, Z. X. (1998). Contamination of DDT and BHC in water, sediments and fish (Carassius auratus) muscle from Baiyangdian Lake (in Chinese). Acta Scientiae Circumstantiae, 18, 308–312.
Dua, V. K., Kumari, R., & Sharma, V. P. (1996). HCH and DDT contamination of rural ponds of India. Bulletin of Environmental Contamination and Toxicology, 57, 568–574.
Feng, H., Cochran, J. K., Lwiza, H., Brownawell, B. J., & Hirschberg, D. J. (1998). Distribution of heavy metal and PCB contaminants in the sediments of an urban estuary: the Hudson River. Marine Environment Research, 45, 69–88.
Fernandez, M. A., Alonso, C., Gonzalez, M. I., & Hernandez, L. M. (1999). Occurrence of organochlorine insecticides, PCBs and PCB congeners in water and sediments of the Ebro River (Spain). Chemosphere, 38, 33–43.
Golfinopoulos, S. K., Nikolaou, A. D., Kostopoulou, M. N., Xilourgidis, N. K., Vagi, M. C., & Lekkas, D. T. (2003). Organochlorine pesticides in the surface waters of Northern Greece. Chemosphere, 50, 507–516.
Gomez-Gutierrez, A. I., Jover, E., Bodineau, L., Albaiges, J., & Bayona, J. M. (2006). Organic contaminant loads into the Western Mediterranean Sea: estimate of Ebro River inputs. Chemosphere, 65, 224–236.
Gong, X. Y., Qi, S. H., Wang, Y. X., Julia, E. B., & Lv, C. L. (2007). Historical contamination and sources of organochlorine pesticides in sediment cores from Quanzhou Bay, Southeast China. Marine Pollution Bulletin, 54, 1434–1440.
Gong, Z. M., Xu, F. L., Dawson, R., Cao, J., Liu, W. X., Li, B. G., Shen, W. R., Zhang, W. J., Qin, B. P., Sun, R., & Tao, S. (2004). Residues of hexachlorocyclohexane isomers and their distribution characters in soils in Tianjin area, China. Archives of Environmental Contamination and Toxicology, 46, 432–437.
Hitch, R. K., & Day, H. R. (1992). Unusual persistence of DDT in some western USA soils. Bulletin of Environmental Contamination and Toxicology, 48, 259–264.
Hoke, R. A., Ankley, G. T., Kosian, P. A., Cotter, A. M., Vandermeiden, F. M., Balcer, M., Phipps, G. L., West, C., & Cox, J. S. (1997). Equilibrium partitioning as the basis for an integrated laboratory and field assessment of the impacts of DDT, DDE and DDD in sediments. Ecotoxicology, 6, 101–125.
Hu, G. C., Luo, X. J., Li, F. C., Dai, J. Y., Guo, J. Y., Chen, S. J., Hong, C., Mai, B. X., & Xu, M. Q. (2010). Organochlorine compounds and polycyclic aromatic hydrocarbons in surface sediment from Baiyangdian Lake, North China: concentrations, sources profiles and potential risk. Journal of Environmental Science, 22, 176–183.
Hu, W. Y., Wang, T. Y., Khim, J. S., Luo, W., Jiao, W. T., Lu, Y. L., Naile, J. E., Chen, C. L., Zhang, X. A., & Giesy, J. P. (2010). HCH and DDT in sediments from marine and adjacent riverine areas of North Bohai Sea, China. Archives of Environmental Contamination and Toxicology, 59, 71–79.
IPCS. (2001). The framework of the Inter-Organization Programme for the Sound Management of Chemicals (IOMC). http://www.bluegreenearth.us/archive/reviews.
Iwata, H., Tanabe, S., & Tatsukawa, R. (1993). A new view on the divergence of HCH isomer compositions in oceanic air. Marine Pollution Bulletin, 26, 302–305.
Iwata, H., Tanabe, S., Ueda, K., & Tatsukawa, R. (1995). Persistent organochlorine residues in air, water, sediments, and soils from the Lake Baikal region, Russia. Environmental Science & Technology, 29, 792–801.
Janiot, L. J., Sericano, J. L., & Roses, O. E. (1994). Chlorinated pesticide occurrence in the Uruguay River (Argentina–Uruguay). Water Air and Soil Pollution, 76, 323–331.
Jiang, X., Xu, S. F., Martens, D., & Wang, L. S. (2000). Polychlorinated organic contaminants in waters, suspended solids and sediments of the Nanjing section, Yangtze River (in Chinese). China Environmental Science, 20, 193–197.
Jones, K. C., & de Voogt, P. (1999). Persistent organic pollutants (POPs): state of the science. Environmental Pollution, 100, 209–221.
Khim, J. S., Lee, K. T., Kannan, K., Villeneuve, D. L., Giesy, J. P., & Koh, C. H. (2001). Trace organic contaminants in sediment and water from Ulsan Bay and its vicinity, Korea. Archives of Environmental Contamination and Toxicology, 40, 141–150.
