Abstract
The aim of the study is to assess the evolving mine water quality of closed uranium mines (abandoned between 1958 and 1992) in the Czech Republic. This paper focuses on the changes in mine water quality over time and spatial variability. In 2010, systematic monitoring of mine water quality was performed at all available locations of previous uranium exploitation. Gravity flow discharges (mine adits, uncontrolled discharges) or shafts (in dynamic state or stagnating) were sampled. Since the quality of mine water results from multiple conditions—geology, type of sample, sampling depth, time since mine flooding, an assessment of mine water quality evolution was done taking into account all these conditions. Multivariate analyses were applied in order to identify the groups of samples based on their similarity. Evaluation of hydrogeochemical equilibrium and evolution of mine waters was done using the Geochemist’s Workbench and PHREEQC software. The sampling proved that uranium concentrations in mine waters did not predominantly exceed 0.45 mg/L. In case of discharges from old adits abandoned more than 40 years ago, uranium concentrations were below the MCL of US Environmental Protection Agency for uranium in drinking water (0.03 mg/L). Higher concentrations, up to 1.23 mg/L of U, were found only at active dewatered mines. Activity concentration of 226Ra varied from 0.03 up to 1.85 Bq/L except for two sites with increased background values due to rock formation (granites). Radium has a typically increasing trend after mine abandonment with a large variability. Concerning metals in mine water, Al, Co and Ni exceeded legislative limits on two sites with low pH waters. The mine water quality changes with a focus on uranium mobility were described from recently dewatered mines to shafts with water level maintained in order to prevent outflows to surface water and finally to stagnating shafts and discharges of mine water from old adits. The results were in good agreement with published experience on mine water stratification, its disturbance by pumping or natural water decant and the “first flush” phenomenon after mine flooding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the 1940s, uranium mining and milling started its rapid development. As was commonly the practice in the 1940s, little was known, nor attention paid to the environmental impacts of mineral exploitation and production. From 1945 to the middle of the 1990s, uranium mining was a significant industry in the Czech Republic, and as far as the production of uranium concentrate was concerned, the Czech Republic held a foremost position in the world. At present, mining of uranium is performed in one underground mine in the deposit of Rožná with termination of mining operations planned in 2015.
Uranium deposits in the Czech Republic are connected with the Bohemian Massif, which represents a fragment of Variscan orogeny. Endogenous deposits of uranium mineralization are mainly bonded to metamorphic series and granitic massifs; younger exogenous deposits are associated with platform Permian-Carboniferous, and Cretaceous and Tertiary formations (Kafka et al. 2003). This paper relates only to the issue of hydrogeochemical changes of mine waters in abandoned mines and flooded mine workings in endogenous deposits.
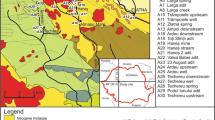
Endogenous deposits in pre-platform formations of the Bohemian Massif (for location, see the map in Fig. 1) exceed borders of the Czech Republic (deposits in Poland/Kowary/Germany/Schlema-Alberoda, Pöhla). In view of geological structure, morphology of ore bodies and metalliferous accumulation of the deposits, there are three genetically distinguished types of hydrothermal deposits (Bernard and Pouba 1986):
-
Type I
Graphitized crushing zones in rocks of metamorphic fundament. Metalliferous zones of low to medium temperature hydrothermal deposits appear in strongly altered rocks (gneisses) of the Bohemian Massif crystalline complex. The main uranium minerals are uraninite, coffinite and brannerite.
-
Type II
Veins and vein systems in rocks of Variscan structural level. Veinous low to medium temperature hydrothermal deposits are localized in large granitic massifs and crystalline schists, sandstones and siltstones of the Variscan structural level. The main uranium mineral is uraninite.
-
Type III
Chloritized tectonic zones in Variscan granitoids. Deposits are bonded to tectonic of granitoids. Uranium minerals are represented by uraninite, coffinite and brannerite.
Main uranium mining locations in the Czech Republic. Small deposit 50 to 1,000 t of U, medium deposit 1,000 to 10,000 t of U, large deposit over 10,000 t of U. IDs in Table 1
Czech uranium deposits were abandoned, with the exception of Rožná deposit between 1958 and 1992. When uranium ore exploitation was finished and the mine dewatering stopped, the process of spontaneous mine flooding started. Depending on excavated volumes, the depression cone area and the hydrogeological conditions of the deposit, this process took several years for each mine. In the case of deposits abandoned in the 1990s, conditions for proper mine water management were created in advance. Groundwater is pumped from the shafts, in order to preserve groundwater level below the drainage base, to surface water. After treatment, it is discharged to surface streams. This ensures that shallow groundwater and surface water are not threatened by uncontrolled discharges of contaminated water from flooded mines. All these deposits are systematically monitored, and as soon as water quality corresponds with the limits approved by the Czech Inspectorate of Environment for mining site under closure, the groundwater pumping can be stopped (Hájek et al. 2006).
Monitoring of uranium mine waters’ chemical composition in the Czech Republic has been carried out systematically since the beginning of their opening and exploitation. It should be highlighted that in the Czechoslovak political system of that time (1950s), the data were secret and were not freely available in the whole range of monitored spectra up to the 1980s. Part of the information is currently archived in the state-owned enterprise DIAMO in Stráž pod Ralskem which has been managing all abandoned uranium mines in the Czech Republic since the 1990s, i.e. it also bears the responsibility for consequences of former uranium exploitation and processing. DIAMO, s.p. carries out systematic monitoring of abandoned mines’ selected locations and the results are published in annual reports (Neubauer et al. 2011; Váša et al. 2011; Brůček et al. 2011).
Due to the tradition of uranium mining in the Czech Republic and the existence of uranium deposits abandoned from 1958 to 1992, the studies of uranium mining impacts on hydrosphere constitute valuable experience for countries with developing uranium mining. The aim of this article is to assess impacts of uranium mines’ closure and abandonment on groundwater in the Czech Republic including the evolution of mine water quality after the deposit is abandoned.
