Abstract
Perfluorinated chemicals (PFCs) have been used for many years in numerous industrial products and are known to accumulate in organisms. A recent survey showed that tissue levels of PFCs in aquatic organisms varied among compounds and species being undetected in freshwater zebra mussels Dreissena polymorpha. Here we studied the bioaccumulation kinetics and effects of two major PFCs, perfluorooctane sulfonic acid compound (PFOS) and perfluorooctanoic acid (PFOA), in multixenobiotic transporter activity (MXR) and filtration and oxygen consumption rates in zebra mussel exposed to a range of concentrations of a PCF mixture (1–1,000 μg/L) during 10 days. Results indicate a low potential of the studied PFCs to bioaccumulate in zebra mussel tissues. PFCs altered mussel MXR transporter activity being inhibited at day 1 but not at day 10. Bioaccumulation kinetics of PFCs were inversely related with MXR transporter activity above 9 ng/g wet weight and unrelated at tissue concentration lower than 2 ng/g wet weight suggesting that at high tissue concentrations, these type of compounds may be effluxed out by MXR transporters and as a result have a low potential to be bioaccumulated in zebra mussels. Oxygen consumption rates but not filtering rates were increased in all exposure levels and periods indicating that at environmental relevant concentrations of 1 μg/L, the studied PFCs enhanced oxidative metabolism of mussels. Overall, the results obtained in this study confirm previous findings in the field indicating that an important fraction of PFC accumulated in mussel tissues is eliminated actively by MXR transporters or other processes that are metabolically costly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluorinated chemicals (PFCs) such a perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), or perfluorononanoate (PFNAs) have been used for many years in numerous industrial formulations, known widely for their use in polytetrafluoroethylene (PTFE) products, although they are also used as additives in detergents, soaps, as surfactants, explosives, and as flame retardants (Fromme et al. 2009). They are considered a new generation of contaminants that have aroused concern because they are globally distributed in the environment, especially in the aquatic environment, have a high bioaccumulation potential, and can have an impact on aquatic organisms (Giesy and Kannan 2001).
The information on the accumulation levels and toxicological effects of PFCs is to date limited and has focused primarily on PFOS and PFOA (Hekster et al. 2003; Kennedy et al. 2004). In addition, most studies have been focused on mammals, fish, and birds (Houde et al. 2011; Kannan et al. 2001; Vicente et al. 2012). In a recent study, we evaluated the presence of five PFCs in aquatic organisms such as fish, oysters, crabs, marin mussels, and zebra mussels collected in Catalan river basins and in coastal areas to define a suitable biomonitoring matrix that can be used to assess the presence and potential effects of PFCs (Fernández-Sanjuan et al. 2010). Our results indicated a different accumulation behaviour of PFCs in the studied species being undetected in bivalves. Specifically, zebra mussels collected across a polluted area likely affected by PFCs contamination did not contain PFCs (Fernández-Sanjuan et al. 2010). This behaviour was also observed by Kannan et al. (2005), where PFOS and PFOA were only detected in zebra mussels in one out of three rivers analyzed in the Great Lakes (USA) and at very low concentrations (3 ng/g wet weight).
