Abstract
Uranium in acidic mine drainage from the former Ogoya Mine in Ishikawa Prefecture, Japan, and in neutral surface waters from its surrounding rivers was investigated from the viewpoint of radioactive disequilibrium in the uranium decay series. Water samples were periodically collected from the mine pithead and its surrounding rivers and their U isotopes (238U and 234U) were measured together with chemical components. The 238U concentrations in the water samples varied widely from 0.0036 to 0.78 mBq/L with a factor of about 200. High 238U concentrations were observed in the strongly acidic drainage (pH: around 3.5) from the pithead and the 234U/238U activity ratios showed significant values of as high as 10–15. By taking into account of the measurement of Th isotopes, it appeared that probable processes controlling the high 234U/238U activity ratios in acidic mine drainage were due to that the acidic water flowing from the mine pithead was formed only in the upper water layer of the pits and 234U was preferentially leached in the deeper underground water under the neutral and reducing conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, radioactive disequilibrium (i.e., activity ratios among nuclides that are not unity) in the uranium decay series is a quite common and well-investigated phenomenon [1]. The 234U/238U radioactive disequilibrium is still of interest in connection with hot atom phenomena, and the extent of this disequilibrium has been used as a tool in hydrologic investigations such as water circulation, mixing of water masses between various aquifers and geochronology [1–9].
In Japan, since Sakanoue and various co-workers [10, 11] first confirmed this anomaly for hot spring water of the Sanin district about 35 years ago, many studies have been carried out. We have also found anomalously high 234U/238U activity ratios close to 50 in hot spring water at Tatsunokuchi town near the Low Level Radioactivity Laboratory (LLRL) [12]. This hot spring water is approximately neutral, and originates in a volcanic tuff breccia and contains a large quantity of sodium sulfate with sodium chloride. During the course of this study, we found high 234U/238U activity ratios of around 13 in acidic mine drainage (pH: around 3.5) from the former Ogoya Mine where is ca. 20 km from the LLRL. Now, an underground tunnel of this mine is being used by us to measure extremely low-level radioactivity levels [13]. Generally, anomalous ratios of 234U/238U have been found in neutral water samples obtained mainly under a reducing condition where weathering (dissolution) of aquifer rock is suppressed, and two distinct mechanisms have been proposed [6–8]. One is the direct ejection of the recoiling nucleus, 234Th, into the aquatic system from a mineral grain (physical model). The other relates the chemically unstable state of the recoiling atom which is more liable to be oxidized and reachable due to its unusual lattice position in the mineral (valence change or chemical state change model). Therefore, 234U in such a site is preferentially liable to be leached in water, especially if the oxidation of U4+ to U6+ (UO2 2+) occurs. An intermediate model has also been proposed, which considers the removal of implanted recoil nuclei in the alpha recoil track produced in an adjacent grain, as due to subsequent etching by chemical solution (radiation damage model). Therefore, the appearance of high 234U/238U activity ratios in water under the acidic condition is a striking feature, because the 234U/238U activity ratio is considered to be near unity if the dissolution of rocks is accelerated under the acidic water condition, which makes a better understanding of the processes controlling high 234U/238U activity ratios scientifically very interesting.
In this paper, we measured uranium isotopes in mine drainage from the former Ogoya Mine in Ishikawa Prefecture, Japan, and in surface waters from its surrounding rivers from the viewpoint of radioactive disequilibrium in the uranium decay series. Based on the measurements, we discuss probable processes controlling the high 234U/238U activity ratios in acidic drainage from the mine.
Experimental
Outline of study site
In Japan, many mines are no longer in operation and their pits have been filled by underground water. This water flows out as mine drainage which includes a huge amount of heavy metals, and has become an environmental problem. In this regard, the Ogoya Mine is much more the rule than an exception.
