Abstract
There is evidence that over the last 30 years, there have been mass declines in diverse geographic locations among amphibian populations due to disease outbreaks. Multiple causes have been suggested to explain this increase in disease incidence. Among these, climate changes, environmental pollution and reduced water quality are gaining attention. Indeed, some chemicals of environmental concerns are known to alter the immune system. It is possible that exposure to these pollutants could alter the immune system of anurans and render them more susceptible to different pathogens. In this study, we sampled Rana pipiens in five different sites near St. Lawrence River (Quebec, Canada) during the months of July and September in 2001. Two of these sites were protected areas, in which low levels of pesticides were detected, while the remaining three sites were located in areas with intensive corn and soybeans cultivations. Our results demonstrated that frogs living in agricultural regions are smaller in size and weight than frogs living in areas with lower levels of pesticides at both sampling times. Moreover, we have observed a significant decrease in the number of splenocytes (cellularity) and the phagocytic activity in frogs sampled in impacted sites. Taken together, these results suggest that frogs living in agricultural regions might be more vulnerable to infections and diseases through their smaller size and alteration of their immune system. Our results also contribute to the overall discussion on factors involved in amphibian declines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The northern leopard frog Rana pipiens is part of the leopard frog species complex, the most widespread anuran group in North America (Cook 1984). From the mid-1960 to the mid 1980s, populations of the northern leopard frog have declined dramatically over most of its range (Stebbins and Cohen 1995). For example, in the USA in general, there was an approximate 50 % decline in populations during the 1980s (Kagarise Sherman and Morton 1993; Pechmann and Wilbur 1994; Drost and Fellers 1996). In eastern Canada, declines have been less precipitous but commercial catches in Quebec have dropped over the last 25 years (Gilbert et al. 1994). Canada and USA are not the only countries to suffer from declines in frog populations. Indeed, since the 1970s, an important decline in amphibian populations in general has been observed in many countries including Puerto Rico (Burrowes et al. 2004), Northeastern Australia (Czechura and Ingram 1990), Costa Rica, Ecuador and Venezuela (Pounds and Crump 1994; Pounds et al. 1997; Houlahan et al. 2000; Young 2001; Ron et al. 2003). The decline is perceived to be a multifarious problem that is linked to several additive causes (Stuart et al. 2004; Hof et al. 2011; Wake 2012), such as habitat loss (Blaustein et al. 1994; Dunson et al. 1992), UV radiation (Kiesecker and Blaustein 1995; Pounds et al. 2001; Tinsley 1995; Selgrade et al. 1997), climate changes (Pounds et al. 2001), cold (Cooper et al. 1992; Maniero and Carey 1997), introduction of exotic species (Hayes and Jennings 1986), pathogens (Boyer and Grue 1995; Johnson et al., 1999; Carey 2000) and pollution (Carey and Bryant 1995; Rollins-Smith 1998; Carey et al. 1999); all have been hypothesised to explain the decrease in amphibian populations. It is likely though that no single factor is responsible for the decline; each locality may have its own particular cause or causes (Carey and Bryant 1995; Stuart et al. 2004; Hof et al. 2011; Wake 2012).
In numerous regions, the decline of amphibian populations was linked to disease outbreaks caused by different pathogens such as the chytrid fungus (Berger et al. 1998; Crawford et al. 2010; Voyles et al. 2011), irridoviruses (Green et al. 2002) or parasites (Tinsley 1995). It is already well documented that environmental stressors such as pollution can modulate immune responses (Boyer and Grue 1995; Berger et al. 1998; Johnson et al. 1999; Carey et al. 1999; Christin et al. 2003, 2004; Rollins-Smith et al. 2004; Davidson et al. 2007; Gibble and Baer 2011). It is therefore possible that certain environmental agents could cause immunosuppression in amphibians and render them more susceptible to various pathogens.
For the moment, limited data are available on the effects of pesticides and other environmental contaminants on the amphibian immune system. Many studies focussed on the effects of a single pollutant at high doses on the immune system of amphibians (Zettergen et al. 1991; Devillers and Exbrayat 1992; Gromysz-Kalkowaska and Szubartowska 1993; Sharon et al. 1999; Jelaso et al. 1997; Taylor et al. 1999). However, wild animals are usually exposed to low doses of multiple toxic substances at the same time. It is clear from studies with other species that environmental pollutants, including pesticides, can alter the function of the immune system (Sharma and Reddy 1987; Luebke et al. 1997; O'Halloran et al. 1996; Zelikoff et al. 1996; Daszak et al. 1999, 2003). Nevertheless, more recent studies have revealed the immunosuppressive property of various single and combination of pesticides at environmentally relevant concentrations (Gilberston et al. 2003; Hayes et al. 2006, 2010; Shutler and Marcogliese 2011). These xenobiotics could be immunotoxic by acting directly on cells and/or could induce immunosuppression through their action on the neuroendocrine system that, in turn, negatively signals the immune system (Rollins-Smith and Cohen 2004). Modulation of immune functions could further diminish the ability of animals to respond adequately against pathogens (Krzystyniak et al. 1985; Fournier et al. 1988, 2005; Brousseau et al. 2002, 2012). Immunosuppression caused by environmental pollutants could then partly explain the increase of infectious disease and the decline of some amphibian populations.