Impact of uranium mine closure and abandonment on groundwater
Mine waters present a challenge to conventional underground mines. It complicates all mining activities on the deposit, cannot be easily discharged and pose an unnatural load on the environment in the area involved in mining. The same applies to the phase after the mining termination, and the problems can, as shown by number of locations, persist for decades. Various impacts of mining activities on hydrological and hydrogeological conditions are summarized for example in Younger et al. (2002) and Wolkersdorfer (2008).
Mine water is the result of mixing what are usually hydrogeochemically different water sources (formation water, water infiltrating from the surface and operational water) as well as the result of hydrogeochemical reactions of the water-rock-mine air interaction. In addition, it is significantly impacted by mining technologies. The chemistry of mine water changes in the course of the opening and exploitation of each uranium deposit depends on the extent of recharge area, total area of mine working exposed surface, mineral composition of the rock environment and reached depth of mine excavations. At the moment when the mine water pumping stops due to mine closure, uranium deposit levels are gradually flooded. Recovered water body is enriched by easily extractible compounds from the bare rock environment that was kept dry and exposed to oxidation, and also by dissolution of precipitated salts deposited in mine workings. As the mine is gradually flooded, total dissolved solids in mine water rapidly increases which results in increased concentrations (orders of magnitude) in uranium and other associated metals requiring treatment prior to discharge to the surface water bodies.
In the course of mine flooding in early stages, the dissolution of secondary uranium minerals that precipitated on mine working surfaces during exploitation (oxygen access) occurs. Solubility of (especially) U oxides is significantly affected by the degree of crystallization. The solubility coefficient of crystalline uraninite can be eight to nine orders lower than the solubility coefficient of amorphous UO2 (pitchblende).
After the increase of solutes concentrations in mine water (including uranium) shortly after mine flooding (the “first flush”), stratification of mine water quality evolves with a change from oxidising to reducing conditions in the deeper levels of stagnating waters. Hexavalent uranium (uranylic ion \( \mathrm{UO}_2^{2+ } \)) which in natural conditions is much more mobile than U4+ stabilize by reduction according to the following equation:
Mine water composition is highly dependent also on pH of environment. Reimann and Caritat (1998) characterise uranium mobility in acid environment as high, in neutral and alkaline environment as very high. In the case of Czech uranium deposits, occurrence of acid mine drainage is exceptional (Licoměřice); mine waters are mostly neutral.
Reimann and Caritat (1998) mention reduction, adsorption and possibly also precipitation as the main geochemical barriers of uranium transport. While in an oxidising environment, uranium is rather mobile, in reducing conditions it stabilizes. Arnold et al. (1998) describe sorption on mineral and rock surface as one of the dominant factors affecting uranium mobility. His experiments imply that maximum sorption is achieved at a pH close to 7, which are typical conditions of Czech uranium deposits. Considerable increase of sorption is caused by the presence of Fe oxides and hydroxides. Ames et al. (1982) mention rather significant sorption sensitivity by uranium phases (especially carbonate complexes) to temperature changes.
Studying the natural processes that take place in mine water on flooded deep mines is very difficult due to the inaccessibility of the remote parts of the mine after flooding. More information is known about the regime of shallow circulation water chemistry, whose regular monitoring is possible. However, we still do not know exactly all of the processes in quasi-stagnant water accumulated in deeper parts of former mines. In a majority of cases, it is not technically possible to monitor this water and describe the evolution of its physical–chemical properties and natural processes that affect them. Experience tells us that the quality of mine water on flooded deposits stratifies (Zeman et al. 2009; Nuttall and Younger 2004).
Explanation of physical and chemical processes leading to the increase of solutes (potential pollutants) in mine water in the course of flooding and their gradual decrease after mine abandonment is very complex (Younger et al. 2002; Gzyl and Banks 2007). It comprises flow dynamics of ground and surface waters, changes of drainage levels, hydraulic gradients and existence of preferential water pathways. The natural decrease of mine water pollution is moreover connected with processes of natural attenuation in a rock environment. Processes of natural attenuation include hydrodynamic dispersion, sorption, ion exchange, biotic and abiotic degradations (radioactive decay in the case of radionuclides), geochemical reactions, dilution, etc. (Fetter 2001; Appelo and Postma 2005; Domenico and Schwartz 1998; Fetter 1999). These processes generally lead to a decrease of mass or concentration of pollutants in groundwater. Natural attenuation of uranium and metals in general is a very sophisticated issue requiring application of geochemical and reactive transport modelling. A review of modern multicomponent reactive transport codes indicates a relatively high level of maturity (Kerry et al. 2005). However, the predictive potential of such models is, in practice, limited by availability of geochemical parameters such as the presence and quantities of primary and secondary mineral phases (demonstrated by Bain et al. 2001). Knowledge of the heterogeneity of the rock environment and variability of transport parameters limit the reliability of studies in the regional scales.
It is therefore obvious that water management of abandoned mines requires a combination of theoretic model studies and long-term monitoring of abandoned uranium mining sites that can bring empirical experience usable for validation of conceptual and numerical geochemical and transport models. The issue of water management of abandoned mines was dealt with in detail in monographs by Wolkersdorfer (2008) and Younger et al. (2004).
In literature, we can find a number of experiences with detailed (sometimes even systematic) monitoring of individual abandoned uranium mining sites. The monitoring is very often connected to remediation activities on site. Carvalho et al. (2007a) published an extensive regional study focused on detection of radionuclides’ concentrations in soils in 60 areas of former radium and uranium mining in Portugal. Nevertheless, systematic regional study focused on uranium mining impact on groundwater has not been found.
Given the number of available studies dealing with the impact of uranium mining on the surface water (e.g. Meinrath et al. 2003; Baborowski and Bozau 2006; Carvalho et al. 2007b; Bister et al. 2010), this paper focuses only on groundwater.
