Abstract
Polypropylene (PP) and poly(butylmethacrylate-co-hydroxyethylmethacrylate) (PBMA-co-HEMA) nonwoven materials as oil absorbents have been fabricated for the first time via melt blown method. As-prepared nonwovens were investigated in terms of mass per unit area, density, air permeability, contact angle, and morphology observations for fiber diameter distribution and single fiber surface by a field emission scanning electron microscope. The nonwovens are demonstrated as fast and efficient absorbents for various kinds of oils with oil absorbency up to seven to ten times their own weight. The nonwovens show excellent water repulsion but superoleophilic properties. The measured contact angles for water and toluene are more than 127° and ca. 0°, respectively. The addition of PBMA-co-HEMA makes the nonwoven surface more hydrophobic while conserving superoleophilicity. Compared with PP nonwoven, broad diameter distribution of the blend nonwoven is attributed to poor melt fluidity of PBMA-co-HEMA. In terms of single fiber, coarse surface and the presence of point-like convexities lead to the fibers being more readily wetted by oil. More interesting, oil–water separation and oil recovery can be easily carried out by filter and absorption–desorption process, the recovered materials contained hardly any oil droplet and could be reused for next cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accidents involving tanker transport and offshore oil-drilling can often result in the release of large volumes of crude oil (Cestari et al. 2012; Allan et al. 2012). Oil spill causes a series of catastrophic pollution to aquatic animals and plants, and even human beings. Increasing environmental concern, especially after several hazardous incidents in the past few years, make removal of oil spill become a hot research topic. Among existing techniques for oil spill cleanup, the use of absorbents is generally considered to be one of the most efficient techniques due to recycle and without side effects (Korhonen et al. 2011). Several oil-absorptive materials have been used such as straw or vermiculite and ashy materials to spread on the surface of the water where the oil leakage occurs. However, these materials are recovered and they ultimately become waste products, for the absorbed oil cannot be recovered.
Generally speaking, the desirable oil cleanup materials have the following characteristics: fast oil absorption, large absorption capacity, good absorption selectivity and reuse, lower density than water, swell but insoluble in oil, as well as neat storage with or without oil (Jang and Kim 2000). Several novel materials for oil–water separation have been recently reported by other researchers (Nguyen et al. 2012; Xue et al. 2011; Darmanin et al. 2008). Reported absorption capacities are noted when possible to give new idea of oil absorbents fabricated with polyethynylbenzene derivates (Li et al. 2011) and hydrophobic grapheme (Nguyen et al. 2012). The utilization of such precious materials as absorbents is quite uneconomic for a substantial treatment processing. A proper candidate of the existing absorbents on oily-waste water cleanup may prefer to low-cost materials (Gupta et al. 2009). With this awareness, polypropylene (PP) as a commercial synthetic material in oil spill cleanup has good performance and good efficiency-absorbing organic constituents (Zhu et al. 2011). Commonly used PP absorbents are structured into webs, fabrics, sheets, and pads, which are reusable and easier to control in either open or confined spaces (Choi et al. 1993). An object of the desirable material is to provide a simple and reliable manner of controlling oil spills and recovery of the oil thereafter. It is known to utilize a melt blown web of polypropylene material for oil absorption, which is sufficiently inexpensive, easily recycled, efficiently strong, and existence of oil-absorptive property. Moreover, disposal or recovery of oil may be effected by porosity in fiber web, aggregation state, and physical configuration of the fiber such as twist, crimp, surface roughness, porosity, and fineness (Choi and Moreau 1993).
Recently, alkyl acrylate copolymers, which have hydrophobicity, is oil-swellable, lipophilic, and gel-type structure consisting of a three-dimensional elastic network, have been attracted much interest in the research area (Zhao et al. 2012; Erandimala and Neckers 2010; Rengasamy et al. 2011). However, poor processability, slow absorption, and higher gravity than water cause their limited application and bleaker future in industrial product. Also, it is difficult to retrieve and assemble in most areas where they are applied.
