Abstract
Amphibians are the most important vulnerable non-target vertebrate group that are affected by pesticides. Most previous studies have confirmed the destructive effects of pesticides. But, so far, no comprehensive studies have been carried out in Iran. Therefore, to estimate the mortality rate of frogs during the growing season in different cultivating systems, we examined the presence of pesticides in water and substrate as indicators of habitat quality and in the liver tissue of Marsh frog Pelophylax ridibundus (Pallas, 1771), enclosed in the prepared cages at five rice paddy fields in Mazandaran province, Iran. The measurement of pollution was done using mass gas chromatography method and statistical analyses by Minitab software. Furthermore, the probable movement pattern of free frogs was analyzed using capture-mark-recapture method. Thirteen pesticides were detected both in the habitat and in frogs’ liver tissue. Among them ß-Mevinphos, Fenitrothion, Bromofos, and Trifluralin had the most frequent occurrence in liver tissue, and Diazinon with concentrations up to 517.8 μg/Kg had the highest concentration. Furthermore, there is a significant correlation (R2 > 0.96) between water quality and frogs’ contamination, whereas, no correlation was observed between substrate pollution and frogs’ contamination. Pesticide concentrations were higher in two stations but lower than lethal doses to frogs, so that no mortality was observed at any of the stations. However, some specimens had a considerable muscle atrophy. Despite no significant movement pattern was detected, we can expect that if this trend continues, in a long term, they will face a reduction in the survival rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural land development is one of the major alteration in nature made by humans over the past century (Matson et al. 1997; Tilman et al. 2002; Green et al. 2005). The conversion of natural habitats to permanent fields and pastures has led to a reduction of 50% of these habitats to be turned into agricultural lands (Green et al. 2005). This habitats destruction is a threat to biodiversity in the present and in the future (Sala et al. 2000; Dirzo and Raven 2003).
Agricultural intensification has led to increased use of insecticides in recent decades (Konstantinou et al. 2006). Insecticides are known to contaminate surface and groundwater and are considered a serious environmental concern in Western Europe (Albanis et al. 1998; Müller et al. 2003). On the other hand, direct exposure to pesticides is a serious risk to health of people who are profoundly exposed to pesticides, especially farmers and rural populations (Elbaz et al. 2009).
In this regard, the use of organophosphate and carbamate pesticides has increased in Iran. Hence, monitoring of these pesticides has increasingly become important as a necessary challenge to increase food safety (Dehghani et al. 2017; Sharifzadeh et al. 2018). Studies indicate that about one-third of agricultural pesticides are consumed in north of Iran, resulting in the fact that farmers in Mazandaran Province are exposed to pesticide hazards 30 times more than other farmers in other areas (Pishgar-Komleh et al. 2011). Weak management in planning schedules for spraying and farmers’ lack of awareness about adverse effects of chemical pesticides have led to an increase in environmental pollution, especially in aquatic ecosystems and aquatic resources (Coronado et al. 2004).
These pesticides, through mechanisms such as inactivating cholinesterase enzyme and causing genetic mutations, can lead to impaired central nervous system function (Dehghani et al. 2011). So, using organochlorine pesticides is banned in many countries since they are highly toxic and have adverse health effects (Rezaeigolestani and Hashemi 2018). In spite of the many mentioned disadvantages of chemical pesticides, it is worth noting that using pesticides is a suitable approach to increase the agricultural and horticultural productivity by minimizing the detrimental effects of pests and weeds (Lichtenberg 2013).
On the other hand, using insecticides to control insect larvae in rice paddy fields can be harmful for non-target vertebrates and invertebrates (Subrero et al. 2019). Some characteristics of amphibians such as the existence of two stages in their lives, permeable skin, and their susceptibility to absorb materials increase the amphibian vulnerability to chemical compounds in compare to other vertebrates (Stuart et al. 2004; Brühl et al. 2013). The effect of organophosphorus pesticides on frogs’ hepatocytes has been studied in several researches (Ezemonye and Tongo 2010; Ghasemzadeh et al. 2015). Hegde and Krishnamurthy (2014) have demonstrated that in organic rice paddy fields which has no chemical contamination, there is a positive correlation between chemical pesticides presence in rice paddy fields and frogs’ health. So, increased contamination of rice paddies is corresponded to a decrease of frogs’ health and the levels of acetylcholinesterase enzymes and also deformity of frog (Hegde and Krishnamurthy 2014). The study by Tongo et al. and colleagues showed that the accumulation of Diazinon in African common toad (Bufo regularis) liver tissue is more than serum and other tissues of brain, lung, and digestive system, which results in important changes in the biochemical indices of various animal tissues (Tongo et al. 2012). Although the liver plays an essential role in animals’ detoxification, Diazinon has the ability to damage cells of vital liver organs, which can disrupt the function of the liver tissue (Kappers et al. 2001; Sams et al. 2003; Tongo et al. 2012).
The effects of pesticides on the global decline of amphibian populations has also been remarked (Fellers et al. 2004; Davidson and Knapp 2007; Collins and Crump 2009). Pesticides have been shown to increase mortality rates of amphibians (Blaustein et al. 2003; Carey and Alexander 2003; Collins and Storfer 2003; Jensen and Camp 2003; Morehouse et al. 2003; Knapp 2005). In other studies, several side effects have been identified during the developmental stages, including delaying in metamorphosis, growth disturbance, and malformation (Sparling et al. 2001; Greulich and Pflugmacher 2003; Davidson 2004; Fellers et al. 2004; Davidson and Knapp 2007). Also, in other studies on samples of adult individuals, behavioral changes and occurrence of certain diseases have been reported (Taylor et al. 1999).
However, regarding on in situ analyses conducted in agro ecosystems with their test species, the marsh frog Pelophylax ridibundus (Pallas, 1771), and proving that despite the damaging impact of the pesticides on their various morpho-physiological and life history parameters, this frog species adapts and survives in rice fields (Zhelev et al. 2017, 2018). Also, this frog species is quite studies in Eurasia, and there is conclusive evidence that it is very suitable bioindicator species for anthropogenic pollution (Zaripova and Fayzulin 2012; Stakh et al. 2017; Snegin and Barkhatov 2019; Zhelev et al. 2019).
