Abstract
The use of organophosphorus flame retardants (PFRs) as flame retardants and plasticizers has increased due to the ban on common polybrominated diphenyl ether mixtures. However, only limited information on PFR contamination is available so far from Southeast Asia. In the present study, residual levels of PFRs in house dust and exposure through dust ingestion were investigated in the Philippines. House dust samples (n = 37) were collected from Malate (residential area) and Payatas (municipal dumping area) in the Philippines and analyzed using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry. Among the targeted seven PFRs, triphenyl phosphate (TPP) was the predominant compound. Median levels of ΣPFRs in Malate (530 ng/g) were two times higher (p < 0.05) than in Payatas (240 ng/g). The estimated daily intake of PFRs in the Philippines (of areas studied) via house dust ingestion was below the guideline values. House dust may be an important contributor in the overall exposure of humans to TPP even when considering dietary sources. To our knowledge, this is a first report on PFR contamination in house dust from developing country. PFRs were ubiquitously detected in the home environments in the Philippines. Although estimated exposure levels through dust ingestion were below the guideline, it was suggested that toddlers are at higher risk. Therefore, further investigations to understand the behavior of PFRs in house and other microenvironments and overall exposure pathways for the country’s populace to PFRs are necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of organophosphorus compounds (PFRs) as flame retardants and plasticizers has increased extensively due to the ban on polybrominated diphenyl ether (PBDE) mixtures (i.e., PentaBDE, OctaBDE, and DecaBDE mixtures), which are a class of brominated flame retardants (BFRs) that were added as persistent organic pollutants at the fourth meeting of the Conference of the Parties (COP4) to the Stockholm Convention (COP4 2009). As a result, the consumption of BFRs in Europe decreased from 51,150 to 45,000 t between the years of 2004 and 2006 (EFRA 2007). In contrast, the annual consumption of chlorinated and non-chlorinated PFRs in Europe increased from 83,700 t in 2004 to 91,000 t in 2006 (EFRA 2007).
Among the PFRs, chlorinated alkyl phosphate (tris-2-chloroethyl phosphate (TCEP)) is mostly used as a flame retardant in both flexible and rigid polyurethane foams (EFRA 2006a). The presence of both chlorine and phosphorus is advantageous for the optimum non-flammability, working in both the solid and gaseous phases. TCEP is also included in the European Commission second priority list (EEC 1995). On the other hand, non-chlorinated aryl phosphates (triphenyl phosphate (TPP) and tricresyl phosphate (TCP)) are widely used as flame retardants in PVC, artificial leather, tents, tarpaulins, electrical cables, and conveyor belts. They are also found in cellulosic polymers (cellulose acetate), engineering thermoplastics, and synthetic rubber (EFRA 2006b). The current production of TCP mainly includes a mixture of the meta- and para-isomers, and the ortho-isomer content is usually minor (WHO 1990). However, it was reported that ortho-TPP showed adverse biological effect (Chapin et al. 1988, 1990). TPP can cause contact dermatitis in humans and is a potent inhibitor of human carboxyl esterase (WHO 1991a). Tri-n-butyl phosphate (TnBP), and tris(2-ethylhexyl) phosphate (TEHP) were neurotoxic and skin irritators (WHO 1991b, 2000). TCEP has been found to have teratogenic and hemolytic effects and has carcinogenic potential in rats and mice (Beth-Hubner 1999; Sato et al. 1997).
After their use, large amounts of PFRs are discharged into environment, and they have already been detected ubiquitously in various environments including drinking water, river water, wastewater, sediment, air, and indoor dust (Andresen et al. 2004; Martínez-Carballo et al. 2007; Kanazawa et al. 2010; Van den Eede et al. 2011; Stapleton et al. 2009). Furthermore, PFRs have also been detected in wildlife and human breast milk (Sundkvist et al. 2010; Kim et al. 2011a). Although some studies have been conducted in some developed nations including those in Europe as well as the US and Japan, to our knowledge, almost no information is available on the occurrence and behavior of PFRs in the indoor environments of developing countries in Asia such as the Philippines. Various environmental pollutants are released into the environments due to the lack of treatment facilities for the pollutants derived from rapid industrial development and urbanization in the Philippines (Malarvannan et al. 2009). Notably, large-scale open dumping of municipal wastes in the suburbs of cities in Asian developing countries is receiving considerable attention as a potential source of anthropogenic contaminants (Kunisue et al. 2004). People in these areas (in and around dump site) may be exposed to high levels of contaminants through various exposure pathways including water, air, food, and dust.