The Ogoya Mine is located at ca. 20 km from the LLRL. The surrounding area of this mine consists of green-tuff formation and the mineral vein was formed by the intrusion of hydrothermal deposits into a fault and/or craft developed by the volcanic activity. The main minerals present are chalcopyrite, zinc and lead ore deposits, and iron sulfide. Mining was begun in 1682, with only the copper ore deposits being refined. The mine operation peaked in about 1955 and it was closed in 1971. During its operation, all of the mine drainage was discharged into the Kakehashi River near the mine, and high levels of Cd, which exceeded the proposed environmental guideline for river water, were detected in this river in the last decade of mine operation. Since closing, acidic water containing high levels of heavy metals still flows out mainly from the pithead of the mine drainage, although neutralizing coagulation treatment has been carried out using slaked lime (calcium hydroxide: Ca(OH)2) since 1953. The treated wastewater flows into a disposal pond, and, after rechecking the heavy metal concentrations near the pond outlet, the water is solely released into a small river of the downstream. A number of studies on calcification treatment of mine drainage and depositional formula of heavy metals, and mineralization and heavy metal interaction after lime treatment of mine drainage have been performed [14, 15].
Sample collection
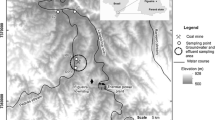
The sample collection sites are shown in Fig. 1. Water samples were collected in 1994–2003 in 20 L polyethylene bottle without filtration. The pH in the water was immediately measured. For measuring chemical components and heavy metals, 200 mL of water were also collected from different sources: (A) drainage from the mine pithead (number of samples: n = 5); (B) leakage water from the mine (n = 5); (C) downstream water from the disposal pond outlet (n = 2); (D) and (E) water running through a tunnel (n = 2) (this tunnel was ca. 500 m long and was not covered with concrete or other materials, and had been used as a road for the ore carts and miners); (F) leakage water from ceiling of tunnel (n = 4); and (G)–(K) river waters (n = 6). Two water samples of about 170 L of drainage from the mine pithead were also collected in June 2003 to measure Th isotopes, especially 234Th (a daughter nuclide of 238U). Furthermore, to get information on the age of the mine drainage, a sample for measuring 14C and δ13C was collected in June 2003 in situ as precipitate of SrCO3 to fix total dissolved carbon by adding 50 mL of saturated SrCl2 solution to 1 L water in a 1 L glass bottle [16].
Analyses of water samples
For U (238U and 234U) analysis of water samples, the samples (ca. 20 L) were acidified to pH less than 1 by adding HNO3, while being heated with stirring for more than two hours, Fe3+ carrier and 232U as a yield tracer were also added, and then the samples were allowed to equilibrate overnight. Each water sample was then neutralized by NH4OH solution to coprecipitate U isotopes with Fe(OH)3. The obtained precipitate of Fe(OH)3 was separated from the solution by decantation and centrifugation, and then was treated with HF + HNO3 + HClO4 in a Teflon beaker to decompose mainly dissolved silica. The residue was dissolved with concentrated HCl, and U isotopes in the solution were radiochemically separated by anion exchange column method after Fe extraction with diisopropylether. The purified U isotopes were electroplated onto a polished stainless steel disc and their alpha-activities were determined by alpha-particle spectrometry with a surface barrier Si detector (Tennelec TC256 spectrometer coupled to a 1 k channel pulse height analyzer) [12].
Using two water samples of drainage from the mine pithead, we attempted to determine Th isotopes. One sample was used to measure 234Th with a yield tracer of 230Th and the other was used to measure 232Th, 230Th and 228Th with a yield tracer of 229Th. The first water sample (170 L) acidified to pH less than 1 by adding HNO3 was allowed in situ to equilibrate overnight with the addition of 230Th as a yield tracer. The water sample was then neutralized by NH4OH solution to coprecipitate U isotopes with Fe(OH)3. The precipitates of Fe(OH)3 were roughly separated from the solution by decantation and brought back to the LLRL. After centrifuging, the obtained precipitates were dissolved with concentrated HCl, and Th isotopes in the solution were separated by anion exchange column method after Fe extraction with diisopropylether. Purified Th isotopes were electroplated onto polished stainless steel disc. The activity of 234Th was measured by low background gas flow counter (Berthold LB-770), whose chemical yield was determined by alpha-particle spectrometry for 230Th. Using the second sample (170 L), we sequentially separated U and Th, and purified them with the addition of 232U and 229Th tracers as yield tracers in the same manner as above. The activities of U (238U and 234U) and Th (232Th, 230Th and 228Th) isotopes electroplated each onto a disc were determined by alpha-particle spectrometry.