Therefore, we wonder whether it is possible to prepare a novel bifunctional material to integrate the advantages of PP and acrylate copolymer, leading to highly efficient and practical oil cleanup material. Here, we present a high performance oil-absorptive nonwoven material by melt blown spinning. Oil absorption of three different as-prepared nonwoven materials is presented in simulated oil–water bath and oil bath pertaining to different types of oil.
Physical configuration of the nonwoven material such as weight, thickness, density, air permeability, diameter distribution, contact angle, and physical mechanical properties has been investigated by a series of measurements. The morphology of the fiber web was monitored by field emission scanning electron microscopy (FESEM). The as-prepared materials showed excellent recyclability in oil–water separation, and the removal of the absorbed oils was readily achieved by a short time desorption process in methanol. The findings of this study offer a facile nonwoven material for oil spill cleanup on the water surface.
Experimental
Materials
PP (MFI = 1,200 g/min, tacticity > 97 %) was supplied by Handan Hengyong Protective & Clean Products Co., Ltd. Poly(butylmethacrylate-co-hydroxyethylmethacrylate) (PBMA-co-HEMA) (monomer ratio, 95/5 wt%) was synthesized using the suspension polymerization, as reported in our previous papers (Xu et al. 2009). Diesel was purchased from Tianjin Petrochemical Co., Ltd., Sinopec Group. Toluene, xylene, and methanol were bought from Tianjin Chemical Regents Co., Ltd. Crude oil was obtained from ConocoPhillips, China. The demineralized water in the experiment was drawn from our campus lake. Solvent Red 24 was provided by Qingdao Sanhuan Colorchem Co., Ltd.
Preparation of nonwoven material by melt blown spinning
PP and PBMA-co-HEMA were dried in a vacuum oven at 60 °C for 48 h before melt blending to remove moisture, completely. PP and PBMA-co-HEMA blends with different compositions were prepared by using a SHJ-20 co-rotating twin-screw extruder (Nanjing Jieya Co., China) at 170–180 °C. The composition ratios by weight of PP and PBMA-co-HEMA were 100/0, 94/6, and 80/20, which was labeled as PP, PP/PBMA-co-HEMA (6 wt%), and PP/PBMA-co-HEMA (20 wt%), respectively. Primary strip blends were pelletized by granulating process for uniformly mixture, while the constituent polymers were mixed to achieve intimate mixing by a grinder into the powder.
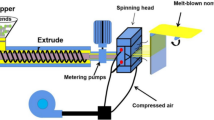
The melt blown process exhibits heating the polymer powder to a molten state and extruding it through a plurality of fine nozzles into a high velocity heated gas stream, and the gas stream attenuates the extrudate to form the melt blown fibers. After the polymer stream attenuation and cooling, the fiber was assembled and solidified on the collector. Figure 1 is a schematic perspective view of the overall melt blown process. A detailed view in longitudinal cross section of nozzles is depicted in the right inset of Fig. 1.
Testing and method
Mass per unit area each sample was displayed on the screen of the equipment according to ISO 9073-1-1989. The thickness measurement was carried out by YG141LA fabric thickness gauge (Laizhou electronic instrument Co., Ltd., China), according to ISO 9073-2-1995. The applied pressure was 0.5 kPa, and the presser-foot has an area of 2,500 mm2, after 10s, the thickness was recorded from the dial-gauge. Air permeability was measured on YG461 permeability fabric tester of medium pressure (Ningbo Textile Instrument Company) using the ISO 9073-15 testing method. Mean value was displayed in Table 1 and each specimen was tested ten times. The density of nonwoven material was measured according to simplified ASTM D792 method. A 25-mL pycnometer with an external cap in a constant-temperature bath (an appropriate constant-temperature bath adjusted to maintain a temperature of 23 ± 0.1 °C) was weighed by precision balance. The density of the plastic (d p ) was calculated as follows:
A, apparent mass of specimen; b, apparent mass of pycnometer filled with 96 % ethyl alcohol at 23.0 °C; c, apparent mass of pycnometer filled with 96 % ethyl alcohol and totally immersed specimen (eliminate bubble) at 23.0 °C; d, 0.7988 g/cm3 , the density of 96 % ethyl alcohol at 23.0 °C.