The present study investigated the distribution in and potential risk to human health of PFRs from house dust. House dust is a repository for various contaminants that are transported into the house from outside or that originate from sources within the house itself and is the subject of growing concern in recent years. Due to their boiling points (approximately 290–400 °C), most of PFRs are categorized as semi-volatile organic compounds (SVOCs) (Wensing et al. 2005). Numerous SVOCs have been found in indoor air and dust (Saito et al. 2007; Van den Eede et al. 2011). Ayoko and Uhde (2003) reported that these substances are essentially adsorbed onto particulate phase. However, it was reported that human exposure to TPP through inhalation is dominated by exposure to the gaseous phase rather than to the particulate phase (Babich 2006). In general, main human exposure to these compounds is inhalative, oral via airborne particles, and/or oral and dermal via settled dust (Wensing et al. 2005). Therefore, persistent compounds such as PFRs in indoor dust represent a potential risk for human health through oral ingestion and inhalation. Klepeis et al. (2001) reported that indoor exposure is an important pathway, since people spend more than 80 % of their time in indoor environments. The concentrations of PFRs in air are several orders of magnitude higher indoor than outdoors, indicating that the major sources of these indoor air pollutants are located in the indoor environment (Wensing et al. 2005). The importance of dust ingestion decreases with age, owing to the higher dust ingestion rates of young children (Jones-Otazo et al. 2005). Therefore, non-dietary exposure via dust inhalation and ingestion may play important roles as exposure routes.
In the present study, the levels and patterns of seven PFRs were analyzed in house dust samples collected from houses in two areas with different characteristics (Malate, which is a common residential area having no specific industrial pollution sources, and Payatas, a municipal dumping area) in the Philippines. The objectives of this study were to (1) provide background information on indoor contamination by PFRs in the Philippines and (2) estimate the non-dietary exposure to PFRs via dust ingestion for children and adults, and (3) compare the relative significance of non-dietary exposures to that of literature estimates of PFR exposure from dietary (e.g., fish) sources.
Materials and methods
Sample collection
House dust samples (n = 37) were randomly collected in August, 2008, from two different characteristic locations in the Philippines, namely Malate (residential area; n = 17) and Payatas (municipal dumping area; n = 20). Volunteers were recruited through the local government or leaders in the community. From the list of candidates, we selected families who agreed to provide all the types of samples requested including floor dust, breast milk, and scalp hair. Floor dust samples were collected by vacuum cleaner bags used in each of the sampled house, which collected dust from the living room, kitchen, and bedrooms. We asked volunteers to use a new vacuum cleaner-bag for the sampling period, and a single vacuum cleaner bag was used for each home. All dust samples collected in vacuum cleaner bag were transferred into clean aluminum foil using a stainless steel spatula. After collection, the samples were stored at −25 °C in the Environmental Specimen Bank (es-BANK) of Ehime University until further analysis (Tanabe 2006). Characteristics of each house including floor area, number of computers/televisions, furniture, and type of flooring were also documented at the time of sample collection.
Chemicals and reagents
All standards were obtained with the highest available purity. 2-Ethylhexyl diphenyl phosphate (EHDPP; 98 %), TnBP (97 %), TPP (97 %), TEHP (97 %), and TCEP (97 %) were purchased from Wako Chemicals (Osaka, Japan). TCP (98 %) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Tripentyl phosphate (TPeP; 98 %) was purchased from Tokyo Chemical (Tokyo, Japan). Of isotope-labeled internal standards, TnBP-d 27 (99 %) and TPP-d 15 (99 %) were purchased from Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich (St. Louis, MO, USA), respectively. LC-grade methanol, dichloromethane, acetonitrile, formic acid, and ammonium acetate were purchased from Wako Chemicals (Osaka, Japan). Deactivated alumina was prepared by heating basic aluminum oxide (Merck, Darmstadt, Germany) at 130 °C overnight and adding water (6 g) to alumina (94 g). Anhydrous sodium sulfate (Extra pure) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Purified water was delivered by Direct-Q (Millipore, Japan) water purification system.