For the measurement of 14C and δ13C, we extracted the CO2 from the precipitates, purified it and then reduced it to graphite. The 14C and δ13C were measured by using Tandem Accelerator Mass Spectrometer (AMS) at the AMS facility of Nagoya University, Japan [16].
Major chemical compositions (Na+, K+, Mg2+, Ca2+, Cl−, NO3 − and SO4 2−) of water samples were determined by ion chromatograph (Dionex ICS-1000). The concentrations of some heavy metals were determined by using inductively coupled plasma atomic emission spectrometry (ICP-AES) and/or inductively coupled plasma mass spectrometry (ICP-MS).
Results and discussion
General chemical characteristics of water samples
The results for major ions in water samples are listed in Tables 1 and 2. The wastewater samples (A) from the pithead and leakage (B) directly related to the mine drainage were strongly acidic (pH: 3.6–4.1), while other water samples were approximately neutral. Total dissolved salt (TDS) concentrations of water samples, which were defined as the sum of both cations and anions measured here, were 10–20 times higher in wastewater from the pithead (A) and leakage water (B) than those in some of the surrounding river water samples (H)–(J). The Ca, Mg and SO4 contents were not significantly decreased at point (C) where a small river containing the wastewater treated at the disposal pond flowed in, nor were they decreased at point (K) of downstream from the junction of this small river and the Kakehashi River. However, as shown in Table 2, the levels of heavy metals at the point (K) decreased drastically up to 1/50–1/10 compared with those at points (A) and (B), indicating the effectiveness of neutralizing coagulation treatment by slaked lime.
Uranium levels and isotopic ratios in water samples
The results of the 238U and 234U concentrations and their 234U/238U activity ratios in water samples are summarized in Table 3. The 238U concentrations in the water samples varied widely from 0.0036 to 0.78 mBq/L with a factor of about 200. Higher values were found in the acidic water samples from the pithead (A) and leakage (B). At points (C) and (K), 238U concentrations decreased to several times lower levels compared with those at points (A) and (B). Lower values (0.0036–0.0071 mBq/L) were observed in leakage water samples from the ceiling of the tunnel, followed by water samples (0.021–0.11 mBq/L) from the surrounding rivers. The activity ratios of 234U/238U varied from 1.69–15.4, with the values of as high as 10–15 in acidic water samples from the pithead (A) and leakage (B). High ratios of 13.6–16.8 were also observed in neutral water samples running through the tunnel, in which 238U contents were low, ranging from 0.022 to 0.032 mBq/L. It seemed likely that this tunnel water was influenced much less by the underground water of the mine. These data was compared with the data on 238U contents and 234U/238U activity ratios for waters which have been measured so far in the underground waters, surface river waters (Tedori and Kakehashi Rivers) and mineral spring waters within ca. 20 km around the Ogoya Mine (Fig. 2) (M. Yamamoto, unpublished data). Until now, the 234U/238U activity ratios over 10 have rarely been observed around here, with the exception of the high value near 50 in the Tatsunokuchi hot spring water. This hot spring was drilled in 1976 and pumped up through the strainer from a depth of 555–575 m. This spring water of about 48 °C has its origin in a volcanic tuff breccia and is mostly likely to be in reducing condition. The 238U content is extremely low, ranging from 0.04 to 0.06 mBq/L. As shown in Fig. 2, the activity ratios of 234U/238U in water samples at points (A), (B), (D) and (E) from the Ogoya Mine were clearly higher than those of waters from other areas measured so far. The values of waters from other points of the Ogoya Mine were within the range from ca. 1 to 5, regardless of their 238U concentrations. Here, it is worth noting that, regardless of being acidic, their 234U/238U activity ratios were very high compared with the values (1.69–5.36) observed for neutral water samples from the surrounding rivers and values measured previously. Such data do not seem to have been reported until now.