Meanwhile, no comprehensive studies have been carried out in the paddy fields of Iran. In the present study, the effects of conventional pesticides on northern rice paddy fields have been investigated on non-target species, Marsh frog Pelophylax ridibundus (Pallas, 1771), and its specific objectives include: (1) to determine water and substrate quality and the relationship between habitat quality and the rate of accumulated pollution in the frogs’ bodies due to different cultivating systems; (2) the population census of frogs in two different cultivating systems and their movement pattern; and (3) to estimate the mortality rate of frog during the growing season in different cultivating systems.
Material and methods
Study area
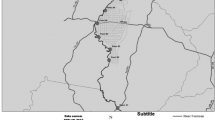
This research was carried out in the summer 2018, in the paddy fields of two districts in cities of Neka and Kiakola in Mazandaran Province. In Neka (36° 43' 40.02"N, 53° 19' 9.49"E), four stations were selected, each in different cultivating system. The stations included A1, A2, A3, and A4 with approximate area of 5000, 6000, 6000, and 2700 m2, respectively. All four stations were selected in the same region in adjacent lands, with the same climatic and agronomic conditions, with a minimum distance of approximately 250 m from each other. The fifth station (K) which was treated as a control station is located in Kiakola city (36° 31' 59.85"N, 52° 45' 29.21"E) with an approximate area of 11,000 m2 (Fig. 1).
This farm has a certificate of European Union standards of organic rice production with certificate number of BINT-3836, which shows that no chemical pesticide or fertilizer has been used for more than 15 years. At the beginning of the growing season, two cages of 2 × 1 m were adjacently located at each of these stations. The cages were made with stainless metal frames, and each of the cages’ six sides was covered with plastic net. The net apertures were large in so far as the water flow, and entry of insects was possible, while the entry and exit of any other animal were impossible. In order to compensate for the decreased of entering insects as food availability due to the enclosure of cages, a 1.5-V fluorescent lamp was used in each cage to attract the insects. The thermoregulation for specimens is provided in the enclosed spaces by setting up an artificial beach in each cage (Dodd 2010) (Fig. 2).
After placing the cages, transplanting of rice was performed within them. After that, fifteen healthy adult frogs (Snout-Vent length, SVL > 60 mm) (Bannikov et al. 1977) were placed in each of these cages. According to different time of transplanting between two regions, frogs were placed into A1-A4 and K cages in May 17 and April 30, respectively. In both regions, frogs were present in the cages for 70 days. Also, in order to compare the amount of pesticides used in farms with the measurement results at the end of the agricultural season, farmers from all five stations were asked to provide information on the type and dose of pesticides they used.
Sampling
The frogs were transported to the cages from a suitable location away from contaminated areas where they were not exposed to agricultural activities. Their sizes and weights were almost equal to each other. Capturing was done using hand net mesh and transporting to cages with latex gloves (Vogiatzis and Loumbourdis 1997; Khan et al. 2003; Christin et al. 2013).
In the end of growing season, specimens of each cage were transported using latex gloves to separate labeled steel boxes containing ice and sent to the laboratory. Then, the frogs’ liver tissues were taken and kept in sterile vials at a temperature of − 20 °C until they were extracted (Kittusamy et al. 2014). Subsequently, stomach contents were examined under stereomicroscope by cutting directly of stomach.
Three different samples (Isworo et al. 2015) were collected to measure the amount of pesticides in water and substrate at each station. Mud samples were removed from the depth of 5 cm near the water sampling locations. The samples were put in separate labeled glass containers and transferred to the laboratory in a case containing ice and maintained at a temperature of − 4 °C until the extraction (Isworo et al. 2015).
Extracting and measuring pesticides
Water samples
pH measured using litmus where necessary was neutralized using sulfuric acid or sodium hydroxide. The samples were then transferred in flasks under cold conditions (6 °C) to the laboratory, and there they were immediately transferred to a refrigerator with a temperature of 6 °C and stored up to 7 days before extraction. Extraction of toxic compounds from water was carried out using liquid–liquid extraction (LLE) approach and separator funnel, according to EPA 3510 (Edgell and Wesselman 1989; Vo 1992).
The extracted specimens were then set up to be concentrated at 15 ml using rotary concentration. Then, it was poured with sodium sulfate into a graduated cylinder. A gentle flow of dry nitrogen caused extra solvent to evaporate and the extracted material volume to be reached at 1 ml. The samples were stored in the refrigerator until performing gas chromatography (Edgell and Wesselman 1989; Hassan et al. 2010).
Substrate and frogs’ liver tissue samples
The extraction of toxic compounds from substrate samples and frogs’ liver tissue by hexane/acetone (1/1) was performed according to EPA 3550 with ultrasonic method. Then, the extracted samples were concentrated and cleaned. Cleaning was carried out using the methods 3610B: Alumina and the 3630C: Silica Gel. Until the stage of gas chromatography, the samples were stored in the (− 20 °C) refrigerator (Hollen and Beugelsdijk 1992; Kittusamy et al. 2014).
Providing standard solutions
Organophosphorus Pesticides Mixture 200 mg/l (Ultra Scientific) was used to prepare standard solutions. Standard solutions were prepared in hexane or iso-octane and kept in the refrigerator away from any source of light. The calibration curve was drawn using these standards at five different concentrations (Pesticides 2007).
Gas chromatography
The compounds, which were extracted by a 6890 N Agilent gas chromatograph and paired with a selective mass analyzer 5975C detector, were analyzed. For this purpose, 2 μl of the sample in splitless mode at 28 °C was injected into a HP-5MS capillary, with size of 30 m × 0.25 mm × 0.25 μm (respectively, length, diameter, and film thickness of the capillary) and controlled by the MSD Chemstation software (Agilent Tecnologies, Inc).
The temperature schedule for chromatographic separation was as follows: first, the column temperature was raised to 60 °C, and the column was held at this temperature for 5 min; then, at every minute, the temperature was increased by 5 °C until the column reached 280 °C; and it was kept at this temperature for 6 min. The carrier gas in this operation was a neutral helium gas (purity greater than 99.99%) that was used in a constant flow of 1 ml/min. GC peaks were identified based on retention time relative to the well individual standard (Pesticides 2007).
Population estimation
Capture-mark-recapture method was used to estimate the population size of free frogs and detect of probably movement pattern during farming season (Seber and Schwarz 2002; Amstrup et al. 2010). Two regions were selected with different conditions: “Neka” area which is spraying frequently during farming season and “Kiakola” paddy field with organic conditions.