Chemicals analysis
Analysis of PFRs was performed following the procedure by Stapleton et al. (2009) with slight modification. We excluded tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and tri(1-chloro-2-propyl) phosphate (TCPP) from our target PFRs due to background contamination problem in our lab. To obtain a representative subsample, dust samples were sieved through a stainless steel sieve (500 μm) to remove fibrous material and large pieces in order to obtain a suitable degree of homogeneity. Approximately 0.1 g of house dust sample was accurately weighted and homogenized with anhydrous sodium sulfate, spiked with 10 ng of internal standard for TnBP-d 27 as a surrogate, and extracted using a high-speed solvent extractor (SE-100; Mitsubishi Chemicals, Japan) with a mixture of acetone and hexane (1:1 v/v) at 35 °C at a 6 mL/min flow rate for 1 h. After extraction, the extract was concentrated to 10 mL using a rotary evaporator (EYELA, Japan). Dust extracts were purified by elution through a glass column (200 × 10 mm i.d.) containing 4 g of 6 % deactivated alumina. All analytes were eluted with 50 mL of 100 % dichloromethane. The dichloromethane fraction was then evaporated using a rotary evaporator until about 1 mL, which was then transferred into a 2-mL glass vial and dried under a gentle stream of nitrogen gas. After complete solvent evaporation, the analytes were reconstituted in 1 mL of methanol, and 1 ng of TPP-d 15 was added as an internal standard. Parallel to the samples, a blank was processed as described above for each batch of seven house dust samples.
Seven PFRs were identified and quantified using an UHPLC (UFLC-XR, Shimadzu Corporation, Japan) coupled with a Applied Biosystems API 5500 electrospray triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA, USA). A 10-μL aliquot of extract was injected onto an Asentis Express C18 analytical column (2.7 μm, 100 × 2.1 mm; Supelco, Bellefonte, USA) and separated with 0.1 % (v/v) formic acid in Milli-Q water (A) and 10 mM ammonium acetate in methanol (B) at a flow rate of 0.2 mL/min. The gradient conditions in positive ionization mode were as follows—(A) 80 %, (B) 20 % as initial conditions and held for 2 min, (A) 5 %, (B) 95 % at 3 min and held for 8 min, and (A) 0 %, (B) 100 % at 9 min and held for 13 min. The MS/MS parameter for the instrument was optimized for individual analytes elsewhere (Kim et al. 2011b).
Quality control and quality assurance
Calibration curves, ranging from 0.01 to 10 ng/mL, were established for each compound. Internal standard response factors were used to establish each calibration curve and used for quantification. The correlation coefficients (r 2) of the calibration curves for all the analytes were over 0.99. The recoveries were determined by comparing the measured concentrations in the standard spiked samples with the initial concentrations of the analytes in the unspiked samples. Average recoveries of TnBP-d 27 were 91 % in all the samples analyzed that were spiked with 1 ng of each standard in order to create a spiked concentration that was three times over the background concentration. The recoveries and relative standard deviations (RSD%) given in parentheses for the compounds in house dust samples were 93 % (0.80 %) for EHDPP, 98 % (2.7 %) for TnBP, 78 % (11 %) for TCP, 89 % (0.60 %) for TPeP, 110 % (6.7 %) for TPP, 100 % (9.1 %) for TEHP, and 96 % (5.3 %) for TCEP. Procedural blanks were analyzed for every batch of seven samples using extract of anhydrous sodium sulfate that was subjected through the entire sample treatment procedure. Concentrations determined for analytes in samples were corrected by subtracting procedural blank values quantified for each batch (seven samples). Method detection limits (MDLs) were calculated as three times the standard deviation of background peaks in the procedural blanks. MDLs with RSDs given in parentheses were 0.75 ng/g (2.5 %), 0.58 ng/g (1.9 %), 0.27 ng/g (0.9 %), 0.42 ng/g (1.4 %), 0.42 ng/g (1.3 %), 0.17 ng/g (0.60 %), and 0.44 ng/g (1.5 %) for EHDPP, TnBP, TCP, TPeP, TPP, TEHP, and TCEP, respectively.
Calculation of PFRs intakes
Concentrations of PFRs in house dust samples in the present study were used to estimate the non-dietary exposure levels of adults and toddlers. Two exposure scenarios were assumed: (1) average/mean dust intake rate and (2) high dust intake rate. In addition, a typical human activity pattern was calculated. The house dust ingestion rate (A, nanograms per day) was calculated using the following equation:
where “B dust” is the median (50th percentile: P50) and high concentrations (95th percentile: P95) in house dust (nanograms per gram); “C intake” is the dust ingestion rate (grams per day) of 20 and 50 mg/day as the mean scenario, and 50 and 200 mg/day for the high scenario for adult and toddler, respectively, and “D pattern” is a typical human activity pattern in the home on a daily basis as 64 % and 86 % for adults and toddlers, respectively, proposed by Klepeis et al. (2001).