The 238U and 232Th concentrations in weathered rocks (about 10 samples) collected from around the entrance of tunnel, which mainly consist of the green-tuff formation, were reported to be 20.8 ± 4.0 and 23.9 ± 5.2 Bq/kg, respectively. The 234U/238U activity ratios for these samples were nearly unity, indicating a radioactive equilibrium [17].
Probable processes controlling high 234U/238U activity ratio in acidic water
Mine drainage discharged to the outside environment has been generally divided into three categories from the viewpoint of water quality: (1) acidic water (pH: 3–5) of Ca–SO4 type including a large amount of iron; (2) mildly alkaline water (pH: 8–9) of NaHCO3–SO4 type; and (3) neutral water of Na–SO4 type. The drainage from the Ogoya Mine is probably category (1). Although the exact mechanism of acidification in mine drainage, containing a large amount of iron, is still not completely elucidated, it can be interpreted as given below [18, 19]. Immediately after the drainage flows onto the earth’s surface, its pH is about 5, and it is acidified to pH of 2–3 rapidly on contact with air. Pyrite (FeS2) is dissolved under the reducing condition, and after contact with air, Fe2+ is oxidized to Fe3+, then a brown precipitate of limonite is produced, and then the water is acidified strongly by the oxidation of S to SO4 2− . By taking into account this interpretation, underground water in deeper layer of mine drainage seems likely to be neutral and under reducing condition. The acidic water may be limited only in the upper part of the water in the pits. If the underground water in a deep layer is made acidic, Th isotopes in rocks are expected to be dissolved to some extent, although this is speculative. In fact, as show in Table 4, although we tried to detect Th isotopes by using water samples of 170 L from the mine pithead, the detected concentrations were very low. The 234Th concentration, which is expected to be ejected directly by recoil due to the α-decay of 238U, was a similar level to that of 238U. The respective concentrations of 230Th, a daughter nuclide of 234U, and 232Th were 100 times lower than that of its parent nuclide 234U, and one order of magnitude lower than that of 238U, respectively, although the activities of 232Th in rocks sampled around here were nearly the same as those of 238U. Thus, the existence of excess 234Th relative to 238U could not be observed and Th dissolution was found to be very low compared with U, indicating that deeper water of the pits was unlikely to be under the acidic condition. Under the neutral condition, Th hydrolyses very easily and is rapidly adsorbed by the surface of surrounding mineral particles. High 234U concentration may be attributed to the preferential leaching of 234U produced mainly from the such 234Th adsorbed on the surface of the mineral particles. It seems to be reasonable to assume that the acidic water flowing from the mine pithead is from only the upper layer of the pits, and its high 234U/238U ratio is attributable to the deeper underground water under the neutral and reducing conditions.
Besides the above U isotopes, we further tried to get information on the age of this drainage by using its 14C record. Measured 14C concentration and δ13C value for sample taken on June 30, 2003 were 51.7 ± 0.4% modern carbon (pmc) and −17.0‰, respectively. The δ13C value indicates that dissolved carbon might be mainly due to the organic carbon in the soil. Although a number of correction schemes for groundwater 14C dating have been discussed, further works for estimating the age of this water is needed, including its validity.