In previous work, we demonstrated that laboratory exposures to a mixture of pesticides have decreased certain immune functions of two frog species: Xenopus laevis and R. pipiens (Christin et al. 2003, 2004). This mixture of pesticides [atrazine (C8H14C1N5, purity of 98 %), metribuzine (C8H14N4OS, purity of 99 %), endosulfan (C12H6C14O2S, purity of 98.8 %), lindane (C6H6C16, purity of 99.5 %), dieldrin (C12H8C16O, purity of 99.8 %) and aldicarb (C7H14N2O2S, purity of 99 %)] was representative in terms of composition and concentrations, to those found in the southwest region of the province of Quebec (Rondeau 1996; Giroux 1999). The immune functions affected following exposure to pesticides were phagocytosis by splenocytes and the proliferation of lymphocytes from the spleen. Furthermore, alteration of these functions appeared to decrease the ability of R. pipiens to defend themselves against parasites as demonstrated in a host-resistance model with the nematode lungworm Rhabdias ranae (Christin et al. 2003). These data clearly demonstrated that pesticides at environmentally relevant concentrations could affect the health status of amphibians.
In order to verify if laboratory results could be extrapolated to natural populations, we sampled young leopard frogs at three contaminated sites and two reference sites along the margins of the tributaries of the St. Lawrence River, in Montérégie, Quebec, Canada. The area of this region covers about 4,700 km2 and approximately 60 % is used for intensive corn and soybeans cultivations (http://www.mddep.gouv.qc.ca/eau/regions/region16) which requires spreading of large quantities of herbicides and insecticides. Frog length and weight were measured and presented as condition index, which is a classical and simple tool to verify the overall health of animals involved in the study, and viability of splenocytes, cellularity and phagocytosis were performed to determine immunocompetence. These immunological endpoints were selected because they are identified as relevant biomarkers to detect impairment of the immune response, enabling us to identify populations at risk (Brousseau et al. 2012; Brousseau and Fournier 2012).
Material and methods
Sites identification
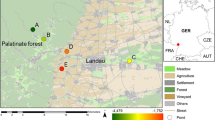
Juvenile R. pipiens were captured at the end of July and at the beginning of September at five different sites near St. Lawrence River in 2001. The first three sites were Fairbanks River in Notre-Dame-du-Mont-Carmel (latitude, 42°29′17″ N/longitude, 72°39′29″ W), Chibouet River in St. Hugues (latitude: 45◦47′59″N/longitude: 72°52′0″ W) and a wetland on Île de la Commune in Boucherville Islands (latitude, 45°36′35″ N/longitude, 73°28′18″ W). These sites were directly adjacent to agricultural land and were exposed to pesticides runoff mainly from nearby cornfields. We also collected animals from two reference sites. They were John Sauro Pond in Venise-en-Québec (latitude, 45°5′4″ N/longitude, 73°9′6″ W) and Le Rocher Park in St-Amable (latitude, 44°39′7″ N/longitude, 73°17′47″ W). The site in Venise en Québec is a protected, relatively pristine, Duck Unlimited, conservation wetland, whereas the site in St-Amable is a wetland within a rural park, with managed landscape and regular human activity nearby (King et al. 2008).
Analysis of physicochemical water parameters
At each site, surface water samples were collected through the summer to monitor concentrations of pesticides during the time of frog metamorphosis as described in King et al. in (2008). In summary, because waterborne concentrations of pesticides can fluctuate considerably with precipitation patterns and the times of application in surrounding crop fields, wetlands exposed to agricultural runoff were sampled repeatedly. Pesticide data for Fairbanks River and Chibouet River were collected as part of a pesticides-monitoring programme of St. Lawrence River tributaries operated by the Ministère du Développement durable, de l'Environnement et des Parcs du Québec. Water samples from these wetlands were collected by the latter department's personnel and sent to the Centre d'expertise d'analyse environnementale du Québec to be analysed for 44 pesticides, including triazines, organophospohorus, carbamates and phenoxyacides, following the methods described in Giroux (2002). For the other three sites (Venise en Québec, St-Amable, Boucherville), water samples were analysed for 13 neutral (uncharged) herbicides by National Laboratory for Environmental Testing at the National Water Research Institute in Burlington (ON, Canada) (King et al. 2008).
Animals, tissue collections and cell suspensions
The methods used in these experiments have been adapted from the Manual of Immunological Methods (Brousseau et al. 1999). Pilot experiments were performed to determine the conditions for each assay prior to the main study.