Statistics
Statistical analyses were performed using the SPSS software (SPSS 12.0 for Windows: SPSS Inc., 2001). Values below the limit of detection were assigned as zero for all statistical analysis and graphic display. Mann–Whitney U test was used to compare the concentration values between the two locations. Spearman’s rank correlation was used to measure the relationship between concentrations of PFRs in house dust samples. Probability values lower than 0.05 were considered as statistically significant.
Results and discussion
PFR levels in house dust
Concentrations of seven PFRs in house dust from residential area (Malate) and municipal dumping area (Payatas) are summarized in Tables 1 and 2, respectively. The detection frequencies of six PFRs, such as EHDPP, TnBP, TCP, TPP, TEHP, and TCEP, were high and ranged from 82 % to 100 % in Malate and from 65 % to 100 % in Payatas, whereas that of TPeP was only about 20 %. These results indicate ubiquitous contamination of PFRs (except TPeP) in Philippine house environments. Similar high detection frequencies (∼70 %) of PFRs (TnBP, TPP, and TCEP) were also reported in indoor dust samples in other studies (Marklund et al. 2003; Stapleton et al. 2009; Meeker and Stapleton 2010; Kanazawa et al. 2010), whereas lower frequencies of TCP were found in house dust from Japan (Kanazawa et al. 2010). Among the seven targeted PFRs, TEHP in Malate and TPP in Payatas were the predominant compounds in house dust at a median concentration of 140 and 71 ng/g, respectively (Tables 1 and 2). TPP is used both as a plasticizer and flame retardant in a variety of applications (plastics, resins, rubber); thus, the high levels detected here could be the result from its use in both applications (Stapleton et al. 2009). Furthermore, the higher concentration of TPP might also be due to adsorption to particles, large past usage, and continuous release into the indoor environment. House dust samples considered for the present study were also analyzed for PBDEs and hexabromocyclododecane (HBCDD) (Malarvannan et al. 2009). When compared with those BFRs, the median levels of ΣPFRs in the present study (530 ng/g in house dust from Malate and 240 ng/g in Payatas, respectively) were higher than those of ΣPBDEs (420 and 100 ng/g, respectively) and ΣHBCDDs (43 and 6.5 ng/g, respectively). Although we did not analyze TDCPP and TCPP, which is another high-production volume PFR, these results suggest higher consumption of PFRs than those of BFRs in the Philippines, which is consistent with their production volumes (BFRs 45,000 t/year and PFRs 91,000 t/year in Europe). Although information on contamination levels of other BFRs and PFRs is needed, contamination by PFRs is ubiquitous in the Philippines. In addition, the relative amounts of PFRs found in dust may reflect not only consumption but also the migration of PFRs from consumer products to ambient air/particulate matter (e.g., dust). Stapleton et al. (2009) reported higher TPP levels in house dust samples (geometric mean, 7,360 ng/g) compared with PBDEs (geometric mean, 4,740 ng/g). Similarly, concentrations of PFRs were also found to be higher than PBDE in indoor air from Tokyo, Japan (Saito et al. 2007). This suggests that exposure to PFRs through dust ingestion is higher than that for BFRs, and so, more attention is needed to identify their potential effects on health and development of toddlers through dust ingestion. To our knowledge, this is the first report on PFR contamination in house dust from a Southeast Asian country.
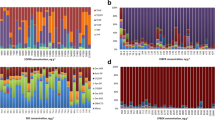
The PFR profiles in the house dust from the Malate and Payatas are illustrated in Fig. 1. Generally, the composition of PFRs was dominated by TPP and accounted for 38 % in Malate and 35 % in Payatas. However, the contributions of the other PFRs were slightly different between the two study areas. For example, in Malate, the second dominant OPFR after TPP was TEHP (22 %) followed by EHDPP (19 % to total PFRs) and EHDPP (25 %) followed by TEHP (18 %) in Payatas. The difference in PFR profiles between locations in the Philippines could be attributed to differences in their sources in the domestic environment. However, it is difficult to identify the specific source of pollutants in indoor dust as houses are furnished and equipped with various products, which contain a variety of chemicals. The types and levels of chemicals that migrated from the consumer products to indoor dust are a function of production process, manufacturers, and year of manufacture. In addition, since the sample size of the present study is small, the results may not representative of the entire country.