Conclusion
From the viewpoint of radioactive disequilibrium in the uranium decay series, uranium in acidic mine drainage from the former Ogoya Mine in Ishikawa Prefecture, Japan, and in neutral surface waters from its surrounding rivers was investigated. Water samples were periodically collected from the mine pithead and its surrounding rivers, and their U isotopes (238U and 234U) were measured together with chemical components. Strongly acidic wastewater (pH: around 3.5) from the pithead contained high concentrations of heavy metals. The 238U concentrations in the water samples varied widely from 0.0036 to 0.78 mBq/L. Higher values were found in acidic water samples of the pithead and leakage from the mine, and their activity ratios of 234U/238U were as high as 10–15. In this acidic drainage, the level of 234Th, which is expected to be ejected by recoil due to the α-decay of 238U, was a similar level to that of 238U. The concentrations of 230Th as a daughter nuclide of 234U and 232Th were 100 times lower than that of its parent nuclide 234U and one order of magnitude lower than that of 238U, respectively. Probable processes controlling the high 234U/238U activity ratio in acidic mine drainage seemed to be due to that the acidic water flowing from the mine pithead was formed only in the upper part of the water from pits, and 234U was preferentially leached in the deeper underground water under the neutral and reducing conditions.
References
Cherdyntsev VV, Chalov PI, Khaidarov GZ (1955) Uranium series disequilibrium dating. In: Trans 3rd session commission for determining the absolute ages of geological formations, Izd. Akad. Nauk SSSR, pp 175–182
Thurber DL (1962) J Geophys Res 67:4518
Osmond JK, Rydell HS, Kaufmann MI (1968) Science 162:997
Kigoshi K (1971) Science 173:47
Osmond JK, Cowart JB (1976) At Energy Rev 14:621
Rossler K (1983) Uranium recoil reactions. In: Buschbeck KC, Keller C (eds) Gmelin handbook of inorganic chemistry, 8th edition, uranium, supplement, volume A6. Springer Verlag, Berlin, pp 135–163
Fleischer RL, Raabe OG (1978) Geochim Cosmochim Acta 42:973
Adloff JP (1981) Radiochim Acta 29:5
Ivanovich M, Harmon RS (eds) (1982) Uranium series disequilibrium; Applications to environmental problems in the earth sciences. Clarendon Press, Oxford
Sakanoue M, Hashimoto T (1964) Nippon-kagaku Zashi. J Chem Soc Jpn 85:622 (in Japanese)
Sakanoue M, Komura K (1971) Nature, Phys Sci 233:80
Yamamoto M, Sato T, Sasaki K, Hama K, Nakamura T, Komura K (2003) J Radioanal Nucl Chem 255:369
Komura K (2002) In: Proceedings of international conference on radioactivity in the environment, 1–5 Sept 2002, Monaco (in CD-ROM)
Sato D, Tazaki K (2000) Chikyukagaku (Geochemistry) 54:328 (in Japanese)
Sato D, Tazaki K (2001) Nendokagaku (Clay Chem) 40:218 (in Japanese)
Mizutani Y, Seto T, Ohta T, Nakai K, Murai Y (1992) In: Proceedings of the symposium on interdisciplinary study of AMS and carbon isotopes at Center for Chronological Research, Nagoya University, pp 15–168
Toguchi A (1994) Master’s thesis of Kanazawa University (in Japanese)
Wtanabe K, Takase K, Sugai T (1975) Suirikagaku (Water Sci) 19:70 (in Japanese)
Yamashita A (1989) Chikasuigatsukaishi (Jpn Assoc Groundw Hydrol) 31:211 (in Japanese)
Acknowledgements
We would like to express our gratitude to the research staff of the Low Level Radioactivity Laboratory, Kanazawa University, for their help with sampling and Prof. Dr. T. Nakamura of Nagoya University for his help with the AMS measurement of carbon isotopes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, M., Sakaguchi, A. & Kofuji, H. Uranium in acidic mine drainage at the former Ogoya Mine in Ishikawa Prefecture of Japan. J Radioanal Nucl Chem 283, 699–705 (2010). https://doi.org/10.1007/s10967-009-0400-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0400-4