R. pipiens, young-of-the-year, were collected at each site using dip nets or by hand. Each frog was weighed and measured (snout to vent). Then, animals were sacrificed using a solution of 0.8 % tricaine methanesulfonate (MS-222, Boreal, St. Catherines, ON, Canada) prepared in distilled water. Spleens were removed aseptically and cell suspensions were prepared in complete amphibian L-15 medium (Biomedia, Drummondville, QC, Canada).
Determination of cellularity and viability
An aliquot of each cell suspension was taken and spleen cellularity as well as viability was determined using trypan blue dye exclusion (Gibco Laboratories, Grand Island, NY, USA). Cellularity and viability were determined microscopically. Then, the cell suspensions were adjusted as required for phagocytosis.
Phagocytosis
Escherichia coli K-12 (Molecular probe, Eugene, OR, USA) conjugated to FITC were opsonised by incubating them, for 45 min at 22 °C, with decomplemented frog serum diluted 1:20 with L-15 medium. Spleen cells were adjusted at 1 × 105 cells/500 μL of L15 and mixed with fluorescent bacteria at a ratio of 1:50 (splenocyte/bacteria) and incubated for 1 h at 22 °C. After incubation, 25 μL of trypan blue (0.4 % w/v) was added to each cell suspension in order to quench the fluorescence of bacteria that were not phagocyted (Brousseau et al. 1999). Negative controls were obtained by incubating the cells in the same conditions without bacteria. The fluorescence of engulfed bacteria was analysed by flow cytometry. The fluorescence emission was collected at 520 nm (FL1). A FACScan (Becton Dickinson, San Jose, CA, USA) with an air-cooled argon laser providing an excitation at 488 nm was used. A total of 10,000 events were acquired for each sample. The data were then analysed from a dot plot showing two parameters: size (forward scatter) on the X-axis and complexity (side scatter) on the Y-axis. Using this dot plot, the phagocytic cell population was electronically gated in order to analyse their phagocytic capacity and a fluorescence frequency distribution histogram (FL-1) was obtained, which is a direct representation of the phagocytic activity of splenocytes. Data collection was performed with Lysis II software (version 1.1) and analyses were performed with WinMDI software (version 2.8).
Statistical analysis
Data for all assays were analysed by Kruskal–Wallis ANOVA on ranks. When significant differences were detected at P < 0.05, pairwise multiple comparisons of medians were done using Dunn's Method. The statistical analyses were performed with SigmaStat (version 3.5).
Results
Abiotic water parameters and pesticide concentrations
Temperature, conductivity, pH and nitrate concentrations were recorded in all sites in summer 2001. Nitrates were at least 2 orders of magnitude higher at Chibouet and Fairbanks River compared to the other sites in July. The lowest conductivity was measured in Venise en Québec. The conductivity measured in St-Amable was similar to the results obtained in the other three sites directly adjacent to agricultural land. In addition to atrazine measured in all the sites and metolachlor measured in the three contaminated sites, (Boucherville, Chibouet, Fairbanks), a suite of organophosphorus, carbamates and phenoxyacides pesticides were looked at. Of these, dimethenamide, dicamba, mecoprop, 2,4-D and bentazone were detected at Fairbanks River and deisopropyl-atrazine, dimethenamide, EPTC, bentazone, dicamba, 2,4-D, mecoprop, MCPA, bromoxynil, 2,4-DB and clopyralid were found at Chibouet River but in small concentrations. Trace levels of atrazine and a few other pesticides were detected at both reference sites. All these results were published in Giroux (2002) and King et al. (2008).
Condition index
The results are presented in Figs. 1 and 2 for sampling done in July and September, respectively. For frogs captured in July, there were no significant differences in body indices between both reference sites. However significant decrease in condition index was obtained for each contaminated site versus the reference sites (P ≤ 0.05). For frogs sampled in September, again there were no significant differences in condition index between both reference sites. However, as for July, there was significant decrease for each contaminated sites versus both reference sites (P ≤ 0.05). When growth was evaluated between the two sampling periods (July and September), the animals captured in reference sites had a 1.5-fold increase growth compared to 2-fold increase for animals captured in contaminated sites.