Changes in mine water chemistry during the course of uranium mine flooding were studied, e.g. by Wolkersdorfer (1996) at the Ezgebirge mine (Germany). He, among others, observed a strong linear increase in concentration of sulphate ions to values of approximately 1,350 mg/L, significant growth of TDS and strong correlation between As and HCO3 −, detected also on other locations (Wolkersdorfer 1994). Uranium concentration in mine water stabilized on the value between 3 and 4 mg/L. 226Ra growth was detected with a correlation coefficient lower than 0.5. The complicated growth tendency of 226Ra also corresponds with findings at our locations of interest. Neves and Matias (2008) present similar results in their assessment of groundwater quality and its environmental implications in the region of abandoned Cunha Baixa uranium mine (Central Portugal). Monitoring carried out between 1995 and 2004 proved a persistent deteriorated quality of groundwater (mine abandoned in 1993) 1 km downstream of the mine. The waters with low pH (3.6 to 5.6) and high values of EC, TDS, SO4, F, Ca, Mg, Al, Mn, Ni, U, Zn and 226Ra were sampled with visible seasonal variations and improvement tendency since 1999.
Similarly, examples of acidic uranium mine water discharges with high content of metals and variable concentration of 238U (0.0036–0.78 mBq/L) were documented by Yamamoto et al. (2010) at the former Ogoya Mine in Japan. Lozano et al. (2000) documented the radionuclide concentrations in groundwater in the area of the abandoned uranium mine Los Ratones (Spain) 25 years after the cease of operation. Both in the case of 226Ra, and uranium seasonal variations connected with baseflow discharge, variations were observed. The activity concentration of 226Ra was below 1 Bq/L, and the average value of uranium concentration was 1.5 Bq/L.
Meyer and Jenk (2010) assessed mine water chemistry development in the Schlema Alberoda deposit and Pöhla uranium mine in Western Saxony of Germany in the period after the deposit flooding. A recently published paper (Földing et al. 2012) presents the experience with flooding of the Pecs deposit in Hungary, which has been flooded since 1997. Also, the abandoned uranium mines in the Kowary region (Poland) still present environmental hazard due to the increased values of radioactivity in surface water and groundwater, as documented by Chau et al. (2011).
Methods
The mine waters of all available sites of previous uranium exploitation with special focus on endogenous deposits were sampled in August 2010. Several of these deposits are systematically monitored, but regularly analysed chemical parameters of mine waters are highly reduced (generally just TDS, U, Ra and sporadically sulphates, Fe, Mn, physical parameters). A basic overview of the mine water sampling sites is presented in Table 1.
Non-filtered water samples were analysed in the laboratories of VŠB-TU Ostrava (major ions and heavy metals), Zdravotní ústav Ostrava (Ag, Al, Ba, Sb and Sr) and DIAMO, s. p., o. z. TÚU Stráž pod Ralskem (U and Ra). Uranium was analysed by photometric analyses with Arsenazo III after separation of uranylic ions on silicagel (ČSN 75 7614 1998). Radium was determined by scintillation determination of 226Ra after co-precipitation with BaSO4 and PbSO4 (PP-LAB-35-02). Ag, Al, Ba, Sb and Sr were analysed by ICP-MS (ČSN EN ISO 17294-2 2005). In the field, conductivity, Eh, pH and temperature were measured by GREISINGER GMH 3430 (conductivity with precision ± 0.5 % of measured value), by WTW pH315i with pH electrode SenTix 21 (pH with precision of ± 0.01 pH) and by GREISINGER GMH 3530 using the electrode GREISINGER GE 105 BNC (Eh). Alkalinity was also determined in the field using HCL titration.
The sampling method always reflected the specific conditions of each location. In some cases (mine adits, uncontrolled discharges), gravity-flow discharges were sampled [Electronic supplementary material (ESM) 1]. In the case of stagnating shafts, shallow-water samples were taken using a peristaltic pump. In the case of shafts in a dynamic state (pumped to maintain water level), pumped water was taken.
Since traditional methods (Piper diagram) failed in differentiating among water samples described by multiple parameters, multivariate methods have been applied. We applied principal component (PCA) analyses (Jolliffe 1986) in order to transform the original set of variables to a new set of uncorrelated variables and visualize the distribution of samples based on two main components. Cluster analysis was conducted with MATLAB using Ward-Wishard clustering strategy (Manly 1994). The code generates dendrogram with similarity/dissimilarity values for samples and a computation log with information on the individual clusters (Cressie 1993). The Geochemist’s Workbench® (Bethke 2007) and PHREEQC for Windows (Parkhurst and Appelo 1999) software were used for geochemical modelling.
Results and discussion
The results of mine water sampling on abandoned uranium mines in Czech Republic are presented in the ESM 2. The description of sampling locations is given in Table 1 (linked by ID).
Apart from the above chemical and physical parameters of mine waters, Al, Ag, As, Ba, Cd, Co, Cu, Ni, Pb, Sb, Sr and Zn were also determined. We do not include their values because, with small exceptions, they correspond to the natural background and do not exceed legal limits given by Decree No. 369/2004 Sb. Concentration limits of Al, Co and Ni (Al ∼ 14.2 vs. 0.25 mg/L limit) exceeded in the Licoměřice site (sample no. 7) and in the Dyleň site (sample no. 31 below mine dump). In both cases, the enhanced metal concentrations are related to low pH (5.30 Licoměřice site, 4.24 Dyleň site), which increases metal mobility.
With respect to the goal of this work, our attention focused on radionuclide concentration in mine waters. The uranium mobility in mine water is controlled by a number of factors, among which pH, Eh and concentration of major ions are the most important.
Uranium concentration in most cases ranges up to 0.45 mg/L. Water discharges from adits of mines abandoned more than 40 years ago showed values below or slightly above the maximum contaminant level of US EPA (2011) for uranium in drinking water (0.03 mg/L) given by the Safe Drinking Water Act. These discharges also mostly met the provisional guideline value of U in drinking water, 0.03 mg/L (WHO 2011). Limits for uranium in drinking water are, in Czech Republic, regulated by the State Office for Nuclear Safety concerning radiotoxicity and by Head Health Officer directives. Radiotoxicity reference and limit values are given by the Decree of State Office for Nuclear Safety 499/2005 Sb, appendix 10. Toxicity limits for uranium concentrations in drinking water are given by the Directive of Head Health Officer ČR OVZ-32.4-19.4.2007 (0.015 mg/L since 1.1.2010).