Capture-mark-recapture surveys were carried out by a two-person team during eight nights between sunset (20:30) and midnight (24:00). The path was randomly selected, and agricultural boundaries were considered as transects. We searched for frogs using headlamp light, captured them manually, and marked them using an individual toe-clipping mark (Donnelly et al. 1994; Ramalho et al. 2013). The toes were disinfected with a solution of Bactine (Martin and Hong 1991) and were released them near to the point of capture.
To avoid of metamorphosis effect on population size estimation, the first capture was done a few weeks after the end of metamorphosis phase at the beginning of summer 2018. Additionally, to counteract the effect of cannibalism, the young individual that may swallow by huge adults was not counted. In each area population, estimating was done once before spraying time and once after that. In fact, “Capture 1” was done 2 weeks before spraying time, and “Recapture 1” was done 1 week later (1 week before spraying). On the other hand, 1 week after spraying time, “Recapture 2” was done in the “Neka” area, and 2 weeks after the “Recapture 1” in the unsprayed “Kiakola” area. Eventually, “Recapture 3” was done 1 week after “Recapture 2” in both areas. In each recapturing, all of the captured frogs (either marked or unmarked) were marked by a new toe-clipping mark, and the number of observations of each frog was recorded.
Statistical analysis
Pesticide contamination data were analyzed by ANOVA at a significant level of 95% (P < 0.05). In addition, two post-hoc tests of Fisher’s least significant differences and Duncan’s multiple range tests were performed for a more accurate comparison. These analyzes were performed using MINITAB® 18.1. Moreover, data from population estimates were analyzed by the open source programming environment R (R Development Core Team 2015), using two mentioned methods (Chapman 1951; Williams et al. 2002).
Results
Farmers’ statements
Six pesticides (one fungicide Tricyclazole; two insecticides Fipronil and Diazinon; and two herbicides Butachlor and Bentazone) belong to five substance group were used. The information of frequency and dozes in each of the five stations is given in Table 1. Farmers of two stations K and A3 were not used any pesticides. However, six pesticides were used in station A1, and all of them except Fipronil were used in both A2 and A4.
Results of pesticides measurements in water samples
Thirteen pesticides are detected in collected samples from the five stations with concentrations up to 26.8 μg/L (Table 2). Among the pesticides, 10 were lower than the limit of quantification (< LOQ), and Diazinon (26.8 μg/L) had the highest concentration compared to other pesticides. Although in the two stations K and A3 the values of all pesticides were < LOQ, in the remaining two stations, ß-Mevinphos and Diazinon, had the most frequent occurrence in water. Based on the results of water quality analysis, it was determined that regardless of the stations, the occurrence of pesticide type in water is significant. Based on the results of Fisher and Duncan post-hoc tests, Diazinon was in a separate group (group A) compared to other pesticides (group B). On the other hand, to understand whether there is a significant difference in overall pollution levels of water quality among different cultivating systems, total concentrations of pesticides in each station were compared. Based on these results, it was found that, regardless of the type of pesticide, the occurrence of total pollution among different cultivating systems is significant. Based on Fisher and Duncan post-hoc tests, the station A4 was categorized separately in group (A), and other stations were placed in group (B). Finally, to understand whether there is a significant difference in the water quality of different cultivating systems based on the type of pesticide, pesticide concentrations in each sample of each station were compared with each other. The results showed that due to the type of pesticide, differences in water quality of different cultivating systems are significant. According to these results, there was a sever contamination pertaining to Diazinon in the water of two stations A2 and A4.
Results of pesticides measurement in substrate samples
Thirteen pesticides are detected in collected samples from the five stations with concentrations up to 292.8 μg/Kg (Table 2). Among the pesticides, the levels of Butachlor (herbicide), Endosulfan, and Fenthion (both insecticides) were < LOQ, but Chlorpyrifos (292.8 μg/Kg) had the highest concentration in compare to other pesticides. Insecticides of ß-Mevinphos and Chlorfenvinphos and Trifluralin (herbicide) had the most frequent occurrence in substrate. Based on the results of substrate quality analysis, it was found that regardless of the stations, the occurrence of pesticide type in substrate is significant. Based on Fisher and Duncan post-hoc tests, Chlorpyrifos was in a separate group (group A) compared to other pesticides (group B). On the other hand, to understand whether there is a significant difference in overall pollution levels of the substrate quality among different cultivating systems, total concentrations of pesticides in each station were compared. Based on these results, it was found that regardless of the type of pesticide, the occurrence of total pollution among different cultivating systems is significant. Based on Fisher and Duncan tests, station A1 was categorized separately in group (A) and other stations placed in group (B). Finally, to understand whether there is a significant difference in the substrate quality of different cultivating systems based on the type of pesticide, pesticide concentrations in each sample of each station were compared with each other. The results showed that the type of pesticide can cause significant differences in the quality of the substrate in different cultivating systems. Based on these results, there was a sever contamination pertaining to Chlorpyrifos in the substrate of station A1.
Results of pesticide measurement in frogs’ liver tissue samples
Thirteen pesticides are detected in frogs’ liver tissue samples collected from five stations with concentrations up to 517.8 μg/Kg (Table 2). Among them, the amounts of the three pesticides of Butachlor, Endosulfan, and Fenitrothion were < LOQ. Insecticides ß-Mevinphos, Fenitrothion, and Bromofos and Trifluralin (herbicide) had the most frequent occurrence in frogs’ liver tissue. Meanwhile, Diazinon (517.8 μg/Kg) had the highest concentration relative to other pesticides. Based on the results of the analysis related to pesticides occurrence in the frogs’ liver tissue, it was ascertained that regardless of the stations, the occurrence of pesticide type in the frog’s liver tissue is significant. Based on the results of Fisher and Duncan post-hoc tests, Diazinon was in separate group (group A) compared to other pesticides (group B). On the other hand, to understand whether there is a significant difference in overall contamination levels of frogs’ liver tissue among different cultivating systems, total concentrations of pesticides in each station were compared. The results showed that regardless of the type of pesticide, the occurrence of total contamination among different cultivating systems is significant. In order to categorize the stations, Fisher and Duncan methods were used. The station A4 with the highest concentration of pollution was placed in group (A), the stations A1, K, and A3 with the lowest pollution were placed in group (B). However, the station A2 was not categorized separately in any of the two groups. In the end, to understand whether there is a significant difference in the contamination of frogs’ liver tissue of different cultivating systems with regard to the type of pesticide, pesticide concentrations in each sample of each station were compared with each other. The results showed that according to the type of pesticide, the occurrence of pollution among different cultivating systems is significant. Based on these results, there was a drastic contamination concerning Diazinon in the frogs’ liver tissue of the two stations A2 and A4.