Condition indices (weight/length) of juvenile R. pipiens animals captured in July. Box-whisker plot of condition indices. The horizontal line in each box is the median, the top and bottom of the box represent th 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. Frogs living in agricultural regions had a significantly lower body indices than frogs sampled in reference sites. The highest mean body index was observed in anurans captured in St-Amable while the lowest was observed in frogs captured in Boucherville. There were no significant differences of the body indices between the two reference sites. Results are expressed in grams per centimeter. Significant differences were detected at P < 0.05
Condition indices (weight/length) of juvenile R. pipiens captured in September. Box-whisker plot of condition indices. The horizontal line in each box is the median, the top and bottom of the box represent th 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. Frogs living in agricultural regions had significantly lower body indices than frogs sampled in reference sites. There were no significant differences of body indices between the two reference sites. Results are expressed in grams per centimeter. Significant differences were detected at P < 0.05
Spleen cellularity
The results are presented in Figs. 3 and 4 for sampling done in July and September, respectively. In July, there were no significant differences for cellularity between both reference sites. For frogs sampled in the three contaminated sites, there were significant decreases in the number of splenocytes (P ≤ 0.05). In September, the results are quite different. Indeed, there was a significant difference between the two references sites (P ≤ 0.05) with a number of splenocytes 6-fold higher in Venise en Québec. The results obtained with frogs collected from Boucherville and Chibouet revealed significant decreases in cellularity when compared to Venise en Québec but not when compared to St-Amable (P ≤ 0.05).
Spleen cellularity of juvenile R. pipiens captured in July. Box-whisker plot of spleen cellularity. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. There was no significant difference between the two references sites. On the other hand, cellularity was significantly lower in frogs captured in all contaminated sites. Cellularity results are expressed in number of cells × 106. Significant differences were detected at P < 0.05
Spleen cellularity of juvenile R. pipiens captured in September. Box-whisker plot of spleen cellularity. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile respectively, and the whiskers define the 5th and 95th percentile observations. When compared to Venise en Québec, cellularity was significantly lower in St-Amable, Boucherville and Chibouet River. Cellularity results are expressed in number of cells × 106. Significant differences were detected at P < 0.05
Viability of splenocytes
The results are presented in Figs. 5 and 6 for sampling done in July and September, respectively. For frogs sampled in July, there were no significant differences in viability between both reference sites. For frogs collected in Boucherville and Fairbanks, there were slight but significant decreases in the viability of splenocytes when compared to the results obtained in Venise en Québec (P ≤ 0.05). For frogs captured in September, there were no significant differences between both reference sites as well as contaminated sites. The main point remains the fact that we have obtained viability over 70 % in all the groups of frogs captured in July and over 80 % in all the groups of frogs captured in September. Therefore, these results confirm that all the splenocyte suspensions were suitable to be used in a subsequent phagocytosis assay.
Viability of splenocytes of juvenile R. pipiens captured in July. Box-whisker plot of splenocytes viability. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. There is a slight, but significant decrease in frogs captured in Boucherville and Fairbanks River. However the percentage of viable cells remains higher than 70 % which means that the quality of the cell suspensions remains adequate to perform phagocytosis with these cells. Viability results are expressed in percentage of viable cells: [(living cells/total cell) × 100]
Viability of splenocytes of juvenile R. pipiens captured in September. Box-whisker plot of splenocytes viability. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. Viability of splenocytes was not significantly different between all the groups and always higher than 70 % which means that the quality of the cell suspensions remains adequate to perform phagocytosis with these cells. Viability results are expressed in percentage of viable cells: [(living cells/total cell) × 100]
Phagocytosis
The results are presented in Figs. 7 and 8 for sampling performed in July and September respectively. For frogs sampled in July, there were no significant differences between both reference sites in terms of number of phagocytes. For frogs collected in contaminated sites, there were significant decreases for all the sites except for Fairbanks. For frogs sampled in September, there was a significant difference between both reference sites with a 6-fold less phagocytes in St-Amable. For frogs collected in Boucherville and Chibouet, the phagocytosis was significantly reduced when compared to the results obtained in frogs captured in Venise en Québec.
Absolute number of active phagocytes present in the spleen of juvenile R. pipiens captured in July. Box-whisker plot of active phagocytes. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. Phagocytosis of fluorescent bacteria was not significantly different between the two reference sites. However, phagocytosis was significantly lower for the three contaminated sites when compared to reference sites. Results are expressed in absolute number of active phagocytes [(cells ingesting fluorescent bacteria × total number of splenocytes)/number of gated events]. Significant differences were detected at P < 0.05
Absolute number of active phagocytes present in the spleen of juvenile R. pipiens captured in September. Box-whisker plot of active phagocytes. The horizontal line in each box is the median, the top and bottom of the box represent the 75th and the 25th percentile, respectively, and the whiskers define the 5th and 95th percentile observations. Phagocytosis of fluorescent bacteria was significantly different between the two reference sites with a significant decrease in fogs captured in St-Amable. Phagocytosis of fluorescent bacteria was significantly lower for Boucherville and Chibouet River when compared to results obtained in Venise en Québec. Results are expressed in absolute number of active phagocytes [(cells ingesting fluorescent bacteria × total number of splenocytes)/number of gated events]. Significant differences were detected at P < 0.05
Discussion
Our results indicate that frogs living in sites directly adjacent to agricultural land, and therefore exposed to pesticides runoff, are smaller in size and weight in July as well as in September. Moreover, in these frogs, the spleen cellularity was lower and the phagocytic activity was significantly reduced in July as well as in September. In parallel, we found that frogs living in St-Amable, a wetland with managed landscape and regular human activity nearby, and at the origin considered as a reference site, have a size and weight equivalent to the frogs captured in Venise en Québec, the pristine site of the study at both sampling times. However with the exception of results obtained at Fairbanks, the spleen cellularity and the phagocytosis activity were significantly reduced for the experiment performed in September.