A 500-mL volume of demineralized water was placed in a 1-L glass beaker. The desired amount of oil was added to the beaker forming oil slicks on the surface of the water bath. After 1 g nonwoven was positioned into the bath, the system was shaken for 10 min at 102 cycles/min in a thermostatic oscillator. The wetted nonwoven was weighed after being drained for 1 min in a strainer above the beaker. The distillation technique as described in ASTM D95-05 was used to measure the amount of water absorbed by the nonwoven material. A mixture of xylene and toluene (80/20, v/v) was used as a carrier solvent for distillation. The oil sorption capacity of the as-prepared nonwoven material was determined as follows:
Where W N, is the weight of the wet sample, W 0 is the initial weight of the sample, and W H is the weight of water absorbed by the sample.
To analyze the amount of oil absorbed on as-prepared material in oil medium without any water, 1 g material was placed in 100 mL 10 % crude oil, diesel, and toluene in a glass beaker of 200 mL. As in the previous procedure, after shaking for 10 min, the material was drained in a strainer for 1 min and weighed. Oil absorbency was calculated as a difference between the weight of wet material and the initial weight of material.
The removal of oils from the water surface was carried out by dipping the as-prepared nonwovens into oil–water mixtures. The toluene dyed red was used in this study for monitoring oil absorption. A 3 cm × 3 cm nonwoven in area was placed in 40 ml water with floating 8 ml dyed toluene. The beaker was placed on a shaker table, set at a frequency of 150 cycles/min and an amplitude of 2 cm for a duration of 1 min. Snapshots exhibited oil cleanup application.
To investigate oil recovery, the wetted oil-absorptive material in red toluene (dyed by Solvent Red 24) was put into methanol for desorption processing. Thereafter, the nonwoven material was taken out, and then 100 ml water was poured into the beaker and was left to settle for 3–5 h. The supernatant oil could be extracted by a separating funnel. Oil will be recovered without disruption of the nonwoven material. To test the reusability, the desorbed sample was dried to a constant weight in a vacuum oven at 60 °C for next cycle. The absorption–desorption cycle was repeated for eight cycles to evaluate the recyclability of as-prepared nonwoven material. Another treatment of oil–water mixture was performed using the nonwovens to evaluate oil–water separation. The nonwoven fabric pieces were fixed as filters for separation. Oil–water mixture were transferred to a syringe barrel, an external force was employed during the separation process. The mixture could be successfully separated, and the whole process was recorded by an optical camera (see details in Online Resource 1, named ESM_1).
FESEM equipment X4800 (Hitachi, Japan) was used for observing surfaces of the nonwovens at an accelerating voltage of 5 kV. The samples were coated in gold and viewed. Using Image Pro Plus 6.0, a general-purpose image analysis soft (Media Cybernetics, USA), images of the fiber diameter were analyzed. The average of 200 readings of a number of specimens was reported and the statistics data were presented.
Contact angle measurements (the sessile drop method of water and oil drops) were carried out under room temperature using a Krüss DSA 100 apparatus (Krüss GmbH, Hamburg, Germany). Water droplets (∼3 μL) and toluene droplets (∼4 μL) were respectively delivered to different points of each specimen. The nonwoven was mounted to a height sufficiently close to the delivery needle of a syringe, and the image of the drop was captured and settled for 3 s to reported water contact angle measurement. The angles were calculated in a Krüss DSA 100 drop shape analysis system using tangent method 2. Oil contact angle was displayed by a series of images at 3 ms interval. The evolution of drop profile was recorded by video camera with every 1 ms during measurements (see details in Online Resource 2 and 3, named ESM_2 and ESM_3).
The test specimens were loaded at a constant displacement rate of 300 ± 10 mm/min according to the ISO 9073-18 standard. Rectangular nonwoven specimens (25 × 150 mm) were machined for the tensile measurement. From each sample, eight specimens in the machine direction (wrap direction) and ten specimens in the cross direction (weft direction) were performed for each testing condition. The distance between the clamps (gauge length) was set as 100 mm.