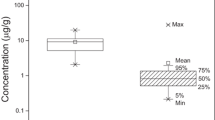
A comparison between concentrations of PFRs in house dust from Malate and Payatas was explored using the Mann–Whitney U test (Fig. 2). A significant difference in median concentrations of ΣPFRs between the Malate and Payatas (p < 0.05) was found. The median of ΣPFRs in dust from Malate (420 ng/g) was twice as high as those in Payatas (190 ng/g). In particular, the levels of EHDPP, TEHP, and TCEP were significantly higher in Malate than in Payatas (p < 0.05). Likewise, EHDPP was three times higher in Malate house dust (median, 110 ng/g) than in dust from Payatas (median, 34 ng/g). Furthermore, the concentration of TCEP in Malate (34 ng/g) was an order of magnitude higher than in Payatas (16 ng/g). On the other hand, levels of TnBP and TPeP were higher in Payatas than in Malate (Fig. 2), though these differences were not statistically significant. This trend is consistent with the results of our previous study, which reported that the levels of PBDEs and HBCDDs in house dust were higher in Malate than those in Payatas (Malarvannan et al. 2009). Although we could not identify any specific reasons from the resident’s questionnaire responses, there could be differences in the use of these compounds between those areas as mentioned above. Further information on the production volume, application patterns, and migration trends of these compounds in household products is needed.
Based on the Spearman’s rank test for correlations, significant positive relationships (p < 0.05) were found among the concentrations of PFRs (Table 3). Statistically significant correlations were found among the concentrations of EHDPP and those for the other compounds including TCP, TPP, and TCEP in Malate (p < 0.05). These correlations between concentrations of individual PFRs in house dust indicate that households in the two study sites may share similar sources of contamination. In the previous study, Takigami et al. (2009) also found positive correlations between TPP and TCEP and between TCP and TPP in indoor dust from Japan.
Global comparison of PFR concentrations in house dust
The levels of PFRs in house dust samples in the present study were compared with those available data in the literature (Table 4). The levels of PFRs in the present study were generally lower than the previous studies reported in United States, Belgium, Sweden, and Japan. For example, a survey of 50 dust samples collected from the Boston, Massachusetts area between 2002 and 2007 reported geometric mean and maximum concentrations of TPP of 7,400 and 1,800,000 ng/g, respectively (Stapleton et al. 2009), which is two to three orders of magnitude higher than the present study conducted in the Philippines (geometric mean, 88 ng/g; maximum, 2,100 ng/g). Although usage and production of PFRs in the Philippins is unknown, it may be generally assumed that the levels of these compounds in house dust are associated with the number, volume, and variety of household products in each home. Van den Eeda et al. (2011) found high concentrations of ΣPFRs (TEP, tris-iso-butyl phosphate: TiBP, TnBP, TCEP, TCPP, tris(2-butoxyethyl) phosphate: TBEP, TPP, TDCPP, and TCP) in house dust samples (n = 33) in the Flemish region of Belgium, ranging from 1,920 to 94,700 ng/g (median, 13,100 ng/g), while it is 21 to 4,300 ng/g (median, 420 ng/g) in the Philippines (present study). Levels of TnBP and TPP in the Philippines dust (median, 19 and 78 ng/g, respectively) were lower than those in Sweden (410 and 920 ng/g, respectively). However, TEHP were higher in the Philippines (median, 75 ng/g) than in Sweden (65 ng/g) (Marklund et al. 2003). The profile of PFRs in dust from the Philippines was different from those from Sweden and Japan (Marklund et al. 2003; Kanazawa et al. 2010). These differences might be due to their variation in the number and types of flame-retarded products (e.g., plastic, printed circuit board, textile, furniture, etc.) utilized in each country.
Exposure to PFRs via dust ingestion
Ingestion of house dust could be an important pathway of exposure for the residents. For instance, the SVOCs with ester bonds, such as PFRs, can be hydrolyzed by lipase in the lung or in the digestive track, allowing the compounds to be absorbed into the human body (Kanazawa et al. 2010). However, information on human exposure to PFRs in the Philippines is not available. Therefore, we estimated the exposure of adults and toddlers to PFRs via house dust ingestion (Table 5). Klepeis et al. (2001) reported that a typical human activity pattern is 64 % and 86 % for adults and toddlers, respectively. Due to insufficient information on human absorption efficiency, we assumed the absorption efficiencies of all PFRs at 100 % (Jones-Otazo et al. 2005; Van den Eede et al. 2011). Exposure to PFRs was calculated for adults and toddlers in two different scenarios of dust ingestion (mean and high dust ingestions) as well as two different scenarios of concentrations in house dust (median and 95th percentile concentrations) to reveal the range of exposures. Mean dust ingestions of 20 and 50 mg/day and high dust ingestion of 50 and 200 mg/day were used for adult and toddler, respectively (Van den Eede et al. 2011). The estimated exposure amounts were then divided by typical body weight of 60 kg for an adult and 15 kg for toddler (2–5 years old) (CDC 2002).