Although referenced but not reproduced in this paper, the abiotic water parameters must be taken into consideration, especially the nitrate concentration and the conductivity which varied from site to site. For conductivity, the lowest measure was obtained in Venise en Québec while the conductivity measured in St-Amable was equivalent to the results obtained in the other three sites directly adjacent to agricultural land with an increase of about 4-fold. It is well documented today that agricultural runoff could lead to increased concentrations of inorganic matter which could be followed by measurement of conductivity (Harwell et al. 2008). As expected, this data confirm the fact that the three agricultural sites were impacted by inorganic matter but also importantly, we must now consider the site located in St-Amable not as clean as expected in order to be qualified as a reference site.
Frogs captured in the three contaminated sites present significant smaller body indices compared to the other two sites. It is tempting to associate this fact with the presence of higher concentrations of pesticides at these sites. It has already been observed that pollutants can reduce tadpoles and amphibian larvae's growth (Larson et al. 1998). Thyroxin is an important thyroid hormone that is involved in amphibian metamorphosis. Following an exposure to atrazine, an increase in corticosterone concentrations was noted in larvae which accelerated metamorphosis by promoting conversion of thyroxin to the more potent triiodothyronine hormone (Larson et al. 1998). Therefore, stress conditions may accelerate metamorphosis. As we observed in our study, in contaminated environments, larvae appear to complete metamorphosis at a smaller size than they would in the absence of contaminants, as the smallest juveniles frogs were captured in contaminated regions. Similar results were obtained with Xenopus tadpoles exposed to a mixture of nine pesticides (Hayes et al. 2006). Metamorphosis of these tadpoles might be faster to hasten escape from a poor environment regardless of the consequences for adult fitness in terrestrial habitat. However, rapid metamorphosis leads to a smaller size which is associated with lower survival and fecundity (Altwegg and Reyer 2003). The smaller size observed in frogs sampled in contaminated regions could be also attributed to other causes such as physiological effects of pesticides on growth itself (Hayes et al. 2006), to food limitation due to poorer food quality in contaminated regions and also to higher larval densities (Smith 1983). We think the latter to be unlikely as a cause of slow growth, as invertebrate productivity was high at all sites sampled. Further studies would be required to understand the way in which these contaminants impair growth.
Frogs living in Venise en Québec have a stronger immune competence than frogs living at the other sites. For both sampling times, the spleen cellularity of these frogs was significantly higher from those measured at the three contaminated sites. A similar pattern was observed for the absolute number of active phagocytes. In parallel we found that the immune status of frogs captured at St-Amable in July was similar to the one obtained in Venise en Québec. However, for frogs collected in September the immune profile of these frogs was similar to the one obtained in contaminated sites. It is well documented, at least in mammals, that spleen mass is a good measure of immune strength (Corbin et al. 2008). Nevertheless, it is interesting to note that immunocompetence increases throughout the summer at all the sites as observed by increasing number of splenocytes as well as phagocytes. These data confirmed that there is maturation of the frog immune system after metamorphosis while there is a shift from the thymus to the spleen as the main haematopoietic organ (Horton et al. 1996; Du Pasquier et al. 1996).
Phagocytosis is a non-specific immune response responsible for the engulfment and destruction of foreign bodies. It constitutes the first line of immunological defence against infectious microorganisms. In addition, phagocytes participate in the elaboration of specific immunity (Janeway et al. 1999). The results of this research have shown that the pesticides used in agricultural sites as well as in an intensively managed park have the potential to suppress the phagocytosis and therefore could interfere in the elaboration of an optimal natural and specific immune response. Indeed, phagocyte disorders often result in recurrent infections of varying severity. We can easily think of patients following a graft, which are under immunosuppressive drugs, and are therefore are at higher risk to develop infections. Animals living at Île de la Commune and Chibouet and Fairbanks Rivers might be at a greater risk to acquire various types of infections because they have a smaller number of active phagocytes. Although, a decrease in the number of phagocytes does not necessarily mean that the efficiency of the remaining phagocytic cells is decreased. Indeed, even if there are a smaller number of cells capable of ingesting fluorescent bacteria, these cells may still keep their ability to destroy foreign bodies by producing reactive oxygen species. In order to determine if this capacity is maintained, specific assays, such as the oxidative burst measuring the production of reactive oxygen species by phagocytic cells should be performed. Moreover, the phagocytosis being the first step in the induction of acquired immunity, we can hypothesise that acquired immunity might be also impaired in the exposed animals (Janeway et al. 1999). In subsequent experiments, humoral and cell-mediated responses must be carefully studied.