The MXR system represents a broad-scale defense mechanism protecting cells and organisms against both endogenous and environmental toxicants, including also anthropogenic chemicals (Epel et al. 2008). MXR is mediated by membrane transport proteins from the ABC (ATP binding cassette) protein family, which recognize a wide variety of potential xenobiotics as substrates, pumping them out of the cell in an energy-dependent, ATP-driven process. The most studied transporter involved in the MXR mechanism is the p-glycoprotein (P-gp), which, in humans, is active in the kidney, adrenal gland, liver, and blood–testes barrier (Cordon-Cardo et al. 1990). P-glycoprotein orthologues have also been identified in many other organisms by gene homology, activity measurements, and immunological probes, and are especially active in aquatic mussels (Epel 1998; Eufemia and Epel 2000; Faria et al. 2011; Minier et al. 2006; Smital et al. 2004; Tutundjian and Minier 2007). Other MXR transporter types such as the multidrug transporter proteins (MRP) are also active in many aquatic invertebrates and aid cells to efflux out xenobiotic metabolites (Luckenbach and Epel 2008; Smital et al. 2004; Faria et al. 2011). MXR defense mechanism can be compromised by many xenobiotics. This increased sensitivity, referred to as chemosensitization, arises from the ability of P-gp to recognize and bind to multiple xenobiotic substrates, resulting in the saturation of the binding capacity; even non-toxic substances can be chemosensitizers and cause adverse effects on an organism by allowing normally excluded toxic substrates to accumulate in the cell (Faria et al. 2011; Luckenbach and Epel 2005; Smital et al. 2004).
Many xenobiotic enhance metabolic costs as a result of detoxification or repair mechanism (Calow 1991). Pollutants may also decrease metabolism and oxygen consumption rates if food intake is inhibited (Barber et al. 1994). Measurements of oxygen consumption rates under normoxic conditions are a good proxy to determine effects on aerobic metabolism. Mussel filter rate is also an important physiological response to pollutants since ensure the circulation of water and food and hence, affects accumulation kinetics (Pan and Wang 2009) and mussel metabolism (Thompson and Bayne 1972). Bivalves have the ability to close their valves and hence stop filtering and thus, decreasing the bioaccumulation potential of pollutants (Kontreczky et al. 1997; Vosloo et al. 2012). Therefore, bivalve filtering rates are also an important physiological response to pollution.
Stevenson et al. (2006) showed a low potential of PFCs to be accumulated in marine mussel gills of the species Mytilus californianus and that longer chain PFCs like PFOS accumulated to a greater extent than short molecules. These results agree with other studies that also reported that long chain PFCs accumulated to a greater extent than short chain ones (Martin et al. 2004). Stevenson et al. (2006) also found that accumulation kinetics of PFNA was independent of efflux P-gp transporter activity but that over 80 % of the accumulated PFNA was rapidly lost from mussel gill tissue once mussels were exposed to clean medium. Furthermore, according to Stevenson et al. (2006), some PFCs inhibited P-gp transporter activity whereas others were able to induce that activity as a general response to stress. Thus the previous results are not conclusive and partly support the argument that MXR transporters may be involved in the fast detoxification of PFCs probably by MRP transporters.
The aim of this present study was to evaluate the accumulation kinetics of two PFCs (PFOA and PFOS) in zebra mussel individuals in laboratory conditions and to study its relation with MXR transporter activity and their potential detoxification route and physiological effects on mussel metabolism.
Experimental
Chemicals
For this work, the following chemicals were used: PFOA 96 % purity and PFOS 98 % purity, cyclosporine A (CycA) and rhodamine B (RB), which were purchased from Sigma-Aldrich (Steinheim, Germany). Stock standard solutions were prepared in acetonitrile at a concentration of 5 ng/μL for all native compounds and were stored at −18 °C. Perfluoro-n-(1,2,3,4-13C4) octanoic acid (m-PFOA) and sodium perfluoro-1-(1,2,3,4-13C4) octane sulfonic acid (m-PFOS), from Wellington Laboratories, were used as surrogate standards. HPLC grade water and acetonitrile were supplied by Merck (Darmstadt, Germany) and glacial acetic acid from Panreac (Barcelona, Spain).