Concentrations of U between 0.45 and 1.23 mg/l were only detected at remediation stations of Rožná and Olší Mine. Rožná is an active dewatered mine, and on the Olší Mine is the mine where uranium is exploited by pumping from a deep well (Michálek et al. 2007; Michálek and Grmela 2010; Rapantova et al. 2007; Rapantová et al. 2008). Only three samples showed anomalously high uranium concentrations. These samples were taken from the pumped Příbram shafts J-19 and J-11A-no. 1 (5.61 mg/L) and no. 2 (5.44 mg/L) and the sample no. 5 from a deep well at the Olší Mine (6.43 mg/L). All these samples quite clearly represent waters of deeper circulation. Increased values of uranium content in these waters correspond well with the conclusions regarding mine water stratification discussed later in this text.
Activity concentration of 226Ra on most sampled locations ranges between 0.03 and 1.85 Bq/L, and elevated values (max. 17.1 Bq/L) were detected at two mining sites: Vítkov and Okrouhlá Radouň. These values relate to a naturally increased background where radium is a product of uranium decay in uranium-bearing granitic rocks. In the case of radium content in mine waters, complicated and indeterminable trends depending on the time passed from the mine flooding are manifested. At a majority of deposits, increases with a low correlation coefficient appear. These findings correspond with the conclusions of Wolkersdorfer (1996).
Hydrochemical data were visualised by means of a standard Piper diagram (Fig. 2). The Piper diagram significantly reduces the amount of information; therefore, it was not possible to define groups of samples upon their similarity. In order to avoid the loss of information, multi-parametric analyses were used for the assessment.
Piper diagram of chemical composition of samples listed in Table 1
The cluster analysis with use of non-standardized samples by the Ward-Wishard cluster strategy resulted in separation of six clusters. The analysis showed that clusters were separated on the basis of sample membership to a geological environment: type of deposit, except for actively pumped shafts. It is therefore apparent that the geological environment differentiates mine water chemistry especially in view of major ion content. The time after mine flooding did not manifest at cluster separation. That is why principal component analyses were elaborated with the results shown in Fig. 3. The components are linear combinations of data file parameters; in our case, two first components account for 55 % variability of the 30-dimensional data file (metals were included into analyses in addition to parameters from table in ESM 2).
Based on the interpretation of the PCA analysis, a hypothesis regarding mine water evolution on abandoned uranium deposits has been developed. The data set was collected under very heterogeneous conditions according to the technical possibilities of the mine, and represents only a short-term monitoring program. In the case of regularly monitored abandoned mines (only selected parameters), the results proved to have very good agreement with a long-term monitoring program. However, the conclusions need to be proven by other detailed studies focused on individual deposits, taking into account site-specific conditions.
In diagrams of the first two components (Fig. 3), several clusters were quite uniquely separated, whose similarity (membership of the cluster) is mainly determined by mine water flow dynamics and time length from deposit flooding. Weights of parameters on the first two components are evident from ESM 3.
The arrow in Fig. 3 represents the presumed evolution of mine water chemistry. In combination with the information on weights of parameters (ESM 3), we can deduce which parameters most rapidly change over time after mine flooding. Those are especially TDS, and mainly the sulphate and some other major ion concentrations, as well as the U concentration. Component 2 is rather significantly affected by a change in pH–Eh conditions and the metal content in waters.
The mine water evolution seems to have a logical relation to the time from the site flooding and the origin of the water sample at the respective depth level. The arrow defines mine water chemistry evolution from dewatered shafts and boreholes (samples correspond to a mixture of mine waters from various depth horizons with prevailing waters pumped from deeper levels) through shafts with a maintained water level up to the shafts with stagnated waters (sampled by peristaltic pump) and finally to gravity-flow discharges from old adits, shafts and uncontrolled discharges (abandoned more than 40 years ago). Group of discharges from adits in Horní Slavkov classed among the group of dynamically sampled stagnating shafts and gravity-flow discharges from old adits. Low Eh of these waters documents their deeper circulation given by geological and geomorphological position of the deposit. This is a reason why this group is separated from other mine water discharges from old adits abandoned more than 40 years ago.
The separation of groups in PCA analysis results according to the time from mine flooding is in good agreement with time evolution of mine water quality reported by a number of authors (e.g. Gzyl and Banks 2007; Younger et al. 2002). During the first flush phase, the contamination decreases in the mine water discharge exponentially. However, in detail, this decrease is site-specific and depends on the volumes of interconnecting workings, their hydraulic connection conductivities, groundwater recharge as well as geochemistry of the site (acidity removal by buffering or dissolution, rate at which acid-containing minerals weather; Younger 2000). The uranium deposits under study mainly represent medium- and small-sized deposits. In the case of medium-sized mines, the volume of flooded mine workings ranges between 0.8 and 3.1 mil m3. This fact could explain the remoteness of the Olsi Mine (medium size—volume 2.3 mil m3) in the group of dewatered shafts from the rest of the group representing a large deposit. The group of shafts with a maintained water level is mutually comparable since all mines are categorized as medium-sized mines. All other groups represent small- and medium-sized uranium mines, and after 20 years from flooding, their behaviour is relatively similar since they form very close groups. This observation could potentially be interpreted such that differences in mine water discharge composition during the first flush (depending on site-specific conditions) are especially pronounced in early phases.
Results of PCA are supported by natural relations observed at mines from the period of flooding through maintaining the water level under the decant level (preventing contamination of surface water) up to natural decant of waters either via adits or uncontrolled discharges. In the course of mine water flooding, hydrochemical stratification (with solutes concentrations in mine water growing with depth) gradually develops due to groundwater inflows from various aquifers and depth levels.