The three ponds present in St-Amable are not irrigated by any agricultural sewage. However, the park was intensively managed and numerous human activities were observed around the ponds. The grass was cut weekly around the ponds, motocross used to come every day and bulldozers were even packing the soil at proximity of the ponds. Therefore, animals may be exposed to other immunosuppressive contaminants other than agripesticides. It is also possible that animals living in this park are under stressful conditions which can affect the immune system (Rollins-Smith 2001). Therefore, human activities around the ponds might be partly responsible for the reduction of the phagocytosis observed in these frogs.
It has become increasingly clear that to predict how pollutants may translate into species loss, we must understand the exact effect of pesticides on the immune system. Our results indicate that pesticides can modulate certain aspects of amphibian immune competencies. The interactions between pesticides and the immune system deserve further studies if we want to understand the impact of agricultural practices on aquatic systems.
In summary, by the present study, we have shown that juvenile R. pipiens, which are exposed to agripesticides, are smaller in weight and length when compared to the frogs captured in the reference pristine site. Although the viability of the splenocytes was not altered by the exposure, the number of active phagocytes was significantly reduced in exposed frogs, process by which the frogs could become much more susceptible to infections.
References
Altwegg R, Reyer HU (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Berger L, Spaeare MJ, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonals KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytriomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036
Blaustein AR, Wake DB, Sousa WP (1994) Amphibian declines: judging stability, persistence and susceptibility of population to local and global extinction. Conserv Biol 8:60–71
Boyer R, Grue CE (1995) The need for water quality criteria for frogs. Environ Heal Perspect 103:352–357
Brousseau P, Fournier M (2012) Aquatic immunotoxicology. In: Férard JP, Blaise C (eds) Encyclopedia of aquatic ecotoxicology. Springer, Heidelberg (In press)
Brousseau P, Payette Y, Tryphonas H, Blakley B, Boermans H, Flipo D, Fournier M (1999) Manual of immunological methods. CRC Press, Boca Raton, 160 pp
Brousseau, P., De Guise, S., Voccia, I., Ruby, S., Fournier, M. (2002) Immune status of St. Lawrence beluga whales. In: L. Measures (ed) The St. Lawrence beluga whales. DFO: 381–403
Brousseau P, Pillet S, Frouin H, Gagné F, Auffret M, Fournier M (2012) Linking immunotoxicity and ecotoxicological effects at higher biological levels. In: Amiard-Triquet C, Amiard JC (eds) Ecological biomarkers: indicators of ecotoxicological effects. CRC Press, Boca Raton (In press)
Burrowes PA, Joglar RL, Green DE (2004) Potential causes for amphibian declines in Puerto Rico. Herpetologica 60:141–154
Carey C (2000) Infectious disease and worldwide decline of amphibian's populations with comments on emerging diseases in coral reef organisms and in human. Environ Heal Perspect 108(suppl1):143–150
Carey C, Bryant CJ (1995) Possible interelation among environmental toxicants, amphibian development and decline of the amphibian population. Environ Heal Perspect 103(suppl4):13–17
Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23:459–472
Christin MS, Gendron A, Brousseau P, Ménard L, Marcogliese DJ, Cyr D, Ruby S, Fournier M (2003) Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ Toxicol Chem 22:1127–1133
Christin MS, Ménard L, Gendron AD, Ruby S, Cyr D, Marcogliese DJ, Rollins-Smith L, Fournier M (2004) Effects of agricultural pesticides on the immune system of Xenopus laevis and Rana pipiens. Aquat Toxicol 67:33–43
Cook FR (1984) Introduction to Canadian amphibians and reptiles. National Museum of Canada, Ottawa, 200 pp
Cooper EL, Wright RK, Klempau AE, Smith CT (1992) Hibernation alters the frog immune system. Cryobiology 29:616–631
Corbin E, Vicente J, Martin-Hernando P, Acevedo P, Pérez-Rodriguez P, Gortazar C (2008) Spleen mass as a measure of immune strength in mammals. Mammal Rev 38:108–115
Crawford AJ, Lips KR, Bermingham E (2010) Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proc Natl Acad Sci USA 107:13777–13782
Czechura GV, Ingram GS (1990) Taudactylus diurnus and the case of the disappearing frogs. Mem OM Museum 29:361–365
Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R (1999) Emerging infectious diseases and amphibian population declines. Emerg Infect Dis 5:735–748