Sampling and culturing conditions
Zebra mussels, which are 2 cm long in shell length attached to rocks, were collected from the Mequinenza reservoir, Ebro River, NE Spain in February 2010 by a scuba diver at approximately 3–5 m depth and transported to the laboratory. Mussels attached to rocks were rinsed and introduced into glass aquaria and maintained under constant oxygenation >90 %, temperature (20 °C), and photoperiod (14:10 h; light–dark). Animals were cultured in local field collected water which was progressively replaced by artificial ASTM hard water (ASTM 1999) and fed with a suspension 1:1 of Scenedesmus subspicatus and Chlorella vulgaris (106 cells/mL, daily). The medium was renewed every other day for 10 days to allow the acclimatization and depuration of animals. After the depuration period, 400 mussels with similar length (2 cm long) were selected for the experiments. They were gently cut off from rocks, placed on glass sheets suspended in a glass aquaria filled with of 20 L medium and maintained a further 10 days in the same conditions described above. The test medium was changed every 2 days and mussels were fed adding food only 2 h before water renewal.
Experimental design
Zebra mussels in groups of 100 were exposed to a control and 3 concentrations, 1, 10, and 1,000 μg/L of an equal mixture of PFOS and PFOA. Stock solution was prepared by adding equal amounts of each contaminant of 0.02, 0.2, and 20 mg/L to a 1-L-glass bottle containing 1 L of ASTM water. The solution was then sonicated for 15 min and added to the experimental tanks filled with 19 L of ASTM water to achieve the desired final concentration.
Chemical analysis
At days 2 and 8, water samples for chemical analysis were collected from all treatments at approximately 10 min after spiking the water with the contaminants (considered W 0 h) and just before changing the medium (considered W 48 h). Furthermore, duplicated composite samples of 20 mussels from each treatment were collected for chemical analysis after 1, 5, and 10 days of exposure. Once collected, mussels in each composite sample were gently washed in ASTM water, their soft tissue removed from their shells and immediately frozen and stored at −20 °C, until further analysis.
Water samples were analyzed as described in Sánchez-Avila et al. (2010). Half liter of unfiltered water samples was spiked with 10 ng/mL of m-PFOS and m-PFOA and was pre-concentrated with Oasis® HLB 6 cc (200 mg) Solid Phase Extraction (SPE) cartridges, previously conditioned with 5 mL of methanol and 5 mL of HPLC water. After pre-concentration, cartridges were washed with 10 mL 10 % methanol solution in HPLC water and vacuum dried for 30 min. Ten milliliters of methanol was used as extraction solvent. Extracts were dried under a gentle N2 current and reconstituted with 200 μL of a 30/70 v/v acetonitrile/HPLC water solution.
Mussel samples were solid–liquid extracted from grinded and homogenized wet samples using acetonitrile, based on a previous method (Fernández-Sanjuan et al. 2010). Three grams were weighted in polypropylene centrifuge tubes, spiked with 100 ng/g of the surrogate standards (m-PFOS and m-PFOA) and incubated for 18 h at 4 °C. Nine milliliters of acetonitrile was added and the sample was vortexed and extracted in an ultrasonic bath for 10 min at room temperature (three times). Afterwards, the samples were centrifuged at 2.500 rpm for 5 min. The supernatant was transferred to a new vial and evaporated to dryness. Then 1 mL of acetonitrile was added to the dried samples and incubated for 10 min in the ultrasonic bath. The samples were purified by adding 25 mg of activated carbon and 50 μL of glacial acetic acid and were vigorously mixed for 1 min. Afterwards, the samples were centrifuged for 10 min at 10.000 rpm. One milliliter of the supernatant was transferred to a clean micro vial and 150 μL of this extract was reconstituted in 350 μL of HPLC water, similar to the initial conditions of the mobile phase.
PFCs were measured using Acquity Ultra performance LC system connected to a Triple Quadruple Detector (TQ Detector; Waters, USA) under negative electrospray ionization. An Acquity UPLC BEH C18 column (1.7 μm particle size, 50 × 2.1 mm, Waters, USA) was used as mobile phase residue trap to remove any contamination from the mobile phase. The analysis was performed on a LiChroCART HPLC RP-18e column (125 mm × 2 mm × 5 μm particle size, Merck, Germany) and the flow rate was set at 0.4 mL/min. Injection volume was of 10 μL. The mobile phase was 2 mM NH4OAc (A)/acetonitrile (B). Gradient elution started from 70 % A and 30 % B, increased to 90 % B in 5 min and to 100 % B in 0.10 min and held for 1 min. PFOS and PFOA were measured using two transitions from parent to product ion and internal standard quantification was performed using m-PFOS to quantify PFOS and m-PFOA to quantify PFOA.