The dynamic mine water stratification develops due to the differences in lateral inflow dynamics at different mine levels. However, stratification itself is always due to the density differences of fluids. In stagnating shafts, static stratification develops over time. Water stratification means that water is separated into horizontal layers of different physical (temperature, density) and/or chemical properties. The stratification may be stable or unstable, depending on the temperature or density differences within the individual layers of water (Gebhart et al. 1988). The stratification is stable when low temperature differences or high density differences occur within a stratified layer.
The stratification can be broken down by external forces (e.g. outflow at the decant level—drainage adit, etc.). Stratification is often characterised by a staircase profile (Wolkersdorfer 2008). Individual mine water bodies can be separated from each other at the levels connecting adits to shafts.
Over a long-term period, thermal convection can stir the stratification within the mine water body. However, the geothermal gradient is not the only driving force for the convective flow. Density differences in the fluid may exist due to effects such as mineralization or turbidity.
Though the mine water stratification in flooded deep mines is a generally accepted fact and has been recognised since the 1970s (Cairney and Frost 1975; Ladwig et al. 1984; Younger and La Pierre 2000; Johnson and Younger 2002; Younger and Charlotte 2004), it is not always appropriately respected when assessing mine water sampling. Water samples taken from shallow parts of shafts or mine water discharges are often assumed to represent mine water quality for the whole flooded mine.
Mine water samples, though they often represent gravity-flow discharges, represent groundwater circulations of various depths. If mine shafts are sampled, the sampling method also has to be taken into account. Table 2 lists the average in situ measured parameters of various groups of mine water samples defined by PCA analyses.
The table indicates that in situ measured parameters may be a simple indicator of the circulation depth, i.e. of the mine water origin. In the case of shafts that actively drain deeper levels of mines (dewatered shafts), higher water temperature was detected (corresponding with local geothermal gradient) as well as reducing conditions typical for zones with limited water exchange with ground surface. Shafts with a maintained water level provide samples of mixed waters integrated through the whole depth profile of the mine, i.e. with a slightly increased temperature and yet low Eh. In the case of stagnating shafts, sampled water quality is given by stratification, depth and the sampling method. Gravity-flow discharges from adits abandoned more than 40 years ago typically show lower temperature, which is stable in the course of the year, and groundwater circulation to the depth of up to approximately 100 m is anticipated (oxidation zone with intensive water exchange with ground surface). Uncontrolled discharges represented very shallow circulation with high temperature of mine waters in summer (sampling in August 2010), oxidation environment and also lower pH (seepage through dump). Temperature stratification of deep mine waters is a typical phenomenon; therefore, it is necessary to take this parameter related to mine water origin into account.
Table 2 further shows an indistinct trend of pH change according to mine water circulation depth. Water chemistry in the oxidation environment is affected by redox processes, e.g. by weathering of accessory pyrite in rocks, which relates to acidity generation.
The evaluation of mine water geochemical development was done using geochemical modelling (Geochemist Workbench and PHREEQC). Uranium mobility was assessed in relation to all hydrodynamic processes at site. The interpretation of physical–chemical processes in mine water by the thermodynamic stability of minerals has been made only as the preliminary study for the explanation of the water quality of abandoned uranium mine discharges in the Czech Republic. It is generally known that contaminants’ dissolution in the mine environment is controlled by kinetic processes. A detailed study for the sites with available long-term monitoring data will represent the next step.
As indicated by results of physical–chemical parameters of monitored mine waters, individual types of mine water at uranium deposits can be differed just upon the standard measurements of pH, Eh and temperature. These parameters also affect total composition of water.
Due to geochemical modelling results, uranium without the presence of other components will have very low mobility. It also applies at rather low uranium concentrations (0.01 mg/L). Figure 4 illustrates uranium stability in water from an active mining period (Fig. 4a—6.4 mg/L) and from mines abandoned 40 years ago (Fig. 4b—0.01 mg/L).
The mobile component is the uranylic ion but only in very acid pH. Schoepite is a very stable mineral in oxidation conditions while uraninite in the reducing environment; stability of these solid phases grows with increasing uranium concentration. It means that if there are no other components in the system, uranium will not freely migrate. However, we also have to mention that uranium mobility depends on temperature. Results of modelling at temperatures lower than 25 °C showed higher uranium mobility (Fig. 4b).
Upon the PCA analysis, waters of monitored uranium deposits were divided into six groups (Figs. 3 and 5) and one outlier (Licoměřice deposit). Geochemical modelling was applied on chemical data representing centroids of groups 1 to 5. Not enough locations were analysed to classify the sixth group.
Eh–pH uranium stability diagrams for individual groups of uranium deposits divided upon the PCA analysis. Eh–pH uranium stability diagrams for individual groups of uranium deposits divided upon the PCA analysis. Diagram++++, a T = 25 °C, P = 1 bars, a[main] = 10.77, a[H2O] = 1, a[SO4] = 102.96, a[HCO3] = 102.8, b T = 18 °C, P = 1.013 bars, a[main] = 10−29, a[H2O] = 1, a[SO4] = 101.4, a[HCO3] = 102.83, c T = 15 °C, P = 1.013 bars, a[main] = 10−65, a[H2O] = 1, a[SO4] = 101.63, a[HCO3] = 102.63, d T = 12 °C, P = 1.013 bars, a[main] = 10−1.88, a[H2O] = 1, a[SO4] = 101.89, a[HCO3] = 102.26, e T = 10 °C, P = 1.013 bars, a[main] = 10−1.36, a[H2O] = 1, a[SO4] = 101.44, a[HCO3] = 102.01, f T = 16 °C, P = 1.013 bars, a[main] = 10−2, a[H2O] = 1, a[SO4] = 101.53, a[HCO3] = 101.38
The first group comprises dewatered shafts and boreholes. Water samples from this group come from deposits which have been abandoned for about 5 years. Waters in this group have the highest concentrations of sulphates, hydrogencarbonates and uranium of all studied locations. These waters are of the Ca-SO4 type. The Eh–pH diagram in Fig. 5a was created for waters from these locations upon the monitored parameters (pH, Eh and SO4 2−, HCO3 − concentration). In these waters, uranium is dissolved and in the form of complex with UO2(CO3)3 4− carbonates.