Daszak P, Cunningham AA, Hyallt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9:141–150
Davidson C, Benard MF, Shaffer HB, Parker JM, O'Leary C, Conlon JM, Rollins-Smith LA (2007) Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ Sci Technol 41:1771–1776
Devillers, J., Exbrayat, J.M. (1992) Ecotoxicity of chemicals to amphibians. Devillers, J., & Exbrayat, J.M (eds). Gordon and Breach Science Publishers, Philadelphia
Drost CA, Fellers GM (1996) Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada, USA. Conservation Biol 10:415–425
Du Pasquier L, Wilson M, Robert J (1996) The immune system of Xenopus: with special reference to B cell development and immunoglobulin genes. In: Tinsley RC, Kobel HR (eds) Biology of Xenopus. Clarendon, Oxford, pp 301–313
Dunson WA, Wyman RI, Corbett ES (1992) A symposium on amphibian declines and habitat acidification. J Herpetol 26:342–349
Fournier M, Chevalier G, Nadeau D, Trottier B, Krzystyniak K (1988) Virus-pesticide interactions with murine cellular immunity after sublethal exposure to dieldrin and aminocarb. J Toxicol Environ Heal 1:103–118
Fournier M, Blakley B, Brousseau P (2005) Toxicological considerations—making the connection between toxicologic and immunotoxicologic studies as these relate to human health. In: Tryphonas H, Brousseau P, Blakley B, Smits J, Fournier M (eds) Investigative immunotoxicology. Taylor and Francis, Boca Raton, pp 407–421
Gibble RE, Baer KN (2011) Effects of atrazine, agricultural runoff, and selected effluents on antimicrobial activity of skin peptides in Xenopus laevis. Ecotoxicol Environ Saf 74:593–599
Gilbert M, Leclair R Jr, Fortin R (1994) Reproduction of the northern leopard frog (Rana pipiens) in floodplain habitat in the Richelieu River, Quebec, Canada. J Herpetol 28:465–470
Gilbertson M-K, Haffner GD, Drouillard KG, Albert A, Dixon B (2003) Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environ Toxicol Chem 22:101–110
Giroux, I. (1999) Contamination de l'eau par les pesticides dans les regions de culture de maïs et de soya au Québec. Direction des écosystèmes aquatiques, Ministère de l'Environnement, 23 pp
Giroux I (2002) Contamination de l'eau par les pesticides dans les régions de culture de maïs et de soya au Québec, Campagnes d'échantillonnage, Ministère de l'Environnement. Direction du suivi de l'état de l'environnement, Montreal, 45 p
Green DE, Converse KA, Schrader AK (2002) Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann N Y Acad Sci 969:323–339
Gromysz-Kalkowaska K, Szubartowska E (1993) Toxicity of tetrachlorinfos to Rana temporaria. Comparative Biochem Physiol C 105:285–290
Harwell MC, Surratt DD, Barone DB, Aumen NG (2008) Conductivity as a tracer of agricultural and urban runoff to delineate water quality impacts in the northern Everglades. Environ Monit Assess 147:445–462
Haye TB, Falso P, Gallipeau S, Stice M (2010) The cause of global amphibian declines: a developmental endocrinologist'sperspective. J Exp Biol 213:921–933
Hayes MP, Jennings MR (1986) Decline of ranid species in western North America: are bulfrogs (Rana catesbeina) responsible ? J Herpetol 20:490–509
Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, Tsui M (2006) Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect 114:A517–8
Hof C, Araujo MB, Jetz W, Rahbek C (2011) Additive threats from pathogens, climate and land-use change for global diversity. Nature 480:516–519
Horton JD, Horton TL, Ritchie P (1996) Immune system of Xenopus: T cell biology. In: Tinsley RC, Kobel HR (eds) Biology of Xenopus. Clarendon, Oxford, pp 279–299
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404:754–755
Janeway CA, Travers P, Walport M, Capra JD (1999) Immunobiology, the immune system in health and disease 4th edition. Garland Publishing, New York, 635p
Jelaso AM, Mackay D, Ide CF (1997) Methylmercury decreases IL-1 immunoreactivity in the nervous system of the developing frog Xenopus laevis. Neurotoxicology 18:841–850
Johnson PTJ, Lunde KB, Ritchie EG, Launer EA (1999) The effect of trematode infection on amphibian limb development and survivorship. Science 284:802–804
Kagarise Sherman C, Morton ML (1993) Population declines of Yosemite toads in the eastern Sierra Nevada of California. J Herpetol 27:186–198
Kiesecker JM, Blaustein AR (1995) Synergism between UV-B radiation and pathogen magnifies amphibian embryo mortality in nature. Proc Natl Acad Sci USA 92:11049–11052
King KC, Gendron AD, McLaughlin JD, Giroux I, Brousseau P, Cyr D, Ruby S, Fournier M, Marcogliese DJ (2008) Short-term seasonal changes in parasite community structure in Northern leopard froglets (Rana pipiens) inhabiting agricultural wetlands. J Parasitol 94:13–22