Physiological effects
MXR activity, filtration rate, and oxygen consumption measurements were conducted at 1 and 10 days of exposure. All measurements were conducted using ASTM hard water and at 20 °C.
To evaluate the functional activity of MXR in zebras, the efflux method according to Pain and Parant (2007) was applied with slight modifications. The assay first consists on exposing mussels to a fluorescent dye that is accumulated in cells and second, on measuring the efflux rate of the dye into the medium which may or not may contain a model MXR inhibitor. If the medium is clear of inhibitor dye, efflux rate (fluorescent units/min) is high; if it contains an inhibitor, then the pump is compromised and dye efflux rate decreases. Rhodamine B (RB), a known substrate for P-gp type MXR transporters, was used for dye and cyclosporine A (CysA) as model MXR inhibitor. This drug is a non-competitive inhibitor, it blocks the ATPase activity of the MXR transporters, affecting the structure of the protein, with allosteric interactions and subsequently suppressing their detoxifying activity. Briefly, two groups of five mussels form each treatment were incubated for 1 h, in a light protected glass beaker containing 200 mL of 5 μM of RB. After the loading period, mussels were washed three times using ASTM water and then left in clean ASTM water for 5 min in order to remove excess dye accumulated. Mussels were washed once more and individually placed in 30 mL of ASTM water with or without 5 μM of CysA. Incubation period lasted 60 min and every 10 min, the medium was stirred and 200 μL was transferred to a 96-well black nunc microplate and the fluorescence measured using a microplate fluorescence reader (Synergy 2, BioTek, USA) at a wave length of 535/590 nm excitation/emission. A standard curve of RB was used to extrapolate RB concentration in the medium. This procedure allowed assessing the MXR related efflux rates of RB. MXR efflux activity was computed as the difference between the total efflux (without CysA) and the remaining efflux rate of RB following CysA (Kurelec et al. 2000; Smital and Kurelec 1997). Calculation of MXR related efflux was possible only when the difference between the two measures were significantly different, indicating a real significant inhibitory effect of CysA. All the measurements performed during the presented experiments were suitable for the calculation of MXR related efflux.
Zebra mussel filtration rates were measured using the method described by Coughlan (1969) and adapted to zebra mussels by Faria et al. (2009), which is based on the loss of neutral red dye particles from the water column due to filtration by the mussels. Immediately following exposures, five mussels from each treatment were placed in 200 mL beakers (1 per beaker) containing 100 mL of neutral red solution (1 mg/L) and left protected from light for 2 h. Just prior to placing the mussels in the solution, an aliquot of water was removed from each beaker representing the initial concentration, C0. After 2 h, mussels were removed and the remaining solution (Ct) along with the initial aliquot (C0) were acidified to pH 5 with HCl. Neutral red concentrations were determined by measuring the optical density at 530 nm. Standards of neutral red were measured along with samples and used to generate a standard curve from which dye concentration could be extrapolated. Filtration rate (f in mL/animal/h) calculation was determined by the equation: \( f = \left[ {{{M} \left/ {{\mathrm{nt}}} \right.}} \right]{\log}\left( {{{{{\mathrm{C}}0}} \left/ {{\mathrm{Ct}}} \right.}} \right) \); where M is the volume of the test solution, n the number of mussels used, t the time in h, C0 and Ct the initial and final concentration of the dye.