Li, H., Zou, H. X., & Xu, O. Y. (1995). Influence of soils and sediments organic matter composition of nonionic compounds (in Chinese). Environmental Chemistry, 14, 124–128.
Li, J., Zhang, G., Guo, L. L., Xu, W. H., Li, X. D., Lee, C. S. L., Ding, A. J., & Wang, T. (2007). Organochlorine pesticides in the atmosphere of Guangzhou and Hong Kong: regional sources and long-range atmospheric transport. Atmospheric Environment, 41, 3889–3903.
Li, X. H., Zhu, Y. F., Liu, X. F., Fu, S., Xu, X. B., & Cheng, H. X. (2006). Distribution of HCHs and DDTs in soils from Beijing City, China. Archives of Environmental Contamination and Toxicology, 51, 329–336.
Liu, X., Zhang, G., Li, J., Yu, L. L., Xu, Y., Li, X. D., Kobara, Y., & Jones, K. C. (2009). Seasonal patterns and current sources of DDTs, chlordanes, hexachlorobenzene, and endosulfan in the atmosphere of 37 Chinese cities. Environmental Science & Technology, 43, 1316–1321.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Martijn, A., Bakker, H., & Schreuder, R. H. (1993). Soil persistence of DDT, dieldrin, and lindane over a long period. Bulletin of Environmental Contamination and Toxicology, 51, 178–184.
Maskaoui, K., Zhou, J. L., Zheng, T. L., Hong, H., & Yu, Z. (2005). Organochlorine micropollutants in the Jiulong River Estuary and Western Xiamen Sea, China. Marine Pollution Bulletin, 51, 950–959.
McCready, S., Birch, G. F., Long, E. R., Spyrakis, G., & Greely, C. R. (2006). Predictive abilities of numerical sediment quality guidelines in Sydney Harbour, Australia, and vicinity. Environment International, 32, 638–649.
Neamtu, M., Ciumasu, I. M., Costica, N., Costica, M., Bobu, M., Nicoara, M. N., Catrinescu, C., van Slooten, K. B., & De Alencastro, L. F. (2009). Chemical, biological, and ecotoxicological assessment of pesticides and persistent organic pollutants in the Bahlui River, Romania. Environmental Science and Pollution Research, 16, 76–85.
New York State Department of Environmental Conservation. (2004). Organic pesticides/herbicides and PCBs soil cleanup criteria table. http://www.dec.state.ny.us/website/der/tagms.
Pedersen, F., Bjømestad, E., Andersen, H. V., Kjlholt, J., & Poll, C. (1998). Characterization of sediments from Copenhagen harbour by use of biotests. Water Science and Technology, 37(6), 233–240.
Posthuma, L., Traas, T. P., & Suter, G. W. (2002). Species sensitivity distributions in ecotoxicology (2nd ed.). Boca Raton: Lewis.
Qiu, Y. W., Zhang, G., Guo, L. L., Cheng, H. R., Wang, W. X., Li, X. D., & Wai, O. W. H. (2009). Current status and historical trends of organochlorine pesticides in the ecosystem of Deep Bay, South China. Estuarine Coastal and Shelf Science, 85, 265–272.
Singh, K. P., Malik, A., Mohan, D., & Takroo, R. (2005). Distribution of persistent organochlorine pesticide residues in Gomti River, India. Bulletin of Environmental Contamination and Toxicology, 74, 146–154.
Stockholm Convention on Persistent Organic Pollutants (SCPOPs). (2009). The nine new POPs-an introduction to the nine chemicals added to the Stockholm Convention by the Conference of the Parties at its fourth meeting. http://chm.pops.int/Programmes/NewPOPs/Publicat-ions/tabid/695/ctl/Download/mid/2784/language/en-US/Default.aspx?id=2.
Tan, L., He, M. C., Men, B., & Lin, C. Y. (2009). Distribution and sources of organochlorine pesticides in water and sediments from Daliao River estuary of Liaodong Bay, Bohai Sea (China). Estuarine, Coastal and Shelf Science, 84, 119–127.
Tang, Z. W., Yang, Z. F., Shen, Z. Y., Niu, J. F., & Liao, R. F. (2007). Distribution and sources of organochlorine pesticides in sediments from typical catchment of the Yangtze River, China. Archives of Environmental Contamination and Toxicology, 53, 303–312.
Tang, Z. W., Yang, Z. F., Shen, Z. Y., Niu, J. F., & Cai, Y. P. (2008). Residues of organochlorine pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan, China. Environmental Monitoring and Assessment, 137, 427–439.
Tao, S., Xu, F. L., Wang, X. J., Liu, W. X., Gong, Z. M., Fang, J. Y., Zhu, L. Z., & Luo, Y. M. (2005). Organochlorine pesticides in agricultural soil and vegetables from Tianjin, China. Environmental Science & Technology, 39, 2494–2499.