Results and discussion
Surface morphology and fiber structure
The FESEM images of the surface of PP, PP/PBMA-co-HEMA (6 wt%), and PP/PBMA-co-HEMA (20 wt%) nonwovens are shown in Fig. 2. The fiber webs are chaotic due to frequent whipping across the air stream. Many voids between fibers provide a huge space for oil absorption and storage. In terms of single fiber, pure PP fiber seems relatively smooth and uniform. The micrographs of PP/PBMA-co-HEMA (6 wt%) fiber show coarse surface and the presence of point-like convexities (see the right inset of Fig. 2), which leads to fibers being much more readily wetted by oil. The features of PP/PBMA-co-HEMA (20 wt%) fiber appear more particles and have an optimal performance in oil absorption. Later in this paper, oil absorbency will be discussed.
Figure 3 illustrates the actual distribution of fiber diameters for the sample shown in Fig. 2. These broad distributions are typical of melt blown. The average fiber diameter size is 4, 2–3, 1–2 μm for PP, PP/PBMA-co-HEMA (6 wt%), and PP/PBMA-co-HEMA (20 wt%), respectively. Moreover, the distribution can be perturbed by the addition of PBMA-co-HEMA. The distribution presents a bell curve but no centralized distribution due to poor melt fluidity of the copolymer caused by hydrogen bonding and entanglements. High-viscosity copolymer in melting state gives rise to the irregular fiber diameter, as depicted in Fig. 3.
Contact angles
The contact angle with the sessile drop method is used as a measure of the degree of attraction of the liquid for the nonwoven. Contact angle measurements reveal that as-prepared nonwovens show hydrophobic (see Online Resource 2) and superoleophilic properties, as shown in Fig. 4. The apparent water contact angle of the nonwovens was higher than 127°; however, toluene contact angle was so small that it is impossible to quantify, showing complete wetting within a very short period of time (more details in Online Resource 3). It was a confirmation that as-prepared nonwovens absorbed oil very quickly but repelled water. Moreover, water contact angle of as-prepared nonwovens increase with the increasing PBMA-co-HEMA content. Judging from the above-mentioned fact, we deduce that a small amount of HEMA has not brought hydrophilic, in reverse, compared with that of the pure PP, water contact of the blend nonwoven slightly increases due to the increased surface roughness caused by needle-like protrusions (Zhao et al. 2006; Herminghaus 2000). The results indicate that the nonwoven surface is more hydrophobic; however, it is of benefit to oil absorption.
Absorption behaviors and oil–water separation
According previous studies (Xu and Xiao 2010), PBMA-co-HEMA with hydrophobic (i.e., oleophilic) nature have predisposition for excellent oil absorption properties. According to ASTM F726-81, 10 % crude oil (crude oil diluted with toluene, 10 wt% oil), in substitution for crude oil, was used for evaluating oil absorption. As shown in Fig. 5, it is verified that the addition of PBMA-co-HEMA bring out better oil absorbency. Maximum absorbency of the nonwoven material with respect to PP/PBMA-co-HEMA (20 wt%) for 10 % crude oil is about 9.02 g/g, higher than for diesel 8.88 g/g and for toluene 7.74 g/g. This can be explaining that the viscosity of 10 % crude oil and diesel are higher than that of toluene. High-viscosity oil can cause two opposite effects: decrease of absorption as the penetration through the interior of the fiber is inhibited and improved sorption since the oil is better adhered to the material surface (Radetić et al. 2003).
As expected, the hydrophobic and superoleophilic nonwovens can easily remove oils from the water surface. The original nonwovens sank beneath the surface of oils but floated on the water surface. It was very beneficial for absorbing oil. By dipping the PP/PBMA-co-HEMA (20 wt%) nonwoven material into a mixture of water and oils, the dyed oils were quickly absorbed by the nonwoven in a few seconds. Then, the oils can be completely removed from the mixture when pulling the floating nonwoven out of the water surface, as illustrated in Fig. 6.