Within the PCA analysis, groups of shafts with maintained water level (group 2) and stagnating shafts (with dynamic sampling—group 3) were formed. Both groups behave almost identically within the geochemical modelling. Locations belonging to the “shafts with maintained water level” group have higher concentrations of total dissolved solids, radium, iron and chlorides (shorter time after flooding). They appear to be shallow circulation waters “contaminated” by rainfall and surface waters. Eh–pH diagram in Fig. 5b, c was created for waters from these locations upon the monitored parameters. Uranium is dissolved and appears in the form of a UO2(CO3)3 4− complex.
The fourth group comprises Horní Slavkov (Eh–pH diagram in Fig. 5d). Due to the concentrations of main compounds, these waters are similar to those from mines that were closed 17 years ago. As for the age and uranium concentrations, waters from this location correspond to groups “old adits and shafts” (Eh–pH diagram in Fig. 5e) and “uncontrolled discharges” (Eh–pH diagram in Fig. 5f). Lower pH and Eh in waters from Horní Slavkov indicate that these waters discharge from deeper levels. Despite that, uranium no longer leaches from the deposit, and therefore does not present a hazard at discharges from adits. Uranium is present also in the form of the \( \mathrm{U}{{\mathrm{O}}_2}\left( {\mathrm{C}{{\mathrm{O}}_3}} \right)_3^{4- } \) complex.
The fifth group represents old adits and shafts. The Eh–pH diagram of waters from this group is shown in Fig. 5e. It includes open adits of mines that were abandoned more than 40 years ago. Waters in these locations evolve in an oxidation environment. Uranium is present also in the form of the \( \mathrm{U}{{\mathrm{O}}_2}\left( {\mathrm{C}{{\mathrm{O}}_3}} \right)_3^{4- } \) complex.
Upon the results of geochemical models, three time phases of deposit evolution can be identified. In the first phase in active mines or shortly after their closure, uranium in waters is relatively immobile depending on concentration of other compounds, especially sulphates, hydrogencarbonates and on Eh–pH condition. In the second phase, uranium is dissolved out of the deposit. In the third phase, more than 40 years after the deposit was abandoned, uranium no longer leaches from the deposit and possesses no hazard to surface water courses. It is a typical description of deposit maturation. Geochemical behaviour of radium has not yet been reliably described, but its mobility in both oxidation–reduction and acid–base conditions is high. Crystallization (with Ba, Ca), precipitation (with Mn, Fe) and adsorption to Fe–Mn oxides, clay minerals and organic matter are considered to be the main geochemical barriers.
Radium is present in water solely in the form of the Ra2+ ion. It does not form individual minerals, but its addition may be contained, e.g. in baryte, uraninite, carnotite and other uranium minerals. Unlike uranium, radium is not dangerous for its chemical toxicity, but it is highly radioactive and carcinogenic. In systems with sulphates, insoluble RaSO4 will precipitate with radium concentrations close to 10−13 mg/L. Carbonates will not affect the Ra2+ behaviour. As indicated by geochemical models, radium does not show dependence on the time from flooding.
Conclusion
Sampling of mine waters on locations of previous uranium exploitation in the Czech Republic provided unique information that can be used when assessing mine water evolution in the period after mine abandonment. This experience may be useful when planning remediation measures with respect to the anticipated duration of mine water treatment after exploitation is terminated. However, applying the results to different locations, complex geology and geochemistry, differing sampling methodologies, QA/QC, duration of mine flooding, etc. must be taken into account.
In relation to radionuclides concentrations, sampling has shown that with the exception of actively dewatered mines, uranium concentration in mine water ranges between 0.01 and 0.45 mg/L. Water discharges from mine adits abandoned more than 40 years ago show values which mostly meet legislation limits for uranium content in drinking water. In the case of radium, it is impossible to identify a clear trend in terms of long-term behaviour because of complex geochemical processes related to host rock (uranium-bearing granitic rocks). Elevated concentrations of metals (Al, Co and Ni) were found only at two sites and had relation to the low pH of mine waters.
Multiparametric analyses (cluster analyses and PCA) were applied on multidimensional data set (30 parameters at 31 locations) in order to reveal natural relations and changes of mine water quality over the time after mine abandonment. Mine water quality evolution was described starting from recently abandoned dewatered shafts to the shafts with maintained water levels (water pumped and treated before discharge to surface streams) and finally to gravity-flow discharges from mine adits abandoned more than 40 years ago. Monitoring proved the mine water stratification and hydrodynamic issues’ importance (depth of groundwater circulation) for the quality of mine water discharges. In situ measured parameters may be a simple indicator of mine water origin, and consequently quality.
Geochemical modelling was applied on six groups of waters separated by PCA analyses with a special focus on uranium mobility in different stages of mine water discharge evolution. In the first phase (active mines and shortly after closure), uranium in mine water has a rather low mobility in dependence on concentrations of other compounds and Eh–pH conditions. In the second phase, uranium is leached out of the deposit. In this period (so-called first flush), it is desirable to prevent the discharge of mine waters to surface waters. The monitoring proved that remedial measures, maintenance of mine water level below the local drainage level and treatment of mine water before discharge, are a very appropriate option. In the majority of cases, this period takes one to two decades after mine flooding. Exceptionally longer periods are usually connected with a thickness of the dry part of the deposit above the decant level (e.g. mine adit) where uranium and other compounds are leached out from the rocks by oxidized infiltrated water. Nevertheless, Czech uranium deposits abandoned more than 40 years ago show evidence that in five decades uranium no longer leaches from the deposit and poses no hazard to environment. Groundwater geochemistry is in an equilibrium state, and under given Eh–pH conditions, dissolution of uranium minerals does not occur.