Tao, S., Yang, Y., Cao, H. Y., Liu, W. X., Coveney, R. M., Xu, F. L., Cao, J., Li, B. G., Wang, X. J., Hu, J. Y., & Fang, J. Y. (2006). Modeling the dynamic changes in concentrations of γ-hexachlorocyclohexane (γ-HCH) in Tianjin region from 1953 to 2020. Environmental Pollution, 139, 183–193.
Tao, S., Li, B. G., He, X. C., Liu, W. X., & Shi, Z. (2007). Spatial and temporal variations and possible sources of dichlorodiphenyltrichloroethane (DDT) and its metabolites in rivers in Tianjin, China. Chemosphere, 68, 10–16.
Tao, S., Liu, W. X., Li, Y., Yang, Y., Zuo, Q., Li, B. G., & Cao, J. (2008). Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environmental Science & Technology, 42, 8395–8400.
Tsydenova, O., Batoev, V., Weissflog, L., & Klaus-Dieter, W. (2003). Pollution of the lake Baikal Basin: organochlorine pesticides. Chemistry for Sustainable Development, 11, 349–352.
Turgut, C. (2003). The contamination with organochlorine pesticides and heavy metals in surface water in Kucuk Menderes River in Turkey, 2000–2002. Environment International, 29, 29–32.
Wang, X. J., Piao, X. Y., Chen, J., Hu, J. D., Xu, F. L., & Tao, S. (2006). Organochlorine pesticides in soil profiles from Tianjin, China. Chemosphere, 64, 1514–1520.
Wang, X. T., Chu, S. G., & Xu, X. B. (2003). Organochlorine pesticide residues in water from Guanting Reservoir and Yongding River, China. Bulletin of Environmental Contamination and Toxicology, 70, 351–358.
Wang, Y., Wang, J. J., Qin, N., Wu, W. J., Zhu, Y., & Xu, F. L. (2009). Assessing ecological risks of DDT and lindane to freshwater organisms by species sensitivity distribution (in Chinese). Acta Scientiae Circumstantiae, 29, 2407–2414.
Weston, D. P., You, J., & Lydy, M. J. (2004). Distribution and toxicity of sediment-associated pesticides in agriculture-dominated water bodies of California’s Central Valley. Environmental Science & Technology, 38, 2752–2759.
Willett, K. L., Ulrich, E. M., & Hites, R. A. (1998). Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environmental Science & Technology, 32, 2197–2207.
Yamashita, N., Urushigawa, Y., Masunaga, S., Walash, M. I., & Miyazaki, A. (2000). Organochlorine pesticides in water, sediment and fish from the Nile River and Manzala Lake in Egypt. International Journal of Environmental Analytical Chemistry, 77, 289–303.
Yang, Q. S., Mai, B. X., Fu, J. M., Sheng, G. X., & Wang, J. X. (2004). Spatial and temporal distribution of organochlorine pesticides (OCPs) in surface water from the Pearl River Artery Estuary (in Chinese). Environmental Science, 25, 150–156.
Yu, Y. J., Huang, H., Wang, B., Wang, X. D., & Wang, L. S. (2004). Level of organochlorine pesticide pollution in the water of the Huaihe River, Jiangsu province (in Chinese). Environmental Chemistry, 23, 568–572.
Zhang, H., Lu, Y. L., Dawson, R. W., Shi, Y. J., & Wang, T. Y. (2005). Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere, 60, 762–769.
Zhang, Z. L., Huang, J., Yu, G., & Hong, H. S. (2004). Occurrence of PAHs, PCBs and organochlorine pesticides in the Tonghui River of Beijing, China. Environmental Pollution, 130, 249–261.
Zhao, L., Hou, H., Zhou, Y. Y., Xue, N. D., Li, H. Y., & Li, F. S. (2010). Distribution and ecological risk of polychlorinated biphenyls and organochlorine pesticides in surface sediments from Haihe River and Haihe Estuary Area, China. Chemosphere, 78, 1285–1293.
Zhou, R., Zhu, L., Yang, K., & Chen, Y. (2006). Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. Journal of Hazardous Materials, 137, 68–75.
Zhou, R. B., Zhu, L. Z., & Chen, Y. Y. (2008). Levels and source of organochlorine pesticides in surface waters of Qiantang River, China. Environmental Monitoring and Assessment, 136, 277–287.
Zhu, Y. F., Liu, H., Xi, Z. Q., Cheng, H. X., & Xu, X. B. (2005). Organochlorine pesticides (DDTs and HCHs) in soils from the outskirts of Beijing, China. Chemosphere, 60, 770–778.
Acknowledgments
The research was financed by the National Science Fund for Distinguished Young Scholars (No. 40725004), the National Natural Science Foundation of China (No. 40671165, No. 41030529), the National Basic Research Program (973 Project) (No. 2007CB407304), the Ministry of Environmental Protection (No. 201009032), and by the Ministry of Education (No. 20100001110035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wu, WJ., He, W. et al. Residues and ecological risks of organochlorine pesticides in Lake Small Baiyangdian, North China. Environ Monit Assess 185, 917–929 (2013). https://doi.org/10.1007/s10661-012-2601-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2601-5