Oxygen consumption assays were performed using standard respirometry methods with 50 mL gas tight syringes (Hamilton, USA) following Agra et al. (2011) procedures. Ten syringes were used per treatment that were filled with 50 mL of the appropriate test solutions; in eight of them, an individual mussel was placed and the remaining two were used as blank controls to correct for the ambient O2 depletion due to factors other than organism’s respiration. The remaining air was expelled from each syringe and left in the dark in a water bath (20 °C) for 4 h. After the first 30 min of exposure, initial O2 concentrations were measured with the aid of an oxygen meter (Model 782) provided with an oxygen electrode model 1302 (Strathkelvin Instruments, Glasgow), and after 4 h from the start of the test, the final O2 concentrations were measured in the same way. Following exposures, individual soft tissue mussel was removed dried for 2 days at 60 °C and weighted. The oxygen consumption was then given by the differences in the oxygen content in water just at the beginning (T0 = 30 min) and after (T final = 4 h) the exposure period. Respiration rate was expressed as μg O2 consumed per dry weight per hour. The depletion in the oxygen content one blank controls was used as a correction factor for the appropriate treatments.
Data analyses
The bioaccumulation factor (BAF) was calculated according to
Where C 0 is the PFC concentration in the organism (ng/g); C w is the estimated measured concentration in water (μg/L) which was obtained from Table 1, BAF is in liters per kilogram. Significant (P < 0.05) treatment effects within and across exposure periods of accumulated and bioaccumulated factors of PFCs, MXR efflux activities, filtration, and oxygen consumption rates of exposed mussels to the studied PFOS were compared using a two-way ANOVA followed by Tukey’s post hoc multiple comparison tests. Data was log transformed prior to analyses to improve ANOVA assumptions of normality and variance homoscedasticity (Zar 1996). Tests were performed with the IBM SPSS Statistics software version 19.
Results and discussion
Accumulation kinetics and bioconcentration of PFCs
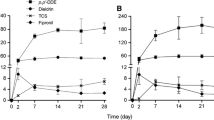
Levels of PFOA and PFOS in water samples taken at days 1 and 8 of the experiments were quite constant in exposed treatments decreasing slightly but not significantly (P < 0.05, based on Student t tests) after 48 h (Table 1). Accordingly, measured concentrations of PFCs were considered not to vary during the experiment and C w for each exposure was estimated as the mean of initial and final values. In control treatments, PFC levels in water and mussel tissue were below detection limits (0.001 μg/L, 0.1 ng/g, respectively). Accumulated levels of PFCs increased significantly (P < 0.05, Treatment effects in Table 2) with exposure levels being for PFOA close to detection limits at 1 μg/L (0.2–0.4 ng/g) and over threefold greater for PFOS (0.6–0.9 ng/g; Fig. 1a, c). PFOS tissue concentrations in mussels remained relatively constant at low spiking levels (1 μg/L), those of PFOA increased at 10 days at moderate levels (10 μg/L), and those of both PFOA and PFOS decreased significantly with time (P < 0.05, period and interaction effects, Table 2) and at the highest concentration tested (1,000 μg/L). Bioaccumulation factors (BAF) of the studied PFCs varied also significantly across exposure levels (P < 0.05, treatment effects, Table 2) being at 1 μg/L 1,000 L/kg for PFOS and 500 L/kg for PFOA (Fig. 1b, d). At 10 and 1,000 μg/L, PFOS showed also between twofold and threefold greater potential to be bioaccumulated than PFOA (Fig. 1b, d), but rarely exceeded bioaccumulation factors of 100 L/kg. Exposure period effected significantly (P < 0.05, period effects, Table 2) bioaccumulation factors of PFOA, being higher at 5 and 10 days in mussels exposed to 1 and 10 μg/L, respectively, and decreasing at day 10 in mussels exposed to 1,000 μg/L (Fig. 2b). Exposure period effects on bioaccumulation factors of PFOS were similar than those of PFOA but less marked (Fig. 2d). Observed bioaccumulation factor ranges of 100–1,000 L/kg for PFOS are of similar magnitude of those reported in the freshwater mussel Perna viridis in laboratory exposures at 1 and 10 μg/L (400 and 200 L/kg, respectively) by Liu et al. (2011) and of benthic