Additional Pearson correlation analysis indicates that there is a significant correlation (R2 > 0.96) between water quality and concentration of pesticides in the frogs’ liver tissue, whereas no significant correlation observes between substrate pollution and concentrations of pesticides in the frogs’ liver tissue (Fig. 3).
Detection of illegal used pesticides
The present study showed that 10 types of 13 detected pesticides are not commonly used in rice cultivation: among these pesticides, Trans-Chlorfenvinphos, cis-Chlorfenvinphos, Parathion, Fenthion, Endosulfan, and ß-Mevinphos are those which have been banned by Iran’s Ministry of Agriculture Jihad mandate for the past 8–30 years. However, the results of this study and consultation with some pesticide vendors revealed that these pesticides are likely to be illegally available to farmers without them knowing and moreover, being directly sprayed along with other four pesticides Trifluralin, Malathion, Chlorpyrifos, and Bromofos, which are commonly used in fruit and vegetable gardens, can enter the rice fields through leaching, runoff, and climatic flows (Kreuger 1999).
Population estimation
The results of population size estimate analysis between two areas are indicated that the size of population increased (P < 0.05) after spraying stage at both areas (Table 3) despite the fact that no spraying was done at the “Kiakola” organic paddy field. This results also showed that some frogs have stayed constantly on the farm and have not abandoned it, even during or after spraying time (P < 0.05).
Discussion
Relation between habitat quality and affected frogs
Among the six pesticides used by farmers, there was no evidence of three Bentazone herbicide, Tricyclazole fungicide, and Fipronil insecticide in water, substrate, and frogs’ liver tissue samples. Since sampling of substrate and water was done in the latest growing season stage of possible, so that the spraying act can be included, identification of the aforementioned pesticides was not possible due to their persistence. According to International Union of Pure and Applied Chemistry (IUPAC), all three of these pesticides showed high degree of persistence in European and American ecosystems. However, according to Iran Plant Protection Organization, the persistence of these pesticides in north ecosystems of Iran is low (less than 30 days and even faster). This difference can be due to variability in the composition, type, and pH of substrates as well as the presence of microorganisms facilitating the decomposition process.
Based on the comparison of farmers’ statements to the observations of the present study, it was discovered that no chemical pesticide was used in the two farms A3 and K. These results are consistent with pesticide measurements in the water samples of these two stations, meanwhile are contradicted by the observed pollution in samples of substrate and frogs’ liver tissue. Regarding the isolation of these farms in terms of accessing to water resources, this can only be done by wind transferring pesticides from the surrounding farms. Also, pesticide persistence in water is less than in substrate (Akerblom 2004). On the other hand, it has been shown that bioaccumulation occurs in substrate microorganisms (Schäfer et al. 2011). So, detecting the higher values of pesticides in substrate rather than in water is not surprising. As well, the same pattern of difference in pesticides amounts was observed at other stations. However, in general, these two stations provide healthier environments than the others. The results of this study indicated that station K, which have been managed under an organic cultivation system with no chemical pesticides for 15 years, showed the lowest amount of pesticides detected in the frogs’ liver tissue compared to other stations. This indicates that there are significant differences between the usage of pesticides in organic farms and the accumulation of contamination in the frogs’ liver tissue and is consistent with the observations of other studies (Hegde and Krishnamurthy 2014).
Among the 6 used pesticides, the concentration and frequency of the three pesticides, Diazinon, Fenitrothion, and Butachlor, corresponded to the observations from habitat and the frogs’ liver tissue. Butachlor is used at approximately the similar concentration and only in one repeat. But the observed values in the habitat and frogs’ liver tissue were < LOQ. Considering the sampling time, this can be due to the speed of biodegrading the pesticide in this environment. According to IUPAC, Butachlor can be biodegraded in environment within 4 to 18 days. Also, Fenitrothion has been used with the similar repeat rate (twice) and approximately the same concentration. However, the observed values in the habitat (except station A3) and frogs’ liver tissue were < LOQ. According to IUPAC, Fenitrothion insecticide can be degraded in water and water sediment in 1–14 days and in less than 30 days, respectively, which justifies the values < LOQ.
The highest significant concentration in water is related to Diazinon insecticide, which is agreed with our observation about frequent usage of it by farmers of non-organic farms. On the other hand, in agreement with previous studies (e.g., Hegde and Krishnamurthy 2014), there is a significant correlation between water quality and concentration of pesticides in the frogs’ liver tissue. However, no correlation was observed between substrate pollution and frogs’ contamination. These results are more consistent with the high permeability of amphibian skin to water (Fryday and Thompson 2012; Christin et al. 2013; Cothran et al. 2013).
Toxicological effects
The effect of insecticides on vertebrates is clearly destructive. The annual mortality rate of non-target birds and small terrestrial vertebrates resulted from some pesticides cannot remain unnoticed, even if such casualties do not reduce the populations for a long term as a result of compensatory effects (Forbes et al. 2001). The relationship between individual and population responses is not clearly obvious, since population-level impacts may be influenced by density-dependent compensatory responses (Forbes et al. 2001; Schmidt 2004). A study demonstrated that pesticides can interact with key processes within aquatic communities and affect the quality of living organisms’ survival (Piha 2006; Benton et al. 2003).