Finally, it is possible to conclude that mining always represents an impact to the environment. However, a number of examples from uranium mines in the Czech Republic prove that decades after uranium mine closure we can observe mine water stratification rejuvenation, shallow groundwater circulation discharges and gradual improvement of their quality to concentration of pollutants meeting the environmental legal limits. Some exceptions need to be further studied by long-term monitoring and research to reveal the reasons of potential problems.
References
Ames LL, McGarrah JE, Walker BA, Salter PF (1982) Sorption of uranium and cesium by Hanford basalts and associated secondary smectite. Chem Geol 35(3-4):205–225
Appelo CAJ, Postma D (eds) (2005) Geochemistry, groundwater and pollution, 2nd edn.. A. A. Balkema Publishers, Leiden
Arnold T, Zorn T, Bernhard G, Nitsche H (1998) Sorption of uranium(VI) onto phyllite. Chem Geol 151(1–4):129–141
Baborowski M, Bozau E (2006) Impact of former mining activities on the uranium distribution in the River Saale (Germany). Appl Geochem 21(6):1073–1082
Bain JG, Mayer KU, Blowes DW, Frind EO, Molson JWH, Kahnt R, Jenk U (2001) Modelling the closure-related geochemical evolution of groundwater at a former uranium mine. J Cont Hydr 52(1–4):109–135
Bernard JH, Pouba Z (1986) Ore deposits and metallogenesis of the Czechoslovak part of the Bohemian Massif. Nakladatelství ČSAV, Prague (in Czech)
Bethke CM (2007) Geochemical and biogeochemical reaction modeling, 2nd edn. Cambridge University Press, Cambridge
Bister S, Koenn F, Bunka M, Birkhan J, Lüllau T, Riebe B et al (2010) Uranium in water of the Mulde River. J Radio and Nucl Chem 286(2):367–372
Brůček P, Bican R, Klierova L, Vávrová L, Juhás D, Jandák R, Kollár K, Vacek J (2011) Report on monitoring results and state of environmental compounds of o.z. SUL in 2010. http://www.diamo.cz/images/stories/files/sul/zp_sul.pdf (in Czech) MS. Accessed 12 June 2012
Cairney T, Frost RC (1975) A case study of mine water quality deterioration, Mainsforth Colliery, County Durham. J Hydr 25(3–4):275–293
Carvalho FP, Madruga MJ, Reis MC, Alves JG, Oliveira JM, Gouveia J, Silva L (2007a) Radioactivity in the environment around past radium and uranium mining sites of Portugal. J Env Rad 96(1–3):39–46
Carvalho FP, Oliveira JM, Lopes I, Batista A (2007b) Radionuclides from past uranium mining in rivers of Portugal. J Env Rad 98(3):298–314
Chau ND, Nowak J, Bialic M, Rajchel L, Czop M, Wróblewski J (2011) Radiological hazard of mine water from polymetallic and uranium deposits in the Karkonosze Mountains, South-West Poland. New Uranium Min Boom. doi:10.1007/978-3-642-22122-4_88
Cressie NAC (1993) Statistics for spatial data—revised edition. Wiley, New York
ČSN 75 7614 (1998) Česká technická norma. Water quality—determination of uranium (in Czech). Český normalizační institut, Praha
ČSN EN ISO 17294–2 (2005) Technical norm. Jakost vod-Použití hmotnostní spektrometrie s indukčně vázaným plazmatem (ICP-MS)-Část 2: Stanovení 62 prvků. EAN code 8590963735641 (in Czech)
Decree No. 369/2004 Sb. Vyhláška o projektování, provádění a vyhodnocování geologických prací, oznamování rizikových geofaktorů a o postupu při výpočtu zásob výhradních ložisek. (in Czech)
Domenico PA, Schwartz FW (1998) Physical and chemical hydrogeology, 2nd edn. Wiley, New York
Fetter CW (1999) Contaminant hydrogeology, 2nd edn. Prentice Hall, New Jersey
Fetter CW (2001) Applied hydrogeology, 4th edn. Prentice Hall, New Jersey
Földing G, Szegvári G, Csővári M (2012) Hydrogeological evaluation of flooded uranium mine cavities in Hungary. New Uranium Min Boom. doi:10.1007/978-3-642-22122-4_36
Gebhart B, Jaluria Y, Mahajan RL, Sammmakia B (1988) Buoyancy-induced flows and transport. Springer, Berlin, pp 1–1001
Gzyl G, Banks D (2007) Verification of the “first flush” phenomenon in mine water from coal mines in the Upper Silesian Coal Basin, Poland. J Cont Hydr 92(1–2):66–86
Hájek A, Lusk K, Všetečka M and Veselý P (2006) Analysis of flooding the uranium mines in the Czech Republic. Archives of Diamo s. p 171 and suppls. (in Czech) MS
Johnson KL, Younger PL (2002) Hydrogeological and geochemical consequences of the abandonment of Frazer’s Grove carbonate hosted Pb/Zn fluorspar mine, North Pennines, UK. In: Younger PL, Robins NS (eds), Mine Water Hydr and Geoch. Geological Society, London, Special Publication 198: 347–364
Jolliffe IT (1986) Principal component analysis. Springer, New York
Kafka J, Aulický R, Ványi K, Kolek M, Kominek V, Kopecký P, Staněk V (eds) (2003) Ore and uranium mining of the Czech republic. Anagram, Ostrava (in Czech)
Kerry TB, MacQuarrie K, Mayer U (2005) Reactive transport modeling in fractured rock: a state-of-the-science review. Earth Sci Rev 72(3–4):189–227
Ladwig KJ, Erickson PM, Kleinmann RLP, Posluszny ET (1984) Stratification in water quality in inundated anthracite mines. Eastern Pennsylvania. US Bureau of Mines Report of Investigations RI-8837. US Bureau of Mines, Pittsburgh
Lozano JC, Vera TF, Gómez EV, Blanco RP (2000) Radiological characterization of a uranium mine with no mining activity. Appl Radiat Isot 53(1–2):337–343
Manly BFJ (1994) Multivariate statistical methods. A primer. Chapman & Hall, London