Krzystyniak K, Hugo P, Flipo D, Fournier M (1985) Increased susceptibility to mouse hepatitis virus 3 of peritoneal macrophages exposed to dieldrin. Toxicol Appl Pharmacol 80:397–408
Larson DL, McDonnald S, Fivizzani AJ, Newton WE, Hamilton SJ (1998) Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiol Zool 71:671–679
Luebke RW, Hodson PV, Faisal M, Ross PS (1997) Aquatic pollution-induced immunotoxicity in wildlife species. Fundam Appl Toxicol 37:1–15
Maniero GD, Carey C (1997) Changes in selected aspects of immune function in the leopard frog, Rana pipiens, associated with exposure to cold. Comparative Physiol B 167:256–263
O'Halloran K, Ahokas JT, Wright PFA (1996) In vitro responses of fish immune cells to three classes of pesticides. In: Ostrander KG (ed) Ecotoxicology: responses, biomarkers and risk assessment. SOS Publication, Fair Haven, pp 535–538
Pechmann JHK, Wilbur HM (1994) Putting declining amphibian populations in perspective: natural fluctuation and human impact. Herpetologica 50:65–84
Pounds JA, Crump ML (1994) Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conserv Biol 8:72–85
Pounds JA, Fogden MPL, Savage JM, Gorman GC (1997) Tests of null models for amphibian declines on a tropical mountain. Conserv Biol 11:1307–1322
Pounds JA, Fogden MPL, Campbell JH (2001) Biological response to climate change on a tropical mountain. Nature 398:611–615
Rollins-Smith LA (1998) Metamorphosis and the amphibian immune system. Immunol Rev 166:221–230
Rollins-Smith LA (2001) Neuroendocrine-immune system interactions in amphibians. Implications for understanding global amphibian declines. Immunol Res 23:273–280
Rollins-Smith LA, Cohen N (2004) Hormones and the immune system of amphibians. In: Heatwole H (ed) Amphibian biology, vol 6, Endocrinology. Surrey Beatty and Sons, Chipping Norton, pp 2377–2391
Rollins-Smith LA, Hopkins BD, Reinert LK (2004) An amphibian model to test the effects of xenobiotics on development of the hematopoietic system. Environ Toxicol Chem 23:2863–2867
Ron SR, Duellman WE, Coloma LA, Bustamante MR (2003) Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J Herpetol 37:116–126
Rondeau, B. (1996) Pesticides dans les tributaires du fleuve Saint-Laurent 1989–1991. Environnement Canada-Région du Québec, conservation de l'environnement, Centre Saint-Laurent. Rapport scientifique et technique ST 62. Environnement Canada, Montreal, QC, Canada
Selgrade MJ, Repacholi MH, Kore HS (1997) Ultraviolet radiation-induced immune modulation: potential consequences for infection, allergic and autoimmune disease. Environ Heal Perspect 105:332–334
Sharma RP, Reddy V (1987) Toxic effects of chemicals on the immune system, in toxicology. Hemisphere Publishing, Washington, pp 555–591
Sharon KT, Williams ES, Mills KW (1999) Effects of malathion on disease susceptibility in Woodhouse's toads. J Wildl Dis 35:536–541
Shutler D, Marcogliese D (2011) Leukocyte profiles of Northern feopard frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands. Copeia 2:301–307
Smith DC (1983) Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510
Stebbins RC, Cohen NW (1995) A natural history of amphibians. Princeton University Press, Princeton, 316 p
Stuart SN, Chanson JS, Cox NC, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Taylor SK, Williams ES, Mills KW (1999) Effects of malathion on disease susceptibility in Woodhouse's toads. J Wildl Dis 35:536–541
Tinsley RC (1995) Parasitic disease in amphibians: control by the regulation of worm burdens. Parasitology 111:S153–S178
Voyles J, Rosenblum EB, Berger L (2011) Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes Infection 13:25–32
Wake DB (2012) Facing extinction in real time. Science 335:1052–1053
Young BE (2001) Population declines and priorities for amphibian conservation in Latin America. Conserv Biol 15:1213–1223
Zelikoff JT, Wang W, Islam N, Flesher E, Twedok LE (1996) Immune response of fish as biomarkers to predict the health effects of aquatic pollution: application of laboratory assays for field studies. In: Ecotoxicology: responses, biomarkers and risk assessment. SOS Publication, Fair Haven, pp 263–279
Zettergen LD, Boldt BW, Petering DH, Goodrich MS, Weber DN, Zettergen JG (1991) Effect of prolonged low-level cadmium exposure on the tadpole immune system. Toxicol Lett 55:11–19
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Christin, M.S., Ménard, L., Giroux, I. et al. Effects of agricultural pesticides on the health of Rana pipiens frogs sampled from the field. Environ Sci Pollut Res 20, 601–611 (2013). https://doi.org/10.1007/s11356-012-1160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1160-1