Based on the initial hypotheses, it was expected that a number of frogs enclosed in the cages would die due to exposure to the pesticides used in the farms. However, no mortality was observed at any of the stations. Cothran et al. (2013) found ascertained that survival rates in amphibians near agricultural areas are higher than amphibians far beyond these areas. According to their results, frogs near the farms had more resistance to Chlorpyrifos, while they did not show significant resistance to roundup herbicide. Yet, studies on carbaryl insecticides verified that the amphibian resistance to pesticides was accompanied with a reduction in fitness, and this resistance was unequal among different species, populations, and individuals (Bridges and Semlitsch 2000; Semlitsch et al. 2000; Bridges et al. 2001). More than half of our studied frogs had varying degrees of muscles atrophy which was more pronounced in A2 and A4 stations. By examining the stomach contents of each individual, it was found that feeding was took place normally. Therefore, this weight loss can be due to exposure to pesticides and not having the possibility of moving from contaminated habitat during spraying. In a normal condition, the energy reserves are depleted in the end of breeding season. Amphibian energy reserves and body condition are connected to their reproductive cycle. Therefore, energy reserves are decreased during breeding season in spring and have the lowest level in early summer (Pider et al. 1992; Brodeur et al. 2020). Cothran et al. (2013) did not find evidence of functional costs when facing with competitors or frightening of being hunted in populations adjacent to the farmland. However, we observe free specimens that they had a considerable muscle atrophy, as well as enclosed specimens in the cages, and suffer severe physical weakness (Fig. 4). These frogs when facing with the collector, they tried to escape, however they were easily caught due to muscle weakness. Additionally, there were specimens that had deformity in motor organs (Fig. 4). Limb deformation has been seen in amphibians living on agricultural fields, where regeneration and growth of frog larvae occur simultaneously with the peak of pesticide usage (Taylor et al. 2005; Fryday and Thompson 2012).
Escape from the polluted areas
In order to investigate the relationship between probable movement pattern of free frogs and spraying, population estimate was made in two regions before and after spraying. Based on the initial hypothesis, it was assumed that the population at stations A1 and A2 would decrease, and the frogs were expected to escape while exposing to chemical pesticides (Dodd 2010). It was also believed that the population size at the station K would be constant. But the results of population estimate indicated an increase in population size at both stations. In a certain distance from the station K, there are rice fields and fruit gardens under the common cultivation system, and in terms of water resources, there is no connection between these fields and gardens and the station K. However, frogs can move freely between fields and gardens. Hence, it is possible to imply that the increased population size at station K was due to the fact that during the spraying period, frogs took shelter in a safe and non-polluted location, station K, from the surrounding fields and gardens. In comparison with station K, stations A1 and A2 faced with a slight increase in population which is probably caused by spray contamination in A1, A2, and the surrounding fields. Considering the fact that spraying in adjacent fields at each stage was conducted almost a week after it was done in the studied farm, the increase in population size at this station could be due to the movement of frogs from other fields during spraying. However, owing to contamination resulted from repeated spraying in the studied field, it cannot be compared with the station K.
It is known that frogs usually have a small home range and often move less than 700 m in crops. It has been observed that large populations of some species are present at the time of using toxic materials and only carry out short movements (Seitz et al. 1992; Tramontano 1997; Hachtel et al. 2005; Kovar et al. 2009). At both stations, a number of frogs did not leave the farm, which, in contaminated farms, could be a result of the frogs’ resistance to pollution and in the organic station, be due to the species preference in staying in a healthier habitat. Actually, this species is highly tolerant to anthropogenic stressors, including pesticides. Zhelev et al. (2017, 2018) were found that the population of P. ridibundus (Pallas, 1771) which inhabited in polluted area have a different hematological parameters of erythrocyte count (RBC), leucocyte count (WBC), hemoglobin concentration (Hb), and packed cell volume (PVC). It can be concluded that frogs took refuge in safe or less polluted areas during the operation of spraying, and this movement leads to a high population density over a short term and a reduction in food supplies and a need for movement back into contaminated areas. Because of each frog had a unique mark (number), we were able to determine the movement pattern of some of them explained above. In fact, contamination of habitat cannot prevent the movement of frogs to make use of food sources. However, it should be noted that population size variation may be related to many complex factors. For instance, due to fluctuations in available food resources, density-dependent factors that affect population size may influence every stressing element in the population such as toxins and negatively affect the survival of infants, immature, and adolescents. To uncover all these complexities, it is necessary to estimate the size of the population in a longer period of time (Gardner 2001; Mann et al. 2009). In fact, the ecological realism in case of in situ analyses in amphibian populations inhabiting environments exposed to anthropogenic stress is often quite different from the one in a controlled environment (in vitro, ex situ, etc., analyses), namely because of the synergic (and/or antagonistic) interactions between the toxicants and the abiotic environment factors (Burraco and Gomez-Mestre 2016; Davis et al. 2017; Gonçalves et al. 2019; Zhelev et al. 2020).
Conclusion
The present study showed that there is a correlation between the concentration and frequency of spraying and the severity of contamination in non-target species of Marsh frog. Therefore, we can expect that if this trend continues in these areas, we will confront a reduction in the survival rate and reproduction success of this species in a long term, while mortality rate is increasing. The results of this study, in consistent with earlier researches, indicate that the survival of amphibians in the north of Iran is at risk; many species, such as salamanders, are now endangered; and environmental contamination accelerates this trend (https://www.iucnredlist.org/). Many Iranian farmers are dependent on excessive use of toxic and sometimes illegal pesticides, which leads workers and consumers of the agricultural products being put in danger.