algae (686–1,000 L/kg), amphipods (828 L/kg), and zebra mussels (885 L/kg) collected in Great Lakes (Michigan, USA; Kannan et al. 2005). The observed low BAF of PFOA also agree with previous lab and field studies that indicate that longer-chain PFCs bioaccumulate in wildlife (Martin et al. 2004). Nevertheless, BAF of 1,000 are quite low when compared with those reported in fish or other aquatic organisms (Fernández-Sanjuan et al. 2010; Kannan et al. 2005; Martin et al. 2004) and means that under realistic exposure levels <1 μg/L, accumulated levels of PFCs may be undetected in zebra mussels (Fernández-Sanjuan et al. 2010). Indeed Kannan et al. (2005) reported that tissue levels of PFOS in zebra mussels and other aquatic invertebrates collected in Great Lakes (Michigan, USA) were low (2.9–4.3 ng/g wet weight) and in most ocasions undetected (<2 ng/g wet weight).
Accumulated (a, c) and bioaccumulation factors (b, d; mean ± SE, N = 2) of PFOS and PFOA in mussels exposed to 1, 10, and 1,000 μg/L of the PFC mixture and collected after 1, 5, and 10 days of exposure. Different letters indicate significant differences among treatment groups according to ANOVA and Tukey’s pos hoc tests
Toxicity effects (mean ± SE, N = 5) of the PFC mixture on MXR activity (a), respiration (b), and filtration rates (c) of mussels exposed to 1, 10, and 1,000 μg/L and collected at 1 and 10 days. Different letters indicate significant differences among treatment groups according to ANOVA and Tukey’s post hoc tests. c This graph has no letters since there were no significant (P < 0.05) treatment effects
Physiological effects
To our knowledge, there are few studies that have assessed effects of PFCs in aquatic life in vivo (Hekster et al. 2003; Farré et al. 2008; Ji et al. 2008; Latała et al. 2009). In this study, PFC exposure affected significantly MXR transporter activity and oxygen consumption rates but not filtering rates (Table 2, Fig. 2). Effects on MXR transporter activity varied with time being inhibited in all exposed mussels after 1 day and enhanced at day 10 in mussels exposed to 10 μg/L of PFCs (Fig. 2a).
It is difficult to provide an explanation for this behaviour but induction and inhibition of MXR activities by different PFCs have been described in marine mussels (Stevenson et al. 2006). The previous authors hypothesized that several PFCs induced MXR transporter activity indirectly as a result of a general stress response, in which the syntheses of other stress proteins were also induced. General stress response is energetically costly (Calow 1991). Oxygen consumption experiments confirmed that PFCs exposure enhanced dramatically aerobic metabolism in all exposure levels and periods (Fig. 2b) and this effect was independent of the filtering process (Fig. 2c), which was unaffected. Thus the hypothesis that PFCs may create a general stress response and hence their detoxification is metabolically costly is supported by our data. Note also that the lowest effects observed for oxygen consumption rates (2 μg/L of the mixture of both PFCs) were in the lower range of reported concentration effects for aquatic biota so far. In a 7-day chronic toxicity test, the cladoceran species Moina macrocopa and Daphnia magna showed significant reproductive changes at 0.31 and 2.5 mg/L for PFOS, respectively (Ji et al. 2008). Among freshwater invertebrates, Chironomus tentans was one of the most sensitive species to PFOS, of which 20-day chronic EC10 based on survival was found at 86 μg/L (MacDonald et al. 2004). In general, fish appear to be more sensitive than invertebrates to chronic PFOS exposure. Ji et al. (2008) reported that the growth of second generation Medaka fish (Oryzias latipes) exposure to 10 μg/L of PFOS was reduced relative to control fish. There is no reported information on sub-lethal responses of mussels to PFCs other than on MXR efflux transporter activity (Stevenson et al. 2006). Lethal concentration levels (96 h LC50) of PFOS to the freshwater mussel Unio complamatu were reported to be 57 mg/L (Beach et al. 2006), which indicate low to moderate toxicity. Our results on both MXR and oxygen consuption rates indicated that PFC mixture of PFOS and PFOA were able to alter mussel physiological responses at concentrations over four orders of magnitude lower than those reported to impair survival.