Another visual separation process was shown in Fig. 7 and Online Resource 1. After the mixture was filtered through the multilayer nonwovens, oils can be trapped in the nonwovens for further recycling. Obviously, as-prepared nonwovens can afford the primary separation of oil–water mixture.
The influence of the initial content of oil in demineralized water bath on absorption of as-prepared materials for diesel is demonstrated in Fig. 8. The results indicate that all investigated samples showed similar increasing trends with the increasing contents of diesel. More than 10 g diesel in 500 ml water needs more nonwoven materials; it can be considered as a reference data in the practical application of oil spill cleanup.
Oil recovery
Here, we present a potential method for oil recovery based on hydrophobic and superoleophilic nonwovens. By immersion processes of dyed toluene, red toluene was quickly absorbed by the nonwoven. The wetted absorbed nonwoven was put into 100 ml methanol and stirred for 1–2 min. Red menthol mixture was observed, confirming the absorbed oils had a desorption process. Indeed, no oil droplet adhered to nonwoven could be seen by the naked eye after washing away oils, as depicted in Fig. 9c. After termination of desorption, menthol and toluene mixture can be separated by distillation (Smallwood 1996). Another simple, economical separation method, water was poured into the mixture, and ultimately a clear separation was formed after letting it stand still for a further 3–5 h, as shown in Fig. 9d. The supernatant, oil content, was coarsely extracted by a separating funnel for reuse.
Reusability
The reusability change in oil absorbency of as-prepared nonwoven material for toluene after eight absorption and desorption cycles is shown in Fig. 10. After the cycling test, the absorbent was dried a total of eight times without any measurable change in the dry weight and oil absorbency did not deteriorate throughout the whole cycles, indicating durability and reusability. Specifically, the decrease of oil absorption capacity does not exceed 1.5 % after 8 cycles of absorption–desorption.
Physical mechanical properties
Typical stress–strain curves for the nonwovens are presented in Fig. 11. Tensile mechanical properties of nonwovens are found to be markedly affected by the blend composition, increasing fraction of the copolymer component in the blends accounted for a decline in the tensile strength, while the strain at break shows a complex change, showing ductile fracture due to decline of cohesive force between the fibers. In terms of fracture, the fibers were usually pullout from the nonwoven but merely few fibers was broken. PP nonwoven exhibits a greater cohesive force than the other two blend nonwovens. Especially, crimp-interchange effects are likely to be very important in fabrics (Kilby 1963). Although it has not been possible to quantify the number of interfilament bonds and entanglement or knots, a qualitative indication of the level of the filament bonding can be obtained from FESEM photomicrographs (Choi et al. 1988). Nevertheless, the fiber assembly and single filament could still provide enough strength to afford oil spill cleanup application.
Conclusions
In this study, we reported a novel melt blown nonwoven, which was a facile material for removing oil from water surfaces and oil recovery. The nonwovens have many practical advantages: low density, ready availability, hydrophobicity, superoleophilicity, sufficient reusability, easy fabrication, and appreciable oil absorption capacities for 7–10 g/g in different oils. Meanwhile, the chaotic fiber assembly provides a huge space for oil absorption and storage. The additions of PBMA-co-HEMA bring about higher performance in oil absorbency, however, the mechanical properties is just passable. The facile nonwoven might be a promising substitute for the oil-absorptive materials used in the large-scale removal of oil spills.