Finally, guideline values of PFRs were set based on available toxicological data. Van den Eede et al. (2011) reported a reference dose of TnBP, TCP, TPP, and TCEP as 2,400, 1,300, 7,000, and 2,200 ng/kg/day, respectively. Based on chronic lowest observed adverse effect level (LOAEL) of TEHP in male and female mice (360 mg/kg/day; WHO 2000) and a safety factor of 10,000 as described by Van den Eede et al. (2011). The guideline values calculated from TnBP, TCP, TPP, TEHP, and TCEP for adult and toddler were 140,000 and 36,000 ng/day, 78,000 and 20,000 ng/day, 420,000 and 110,000 ng/day, 2,100,000 and 540,000 ng/day, and 130,000 and 33,000 ng/day, respectively. Due to a lack of information and high uncertainty regarding dust ingestion and biotransformation, we employed the lowest reported LOAEL and the highest safety factor.
Based on the median (P50) and 95th percentile (P95) concentrations of PFRs measured in house dust samples, we estimated the daily intakes (EDIs) of PFRs by adults and toddlers from the two sites in the Philippines (Table 5). In the present study, the EDIs of each PFR are three to five orders of magnitude lower than guideline values and are lower than those reported in other parts of the world. For example, Van den Eede et al. (2011) estimated a high dietary intake of 6.4, 19.8, 40.7, and 9.6 ng/kg/day for TnBP, TCEP, TPP, and TCP, respectively, for the Belgium population. As such, dust ingestion may be a less important pathway of exposure to PFRs among the general population in both the Philippines and Belgium compared with dietary intake. Furthermore, the total dietary intake of seven PFRs (22 ng/kg/day) in this investigation is also lower than the suggested guideline value of 40,000 ng/kg/day (Sundkvist et al. 2010). However, the EDIs of total PFRs in Malate were 570 ng/day for toddlers and 110 ng/day for adults when high dust ingestion and P95 concentrations were applied. This result implies that toddlers may be at higher risk of exposure to PFRs compared with adults, considering their increased sensitivity during this developmental stage.
Dust ingestion and food intake are relatively important exposure routes for these contaminants. Similar high detection frequencies (<60 %) of PFRs (EHDPP, TnBP, and TEHP) were reported in 58 fish muscle samples from the Philippines (Kim et al. 2011a). This may indicate use of these compounds in the Philippines. Thus, we derived estimates for three exposure scenarios: (1) fish consumption (P50 fish concentrations), and mean dust ingestion (20 mg/day) and normal exposure (P50 dust concentrations) for scenario 1, (2) fish consumption (P50 fish concentrations), and high dust ingestion (50 mg/day) and worst-case exposure (P95 dust concentrations) for scenario 2, and (3) fish consumption (P95 fish concentrations), and high dust ingestion (50 mg/day) and worst-case exposure (P95 dust concentrations) for scenario 3. Daily intake of PFRs by human in the Philippines, through consumption of fish was estimated based on their median (P50) and high (P95) concentrations (wet-weight basis) in fish and per diem fish consumption. The EDIs of EHDPP, TnBP, TCP, TPeP, TPP, and TEHP through fish consumption were 4.9, 44, 0.3, 18, 0.1, and 16 ng/day for the median concentration and 150, 210, 8.4, 91, 48 and 410 ng/day for the high concentration, respectively (Kim et al. 2011a). Based on scenarios 1 and 3, the relative contribution of fish consumption to the total intake of EHDPP, TnBP, TCP, TPeP, TPP, and TEHP except for TPP were higher than that of dust ingestion, while TPP based on the assumptions in scenarios 1 and 3 represented 93 % and 52 %, respectively, of the total intake (Fig. 3). Contributions of EHDPP (78 %), TCP (95 %), TPP (99 %), and TEHP (56 %) to total EDIs for scenario 2 were higher than those of the other scenarios (Fig. 3b), which underscore the significance of house dust ingestion exposure pathways in the daily PFR exposure of the Philippine population. Total human exposure to TnBP, TCP, TPP, and TEHP through fish consumption and house dust ingestion were well below the guideline values. However, due to lack of detailed information on PFRs in other exposure pathways such as milk, various food stuffs and inhalation of air, and dermal absorption parameters, which may also influence body burden, the actual daily intake could not be accurately determined. The available information on the exposure to PFRs through house dust should be supplemented with information on other exposure pathways as part of future research. To our knowledge, estimate on the dietary exposure to PFRs in the Philippines has not been done so far.