References
Akerblom N (2004) Agricultural pesticide toxicity to aquatic organisms: a literature review. Research report no. 16 of Department of Environmental Assessmen, Swedish University of Agricultural Sciences, Sweden
Albanis TA, Hela DG, Sakellarides TM, Konstantinou IK (1998) Monitoring of pesticide residues and their metabolites in surface and underground waters of Imathia (N. Greece) by means of solid-phase extraction disks and gas chromatography. J Chromatogr A 823(1–2):59–71. https://doi.org/10.1016/S0021-9673(98)00304-5
Amstrup SC, McDonald TL, Manly BFJ (2010) Handbook of capture-recapture analysis. Princeton University Press
Bannikov AG, Darevskii IS, Ishtenko VG, Rustamov AK, Shterbak NN (1977) A guide to the amphibians and reptiles of the USSR. Prosveshtenie, Moscow (In Russian)
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18(4):182–188. https://doi.org/10.1016/S0169-5347(03)00011-9
Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC (2003) Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers Distrib 9(2):123–140. https://doi.org/10.1046/j.1472-4642.2003.00015.x
Bridges C, Semlitsch R (2000) Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conserv Biol 14(5):1490–1499. https://doi.org/10.1046/j.1523-1739.2000.99343.x
Bridges CM, Semlitsch RD, Price A (2001) Genetic variation in insecticide tolerance in a population of southern leopard frogs (Rana sphenocephala): implications for amphibian conservation. Copeia 2001(1):7–13. https://doi.org/10.1643/0045-8511
Brodeur JC, Jimena DM, Candioti JV, Poliserpi MB, D'Andrea MF, Bahl MF (2020) Frog body condition: basic assumptions, comparison of methods and characterization of natural variability with field data from Leptodactylus latrans. Ecol Indic 112:106098. https://doi.org/10.1016/j.ecolind.2020.106098
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: an underestimated cause of global decline? Sci Rep 3:1135. https://doi.org/10.1038/srep01135
Burraco P, Gomez-Mestre I (2016) Physiological stress responses in amphibian larvae to multiple stressors reveal marked anthropogenic effects even below lethal levels. Physiol Biochem Zool 89(6):462–472. https://doi.org/10.1086/688737
Carey C, Alexander MA (2003) Climate change and amphibian declines: is there a link? Divers Distrib 9(2):111–121. https://doi.org/10.1046/j.1472-4642.2003.00011.x
Chapman DG (1951) Some properties of the hypergeometric distribution with applications to zoological sample census. Univ Calif Publ Stat 1:131–159
Christin MS, Ménard L, Giroux I, Marcogliese DJ, Ruby S, Cyr D, Fournier M, Brousseau P (2013) Effects of agricultural pesticides on the health of Rana pipiens frogs sampled from the field. Environ Sci Pollut Res 20:601–661. https://doi.org/10.1007/s11356-012-1160-1
Collins JP, Crump ML (2009) Extinction in our times: global amphibian decline. Oxford University Press, New York
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Divers Distrib 9(2):89–98. https://doi.org/10.1046/j.1472-4642.2003.00012.x
Coronado GD, Thompson B, Strong L, Griffith WC, Islas I (2004) Agricultural task and exposure to organophosphate pesticides among farmworkers. Environ Health Perspect 112(2):142–147. https://doi.org/10.1289/2Fehp.6412
Cothran RD, Brown JM, Relyea RA (2013) Proximity to agriculture is correlated with pesticide tolerance: evidence for the evolution of amphibian resistance to modern pesticides. Evol Appl 6(5):832–841. https://doi.org/10.1111/eva.12069
Davidson C (2004) Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14(6):1892–1902. https://doi.org/10.1890/03-5224
Davidson C, Knapp RA (2007) Multiple stressors and amphibian declines: dual impacts of pesticides and fish on yellow-legged frogs. Ecol Appl 17(2):587–597. https://doi.org/10.1890/06-0181
Davis CL, DAW M, Walls SC, Barichivich WJ, Riley JW, Brown ME (2017) Species interactions and the effects of climate variability on a wetland amphibian metacommunity. Ecol Appl 27(1):285–296. https://doi.org/10.1002/eap.1442
Dehghani R, Moosavi SG, Eslami H, Mohammadi M, Jalali Z, Zamini N (2011) Surveying of pesticides commonly on the markets of Iran in 2009. J Environ 2(8):1113–1117. https://doi.org/10.4236/jep.2011.28129
Dehghani MH, Niasar ZS, Mehrnia MR, Shayeghi M, al-Ghouti MA, Heibati B, McKay G, Yetilmezsoy K (2017) Optimizing the removal of organophosphorus pesticide Malathion from water using multi-walled carbon nanotubes. Chem Eng 310(1):22–32. https://doi.org/10.1016/j.cej.2016.10.057
Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Environ Resour 28:137–167. https://doi.org/10.1146/annurev.energy.28.050302.105532
Dodd CK (2010) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, London
Donnelly MA, Guyer C, Juterbock EJ, Alford RA (1994) Techniques for marking amphibians. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 275–284
Edgell K, Wesselman R (1989) USEPA method study 36 - Sw-846 methods 8270/3510 GC/MS method for semivolatile organics: capillary column technique separatory funnel liquid-liquid extraction. U.S. Environmental Protection Agency, Washington, D.C. EPA/600/4-89/010 (NTIS Pb89190581)
Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, Alpérovitch A, Tzourio C (2009) Professional exposure to pesticides and Parkinson disease. Ann Neurol 66(4):494–504. https://doi.org/10.1002/ana.21717
Ezemonye L, Tongo I (2010) Sublethal effects of Endosulfan and Diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 81(2):214–217. https://doi.org/10.1016/j.chemosphere.2010.06.039
Fellers GM, McConnell LL, Pratt D, Datta S (2004) Pesticides in mountain yellow-legged frogs (Rana muscosa) from the Sierra Nevada Mountains of California, USA. Environ Toxicol Chem 23(9):2170–2177. https://doi.org/10.1897/03-491
Forbes VE, Sibly RM, Calow P (2001) Toxicant impacts on density-limited populations: a critical review of theory, practice, and results. Ecol Appl 11(4):1249–1257. https://doi.org/10.1890/1051-0761
Fryday S, Thompson H (2012) Toxicity of pesticides to aquatic and terrestrial life stages of amphibians and occurrence, habitat use and exposure of amphibian species in agricultural environments. EFSA Supporting Publications 9(9):343E. https://doi.org/10.2903/sp.efsa.2012.EN-343
Gardner T (2001) Declining amphibian populations: a global phenomenon in conservation biology. Anim Biodivers Conserv 24(2):25–44