References
Allan SE, Smith BW, Anderson KA (2012) Impact of the deepwater horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol 46:2033–2039
Cestari AR, Vieira EFS, Alves FJ, Silva ECS, Andrade MAS (2012) A novel and efficient epoxy/chitosan cement slurry for use in severe acidic environments of oil wells—structural characterization and kinetic modeling. J Hazard Mater 213–214:109–116
Choi HM, Kwon HJ, Moreau JP (1993) Cotton nonwovens as oil spill cleanup sorbents. Text Res J 63:211–218
Choi HM, Moreau JP (1993) Oil sorption behavior of various sorbents studied by sorption capacity measurement and environmental scanning electron microscopy. Microsc Res Techniq 25:447–455
Choi KJ, Spruiell JE, Fellers JF, Wadsworth LC (1988) Strength properties of melt blown nonwoven webs. Polym Eng Sci 28:81–89
Darmanin T, Nicolas M, Guittard F (2008) Electrodeposited polymer films with both superhydrophobicity and superoleophilicity. Phys Chem Chem Phys 10:4322–4326
Erandimala UK, Neckers DC (2010) Photoresponsive oil sorbers. J Polym Sci A 48:55–62
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas TL (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39:783–842
Herminghaus S (2000) Roughness-induced non-wetting. Europhys Lett 52:165–170
Jang J, Kim B-S (2000) Studies of crosslinked styrene-alkyl acrylate copolymers for oil absorbency application, II. Effects of polymerization conditions on oil absorbency. J Appl Polym Sci 77:914–920
Kilby WF (1963) 2-planar stress–strain relationships in woven fabrics. J Text Inst Trans 54:T9–T27
Korhonen JT, Kettunen M, Ras RHA, Ikkala O (2011) Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces 3:1813–1816
Li A, Sun HX, Tan DZ, Fan WJ, Wen SH, Qing XJ, Li GX, Li SY, Deng WQ (2011) Superhydrophobic conjugated microporous polymers for separation and adsorption. Energ Environ Sci 4:2062–2065
Nguyen DD, Tai NH, Lee SB, Kuo WS (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energ Environ Sci 5:7908–7912
Radetić MM, Jocić DM, Jovancic PM, Petrovic ZL, Thomas HF (2003) Recycled wool-based nonwoven material as an oil sorbent. Environ Sci Technol 37:1008–1012
Rengasamy RS, Das D, Praba Karan C (2011) Study of oil sorption behavior of filled and structured fiber assemblies made from polypropylene, kapok and milkweed fibers. J Hazard Mater 186:526–532
Smallwood IM (1996) Handbook of organic solvent properties. Halsted, New York
Xu N, Xiao C (2010) Swelling and crystallization behaviors of absorptive functional fiber based on butyl methacrylate/hydroxyethyl methacrylate copolymer. J Mater Sci 45:98–105
Xu N, Xiao C, Feng Y, Song Z, An S (2009) Study on absorptive property and structure of resin copolymerized by butyl methacrylate with hydroxyethyl methacrylate. Polymer Plast Tech Eng 48:716–722
Xue ZX, Wang ST, Lin L, Chen L, Liu MJ, Feng L, Jiang L (2011) A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv Mater 23:4270–4273
Zhao J, Xiao C, Xu N, Feng Y (2012) Sandwich oil-absorptive materials by a semi-interpenetrating resin based on poly(butyl methacrylate)-inter-poly(hydroxyethyl methacrylate) in poly(ethylene terephthalate) nonwoven cloth. Polym Polym Compos 20:313–320
Zhao N, Weng L, Zhang X, Xie Q, Zhang X, Xu J (2006) A lotus-leaf-like superhydrophobic surface prepared by solvent-induced crystallization. ChemPhysChem 7:824–827
Zhu H, Qiu S, Jiang W, Wu D, Zhang C (2011) Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ Sci Technol 45:4527–4531
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant Nos.: 50673077 and 51103099). Our work was also supported by Handan Hengyong Protective & Clean Products Co., Ltd., Hebei Province, P.R. China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
The nonwoven as a filter, facilitating a simple separation to oil–water mixture. (MPG 31273 kb)
The video shows a dynamic evolution of water drop on as-prepared nonwoven surface. (MPG 38692 kb)
The video shows a rapid evolution of toluene drop on as-prepared nonwoven surface. (MPG 47252 kb)
ESM 4
(JPEG 61 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Xiao, C. & Xu, N. Evaluation of polypropylene and poly (butylmethacrylate-co-hydroxyethylmethacrylate) nonwoven material as oil absorbent. Environ Sci Pollut Res 20, 4137–4145 (2013). https://doi.org/10.1007/s11356-012-1397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1397-8