Exposure to PFRs through fish and dust (nanograms per day) for adults based on a fish consumption (P50 fish concentrations), and mean dust ingestion (20 mg/day) and normal exposure (P50 dust concentrations) for scenario 1, b fish consumption (P50 fish concentrations), and high dust ingestion (50 mg/day) and worst-case exposure (P95 dust concentrations) for scenario 2, and c fish consumption (P95 fish concentrations), and high dust ingestion (50 mg/day) and worst-case exposure (P95 dust concentrations) for scenario 3
Conclusions
The present study investigated, for the first time in the Philippines, the concentrations of PFRs in house dust samples. The results clearly indicate that PFRs are ubiquitously found in the home environments at these two study sites in the Philippines. High rate of occurrences and concentrations of PFRs, particularly TPP, were found, but their levels were still lower than those in developed countries. The contamination by PFRs in house dust from Malate (median, 550 ng/g) was two times higher than in Payatas (median, 240 ng/g), suggestive of the specific applications of these compounds in Malate. The estimates of the Philippines resident’s exposure to PFRs via dust ingestion were found to be below guideline values. However, intake of total PFRs by toddlers was estimated as five times higher than that for adults, suggesting potential risk for toddlers if PFRs are continuously used in household products because of toddler’s frequent hand-to-mouth contact and tendency to play on floors. Fish consumption, as estimated from literature, was generally the primary source of exposure to PFRs in the Philippines, but, in worst-case exposure, TPP exposure of human was higher through ingestion of dust. Thus, more efforts are needed to understand the behavior of PFRs in house and other microenvironments and overall exposure pathways for the country’s populace.
References
Andresen JA, Grundmann A, Bester K (2004) Organophosphorus flame retardants and plasticisers in surface water. Sci Total Environ 332:155–166
Ayoko GA, Uhde E (2003) Organic compounds adsorbed on particles and settled house dust. In: Morawska L, Salthammer T (eds) Indoor environment: airborne particles and settled dust. WILEY-VCH, Weinheim, pp 147–170
Babich MA (2006) CPSC staff preliminary risk assessment of flame retardant (FR) chemicals in upholstered furniture foam. U.S. Consumer Product Safety Commission. http://www.cpsc.gov/library/foia/foia07/brief/ufurn2.pdf
Beth-Hubner M (1999) Toxicological evaluation and classification of the genotoxic, carcinogenic, reprotoxic and sensitising potential of tris(2-chloroethyl)phosphate. Int Arch Occup Environl Health 72:M17–M23
CDC (2002) Growth charts for 2–5 year old. http://www.cdc.gov/growthcharts/. Accessed 12 April 2011
Chapin RE, George JD, Lamb JC IV (1988) Reproductive toxicity of tricresyl phosphate in a continuous breeding protocol in Swiss (CD-1) mice. Fundam Appl Toxicol 10:344–354
Chapin RE, Phelps JL, Somkuti SG, Heindel JJ, Burka LT (1990) The interaction of Sertoli and Leydig cells in the testicular toxicity of tri-o-cresyl phosphate. Toxicol Appl Pharmacol 104:483–495
COP4 (2009) The 4th meeting of the convention of the parties (COP4) of the Stockholm convention on persistent organic pollutants (POPs). Stockholm Convention on Persistent Organic Pollutants (POPs). http://chm.pops.int/. Accessed 25 April 2011
EEC Directive, European Commission, Regulation (EC) No. 2268/95, 27 September 1995, concerning the second list of priority substances as foreseen under Council Regulation (EEC) No. 793/3, European Chemicals Bureau
EFRA (European Flame Retardants Association) (2006a) Phosphorous–alkyl halogenated phosphate esters.http://www.flameretardants.eu/DocShareNoFrame/docs/3/FDEFAGLBNLDPLEBJDILIMBHJPIB146H7BOHKKK4N1KBO/EFRA/docs/DLS/2HalogenatedPhosphateEstersFactSheetAB-1_00.pdf. Accessed 10 April 2011
EFRA (European Flame Retardants Association) (2006b) Phosphorous–arylphosphates. http://www.flameretardants.eu/DocShareNoFrame/docs/4/FDEFAGLBNLDPLEBJDILIMBHJA4YBYETH6ET9VD63BLVA/EFRA/docs/DLS/3ArylphosphatesFactSheetAB-1_00.pdf. Accessed 20 April 2011
EFRA (European Flame Retardants Association) (2007) Market statistics. http://www.flameretardants.eu/DocShareNoFrame/docs/6/KHAIJIBBHOBJOKBOHNNFGAJL53V443HA4YW3PDB348BT/EFRA/docs/DLS/EFRA_web_11-2007_Market_statistics-1_.pdf. Accessed 10 April 2011
Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B (2005) Is house dust the missing exposure pathway for PBDE? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol 39:5121–5130
Kanazawa A, Saito I, Araki A, Takeda M, Ma M, Saijo Y, Kishi R (2010) Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air 20:72–84
Kim JW, Isobe T, Chang KH, Amano A, Manega RH, Zamora PB, Siringan FP, Tanabe S (2011a) Levels and distribution of organophosphorus flame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ Pollut 159:3653–3659
Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanabe S (2011b) Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A 1218:3511–3520
Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Hern SC, Engelmann WH (2001) The national human activity pattern survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol 11:231–252
Kunisue T, Watanabe W, Iwata H, Subramanian A, Monirith I, Minh TB, Baburajendran R, Tana TS, Viet PH, Prudente M, Tanabe S (2004) Dioxins and related compounds in human breast milk collected around open dumping sites in Asian developing countries: bovine milk as a potential source. Arch Environ Contam Toxicol 47:414–426
Malarvannan G, Isobe T, Sudaryanto A, Takahashi S, Prudente M, Tanabe S (2009) Brominated flame retardants in house dust from the Philippines: levels, profiles and fate. Organohalogen Comp 71:404–407
Marklund A, Andersson B, Haglund P (2003) Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53:1137–1146
Martínez-Carballo E, González-Barreiro C, Sitka A, Scharf S, Gans O (2007) Determination of selected organophosphate esters in the aquatic environment of Austria. Sci Total Environ 388:290–299
Meeker JD, Stapleton HM (2010) House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect 118:318–323
Saito I, Onuki A, Seto H (2007) Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air 17:28–36
Sato T, Watanabe K, Nagase H, Kito H, Niikawa M, Yoshioka Y (1997) Investigation of the hemolytic effects of various organophosphoric acid triesters (OPEs) and their structure-activity relationship. Toxicol Environ Chem 29:277–287
Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF (2009) Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol 43:7490–7495
Sundkvist AM, Olofsson U, Haglund P (2010) Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit 12:943–951
Takigami H, Suzuki G, Hirai Y, Ishikawa Y, Sunami M, Sakai S (2009) Flame retardants in indoor dust and air of a hotel in Japan. Environ Int 35:688–693
Tanabe S (2006) Environmental Specimen Bank in Ehime University (es-BANK), Japan for global monitoring. J Environ Monit 8:782–790
Van den Eede N, Dirtu AC, Neels H, Covaci A (2011) Analytical development and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37:454–461
Wensing M, Uhde E, Salthammer T (2005) Plastics additives in the indoor environment—flame retardants and plasticizers. Sci Total Environ 339:19–40
WHO (1990) Environmental Health Criteria 110. Tricresyl phosphate. Geneva, Switzerland
WHO (1991a) Environmental health criteria 111. Triphenyl phosphate. Geneva, Switzerland
WHO (1991b) Environmental health criteria 112. Tri-n-butyl phosphate. Geneva, Switzerland
WHO (2000) Environmental health criteria 218. Flame retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl) phosphate and tetrakis(hydroxymethyl) phosphonium salts. Geneva, Switzerland
Acknowledgment
Financial support was provided by Grants-in-Aid for Scientific Research (S: 20221003, B: 21310043) and for Young Scientist (B: 23710077) of the Japanese Ministry of Education, Science, Sports, Culture and Technology (MEXT) and Japan Society for the Promotion of Science (JSPS) and the Environment Research and Technology Development Fund (ZRFc-1201) of the Japanese Ministry of the Environment. This research was also supported by MEXT program “Promotion of Environmental Improvement for Independence of Young Researchers” under the Special Coordination Funds for Promoting Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Kim, JW., Isobe, T., Sudaryanto, A. et al. Organophosphorus flame retardants in house dust from the Philippines: occurrence and assessment of human exposure. Environ Sci Pollut Res 20, 812–822 (2013). https://doi.org/10.1007/s11356-012-1237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1237-x