Ghasemzadeh L, Mohajereani H, Nasri S, Rostami A (2015) The effect of Diazinon exposure on hepatic tissue and enzymes in male frog Rana ridibunda. Progress in Biological Sciences 5(2):223–232. https://doi.org/10.22059/pbs.2015.56040
Gonçalves MW, de Campos CBM, Godoy FR, Gambale PG, Nunes HF, Nomura F, Bastos RP, da Cruz AD, Silva DME (2019) Assessing genotoxicity and mutagenicity of three common amphibian species inhabiting agroecosystem environment. Arch Environ Contam Toxicol 77(3):409–420. https://doi.org/10.1007/s00244-019-00647-4
Green RE, Cornell SJ, Scharlemann JPW, Balmford A (2005) Farming and the fate of wild nature. Science 307(5709):550–555. https://doi.org/10.1126/science.1106049
Greulich K, Pflugmacher S (2003) Differences in susceptibility of various life stages of amphibians to pesticide exposure. Aquat Toxicol 65(3):329–336. https://doi.org/10.1016/s0166-445x(03)00153-x
Hachtel M, Ortmann D, Kupfer A, Sander U, Schmidt P, Weddeling K (2005) Return rates and long-term capture history of amphibians in an agricultural landscape near Bonn (Germany). Russ J Herpetol 12(supplement):146–149
Hassan J, Farahani A, Shamsipur M, Damerchili F (2010) Rapid and simple low density miniaturized homogeneous liquid–liquid extraction and gas chromatography/mass spectrometric determination of pesticide residues in sediment. J Hazard Mater 184(1–3):869–871
Hegde G, Krishnamurthy SV (2014) Analysis of health status of the frog Fejervarya limnocharis (Anura: Ranidae) living in rice paddy fields of Western Ghats, using body condition factor and AChE content. Ecotoxicol Environ Contam 9(1):69–76
Hollen RM, Beugelsdijk TJ (1992) A standard laboratory module for automating the EPA 3550 sonication method. In abstracts of papers of the American Chemical Society (Vol. 203, P. 167–Iec). Amer chemical Soc, 1155 16th St, Nw, Washington, dc 20036
Isworo S, Purwanto I, Sabdono A (2015) Impact of pesticide use on organophosphorus and organochlorine concentration in water and sediment of Rawa Pening lake, Indonesia. Res J Environ Sci 9(5):233–240. https://doi.org/10.3923/rjes.2015.233.240
Jensen JB, Camp CD (2003) Human exploitation of amphibians: direct and indirect impacts. In: Semlitsch RD (ed) Amphibian conservation. Smithsonian Institution Press, Washington, DC, pp 199–213
Kappers WA, Edwards RJ, Murray S, Boobis AR (2001) Diazinon is activated by CYP2C19 in human liver. Toxicol Appl Pharmacol 177(1):68–76. https://doi.org/10.1006/taap.2001.9294
Khan MZ, Zaheer M, Fatima F (2003) Effect of lambda cyhalothrin (pyrethroid) and monocrotophos (organophosphate) on cholinesterase activity in liver, kidney and brain of Rana cyanophlyctis. Korean J Biol Sci 7(2):165–168. https://doi.org/10.1080/12265071.2003.9647700
Kittusamy G, Kandaswamy C, Kandan N, Subramanian M (2014) Pesticide residues in two frog species in a paddy agroecosystem in Palakkad district, Kerala, India. Bull Environ Contam Toxicol 93(6):728–734. https://doi.org/10.1007/s00128-014-1351-1
Knapp RA (2005) Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biol Conserv 121(2):265–279. https://doi.org/10.1016/j.biocon.2004.05.003
Konstantinou IK, Hela DG, Albanis TA (2006) The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Environ Pollut 141(3):555–570. https://doi.org/10.1016/j.envpol.2005.07.024
Kovar R, Brabec M, Vita R, Bocek R (2009) Spring migration distances of some central European amphibian species. Amphibia-Reptilia 30(3):367–378. https://doi.org/10.1163/156853809788795236
Kreuger J (1999) Pesticides in the environment: atmospheric deposition and transport to surface waters. Swedish University of Agricultural Sciences, Dissertation
Lichtenberg E (2013) Economics of pesticide use and regulation. In: Shogren JF (ed) Natural resource, and environmental economics, 3rd edn. Elsevier, Waltham, pp 86–97. https://doi.org/10.1016/B978-0-12-375067-9.00092-9
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157(11):2903–2927. https://doi.org/10.1016/j.envpol.2009.05.015
Martin D, Hong H (1991) The use of Bactine® in the treatment of open wounds and other lesions in captive anurans. Herpetol Rev 22(21):462
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science 277(5325):504–509. https://doi.org/10.1126/science.277.5325.504
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12(2):395–403. https://doi.org/10.1046/j.1365-294x.2003.01732.x
Müller K, Deurer M, Hartmann H, Bach M, Spiteller M, Frede HG (2003) Hydrological characterisation of pesticide loads using hydrograph separation at different scales in a German catchment. J Hydrol 273(1–4):1–17. https://doi.org/10.1016/S0022-1694(02)00315-3
Pesticides O (2007) Method 8141b organophosphorus compounds by gas chromatography
Pider AW, Storey KB, Ultsch GR (1992) Estivation and hibernation. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians. The University of Chicago Press, Chicago, pp 250–274
Piha H (2006) Impacts of agriculture on amphibians at multiple scales
Pishgar-Komleh SH, Sefeedpari P, Rafiee S (2011) Energy and economic analysis of rice production under different farm levels in Guilan province of Iran. Energy 36(10):5824–5831. https://doi.org/10.1016/j.energy.2011.08.044
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3–900051–07-0, URL http://www.Rproject.org
Ramalho WP, Jorge RF, Baiocchi LB, Peña AP, Pires RAP (2013) Study on the population structure of the paradoxical frog, Pseudis bolbodactyla (Amphibia: Anura: Hylidae), using natural markings for individual identification. Zoologia (Curitiba) 30(6):623–629. https://doi.org/10.1590/S1984-46702013005000001
Rezaeigolestani M, Hashemi M (2018) A review of pesticide residues in agricultural and food products of Iran. Journal of Nutrition, Fasting and Health 6(1):1–6. https://doi.org/10.22038/jnfh.2018.33593.1125
Sala OE, Chapin FS, Armesto JJ (2000) Global biodiversity scenarios for the year 2100. Science 287(5459):1770–1774. https://doi.org/10.1126/science.287.5459.1770
Sams C, Cocker J, Lennard MS (2003) 544 metabolism of chlorpyrifos and diazinon by human liver microsomes. Toxicol Lett 144(1):s146. https://doi.org/10.1016/S0378-4274(03)90543-1