Meinrath A, Schneider P, Meinrath G (2003) Uranium ores and depleted uranium in the environment with a reference to uranium in the biosphere from the Erzgebirge/Sachsen, Germany. J Envi Rad 64(2–3):175–193
Meyer J, Jenk U (2010) Flooding of the Schlema-Alberoda uranium mines: aspects of the prognosis regarding the uranium concentration in flood waters. GeoSc Eng http://gse.vsb.cz 56(spec. issue 3):27–31
Michálek B, Grmela A (2010) Mine waters of the flooded uranium deposit in Olší. GeoScience Engineering. vyd. VŠB-TU Ostrava. HGF. 2010. 56(3):32–38
Michálek B, Hájek A, Rapantová N, Grmela A (2007) Research on mine waters of the uranium deposit of Olší-Drahonín. J Uhlí, Rudy, Geol průzk 14(12):11–15 (in Czech)
Neubauer L, Straková I et al (2011) Report on monitoring results and state of environmental compounds of o.z. TÚU in 2010. http://www.diamo.cz/images/stories/files/tuu/zp_tuu.pdf (in Czech) MS
Neves O, Matias MJ (2008) Assessment of groundwater quality and contamination problems ascribed to an abandoned uranium mine (Cunha Baixa region, Central Portugal). Environ Geol 53(8):1799–1810
Nuttall CA, Younger PL (2004) Hydrochemical stratification in flooded underground mines: an overlooked pitfall. J Cont Hydr 69(1–2):101–114
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC—a computer program for speciation. batch—reaction. one dimensional transport. and inverse geochemical calculations. U. S. Geol. Surv.. Denver. Colorado. USA. [online] available on: http://water.usgs.gov/software. [cited 1. 3. 2012]
Rapantova N, Grmela A, Vojtek D, Halir J, Michalek B (2007) Groundwater flow modelling applications in mining hydrogeology. J Mine Water Environ 26(4):264–271
Rapantová N, Grmela A, Michálek B, Hájek A, Zábojník P, Zeman J (2008) Utilization of Olší-Drahonín uranium deposit after mine closure. 10th IMWA Congress. Mine Water and the Environment. Karlovy Vary. Proceedings ed. Rapantova & Hrkal. Printed Esmedia DTP s.r.o. Olomouc: 221–224.
Reimann C, Caritat P (1998) Chemical elements in the environment. Springer-Verlag, Berlin
US EPA (2011) National Primary Drinking Water Regulations. http://water.epa.gov/drink/contaminants/index.cfm. Accessed 12 June 2012
Váša J, Toman Z et al (2011) Report on monitoring results and state of environmental compounds of o.z. GEAM in 2010. http://www.diamo.cz/images/stories/files/geam/zp_geam.pdf (in Czech) MS
WHO (2011) Uranium in drinking water. Background document for development of WHO guidelines for drinking-water quality. WHO/SDE/WSH/03.04/118/Rev/1 http://www.who.int/.../uranium_forcomment_2011021
Wolkersdorfer Ch (1994) Changes in mine water hydrology during the flooding of an abandoned uranium mine in the Erzgebirge/Saxonia/Germany. Proc. 5th Int. Mine Water 8 ∼ 1UZ. Congress. Nottingham, 43–55
Wolkersdorfer C (1996) Hydrogeochemical investigations of an abandoned uranium mine in the Erzgebirge/Germany. Appl Geochem 11(1–2):237–241
Wolkersdorfer C (2008) Water management at abandoned flooded underground mines. Springer, Berlin
Yamamoto M, Sakaguchi A, Kofuji H (2010) Uranium in acidic mine drainage at the former Ogoya Mine in Ishikawa Prefecture of Japan. J Rad and Nuclear Chem 283(3):699–705
Younger PL (2000) Predicting temporal changes in total iron concentrations in grundwaters flowing from abandoned deep mines: a first approximation. J Contam Hydrol 44:47–69
Younger PL, Charlotte AN (2004) Hydrochemical stratification in flooded underground mines: an overlooked pitfall. J Cont Hydr 69(1–2):101–114
Younger PL, La Pierre AB (2000) ‘Uisge Me’inne’: mine water hydrogeology in the Celtic lands, from Kernow (Cornwall, UK) to Ceap Breattain (Cape Breton, Canada). In: Robins NS, Misstear BDR. (eds.) Groundwater in the Celtic Regions: studies in hard rock and Quaternary hydrogeology. Geological Society, London, Spec. Publ 182:35–52
Younger PL, Banwart SA, Hedin RS (2002) Mine water—hydrology, pollution, remediation. Kluwer, Dordrecht
Younger PL, Wolkersdorfer C, ERMITE Consortium (2004) Mining impacts on the freshwater environment: technical and managerial guidelines for catchment scale management. J Mine Water Environ 23(Supplement 1):2–80
Zeman J, Černík M, Šupíková I (2009) Dynamic model of long term geochemical evolution of mine water after mine closure and flooding. Water Institute of Southern Africa & International Mine Water Association: Proceedings. International Mine Water Conference. Pretoria (Document Transformation Technologies) 828–836
Acknowledgments
The article has been made in connection with project ICT CZ.1.05/2.1.00/03.0082 (Institute of Clean Technologies for Mining and Utilization of Raw Materials for Energy Use) supported by the European Union and from the means of state budget by the Ministry of Education, Youth and Sports. This research was also financially supported by the Czech Science Foundation (research project no. 105/09/0808).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Stuart Simpson
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rapantova, N., Licbinska, M., Babka, O. et al. Impact of uranium mines closure and abandonment on groundwater quality. Environ Sci Pollut Res 20, 7590–7602 (2013). https://doi.org/10.1007/s11356-012-1340-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1340-z