Schäfer RB, van den Brink PJ, Liess M (2011) Impacts of pesticides on freshwater ecosystems. In: Sánchez-Bayo F, van den Brink PJ, Mann R (eds) Ecological impacts of toxic chemicals. Bentham Science Publishers, Sharjah, pp 111–137
Schmidt BR (2004) Pesticides, mortality and population growth rate. Trends Ecol Evol 19(9):459–460. https://doi.org/10.1016/j.tree.2004.06.006
Seber GAF, Schwarz CJ (2002) Capture-recapture: before and after EURING 2000. J Appl Stat 29(1–4):5–18. https://doi.org/10.1080/02664760120108700
Seitz A, Faller-Doepner U, Reh W (1992) Radio-tracking of the common frog (Rana temporaria). In: Priede IG, Swift SM (eds) Wildlife telemetry: remote monitoring and tracking of animals. Ellis Horwood, London, pp 484–489
Semlitsch RD, Bridges CM, Welch AM (2000) Genetic variation and a fitness tradeoff in the tolerance of gray treefrog (Hyla versicolor) tadpoles to the insecticide carbaryl. Oecologia 125(2):179–185. https://doi.org/10.1007/s004420000443
Sharifzadeh MS, Abdollahzadeh G, Damalas CA, Rezaei R (2018) Farmers’ criteria for pesticide selection and use in the pest control process. Agriculture 8(2):24. https://doi.org/10.3390/agriculture8020024
Snegin EA, Barkhatov AS (2019) Morphogenetic structure of marsh frog populations of Pelophylax ridibundus (Amphibia, Anura) under conditions of urban environment. Theoret Appl Ecol 1:47–53. https://doi.org/10.25750/1995-4301-2019-1-047-053
Sparling DW, Fellers GM, McConnell LL (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20(7):1591–1595. https://doi.org/10.1897/1551-5028(2001)020%3C1591:paapdi%3E2.0.co;2
Stakh VO, Khamar IS, Reshetylo OS, Zabytivskyi YМ (2017) Phenes of water frogs (Pelophylax) as the indicators of water bodies' contamination in pre-Carpathians, roztochia, lesser and western Polissia. Stud Biol 11(1):161–168
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306(5702):1783–1786. https://doi.org/10.1126/science.1103538
Subrero E, Sforzini S, Viarengo A, Cucco M (2019) Exposure to anti-mosquito insecticides utilized in rice fields affects survival of two non-target species, Ischnura elegans and Daphnia magna. Paddy Water Environ 17:1–11. https://doi.org/10.1007/s10333-018-0678-3
Taylor SK, Williams ES, Mills KW (1999) Effects of Malathion on disease susceptibility in Woodhouse’s toads. J Wildl Dis 35(3):536–541. https://doi.org/10.7589/0090-3558-35.3.536
Taylor B, Skelly D, Demarchis LK, Slade MD, Galusha D, Rabinowitz PM (2005) Proximity to pollution sources and risk of amphibian limb malformation. Environ Health Perspect 113(11):1497–1501. https://doi.org/10.1289/2Fehp.7585
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677. https://doi.org/10.1038/nature01014
Tongo I, Ezemonye L, Ochei U (2012) Diazinon mediated biochemical changes in the African toad (Bufo regularis). J Xenobiot 2(1):e4. https://doi.org/10.4081/xeno.2012.e4
Tramontano R (1997) Continuous radio tracking of the common frog, Rana temporaria. In: Böhme W, Bischoff W, Ziegler T (eds) Herpetologia Bonnensis. SEH, Bonn, pp 359–365
Vo A (1992) Use of a centrifugal evaporator to reduce emissions of solvents used in EPA method 3510 prior to GC-MS analysis. J High Resolut Chromatogr 15(8):552–555. https://doi.org/10.1002/jhrc.1240150814
Vogiatzis AK, Loumbourdis NS (1997) Uptake, tissue distribution, and depuration of cadmium (Cd) in the frog Rana ridibunda. Bull Environ Contam Toxicol 59(5):770–776. https://doi.org/10.1007/s001289900547
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and Management of Animal Populations. Academic Press, San Francisco
Zaripova FF, Fayzulin AI (2012) Characteristic of morphophysiological parameters of population of the marsh frog Rana ridibunda (Anura, Amphibia) in urban areas in the republic of Bashkotorstan. Proc Samara Sci Center Russ Acad Sci 14(5):145–149 (in Russian)
Zhelev Z, Tsonev CV, Arnaudova DN (2017) Health status of Pelophylax ridibundus (Pallas, 1771) (Amphibia: Ranidae) in a rice paddy ecosystem in southern Bulgaria: body condition factor and fluctuating asymmetry. Acta Zool Bulg 69(Suppl 8):169–177
Zhelev Z, Tsonev S, Georgieva K, Arnaudova D (2018) Health status of Pelophylax ridibundus (Amphibia: Ranidae) in a rice paddy ecosystem in southern Bulgaria and its importance in assessing environmental state: haematological parameters. Environ Sci Pollut Res 25:7884–7895. https://doi.org/10.1007/s11356-017-1109-5
Zhelev Zh, Tsonev SV, Angelov MV (2019) Fluctuating asymmetry in Pelophylax ridibundus meristic morphological traits and their importance in assessing environmental health. Ecol Indic 107:105589. https://doi.org/10.1016/j.scolind.2019.105589
Zhelev Zh, Arnaudova DN, Popgeorgiev GS, Tsonev SV (2020) In situ assessment of health status and heavy metal bioaccumulation of adult Pelophylax ridibundus (Anura: Ranidae) individuals inhabiting polluted area in southern Bulgaria. Ecol Indic 115:106413. https://doi.org/10.1016/j.ecolind.2020.106413
Acknowledgments
We acknowledge Dr. Nader Bahramifar, Natural Resources and Marin Science faculty member at Tarbiat Modares University, Noor branch, Mazandaran, and Dr. Sedigheh Safaei, curators of Danesh Pazhoohan Payesh Amin Co. for collaborating in extracting and measuring pesticides. Our special thanks is dedicated to A. Sharifi for validation of statistical analyzes, and P. Azizi, E. Esmaeili, and M. Hasanpour for helpful information about pesticide consumption in Iran. Also, the authors are grateful for the kind cooperation of farmers: S. Shokrollah-Pour and Jalali brothers.
Data and material availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
The experiment was planned by Nadimeh Shojaei, Saeid Naderi, and Esmaeil Yasari. Nadimeh Shojaei, Saeid Naderi, and Naeim Moradi planned and conducted the sample preparations, method development, and analyses. The manuscript was drafted by N. Shojaei and N. Moradi. S. Naderi and E. Yasari critically examined and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The publisher has authors consent.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shojaei, N., Naderi, S., Yasari, E. et al. Exposure to common pesticides utilized in northern rice fields of Iran affects survival of non-target species, Pelophylax ridibundus (Amphibia: Ranidae). Environ Sci Pollut Res 28, 33557–33569 (2021). https://doi.org/10.1007/s11356-021-13168-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13168-2