Accumulation versus MXR
Results depicted in Fig. 3a showed an exponential decay relationship between accumulated levels of PFCs and MXR transporter activity in mussels exposed to a large range of concentrations of the studied PFC mixture, 1–1000 μg/L. A closer observation of MXR and PFC accumulation relationships across exposure treatments (Fig. 3b, c, d) indicated that at high exposure treatments, and consequently at high accumulation levels of PFCs, MXR transporter activity was indeed negatively related with PFC tissue accumulation (Fig. 3b). Conversely at exposure treatments where PFC accumulation was low (<2 ng/g wet weight), there was no such relationship (Fig. 3c, d). These results point to the hypothesis that MXR transporter activity may be involved in the detoxification of PFCs not strongly bound to proteins or other cellular structures (the so-called labile fraction sensu Stevenson et al. 2006), and that this labile fraction was only present at high exposure levels in the present study. As shown in Figs. 2 and 3b, MXR transporter activity was inhibited shortly after exposure and as a result accumulation of PFCs was maximal at exposures of 1,000 μg/L. In longer exposure periods, however, MXR activity at 1,000 μg/L was enhanced and as a result accumulation levels of PFCs decreased. The alternative hypothesis that PFC accumulation is independent of MXR transporter activity is also possible but then MXR transporter activity and PFC accumulation relationships observed in Fig. 3b could not be explained. Our first hypothesis contradicts partially the findings of Stevenson et al. (2006) that reported that accumulation and depuration of the PFC perfluorononanoate was independent of P-gp inhibition by verapimil and that its elimination was bi-modal showing a fast elimination rates of the labile fraction at levels higher than 20 ng/g wet weight and lower rates below those levels. Note, however, that in the previous study responses were measured in vitro and assuming that PFCs were only detoxified by P-gp MXR transporters. Recent studies have reported that zebra mussels have both P-gp and MXR putative transporter activities (Faria et al. 2011), thus low accumulation levels of PFCs could also arise from the activity of other efflux transporters. Indeed our first hypothesis also agrees with the fact that PFCs show low affinity for MXR transporters (Stevenson et al. 2006), when compared with that of specific inhibitors like verapimil (Luckenbach and Epel 2005) and consequently efflux of PFCs by MXR transmembrane pumps can only be effective at high exposure/tissue concentration levels.
Relationship between MXR transporter activity and accumulated levels of PFOS and PFOA in mussels exposed to the PCF mixture (1–1,000 μg/L) and collected at 1 and 10 days. Data in a have been fitted to the first order kinetic model PFCs = a × e − b × MXR. b, c, d Inlet graphs depict MXR versus PFC accumulated levels of mussels exposed at 1,000, 10, and 1 μg/L, respectively. Error bars are SE
Conclusions
PFCs accumulate in many aquatic organisms, triggering toxicological effects which are largely unknown. The accumulation potential depends on the environmental conditions but largely on the species and on the biochemical responses against xenobiotics. The results presented here for PFOA and PFOS support previous findings that accumulation of PFCs on zebra mussel is low compared to other aquatic organisms (Kannan et al. 2005; Fernández-Sanjuan et al. 2010). In this study, we have elucidated that the activity of efflux transporters may be involved in PFC detoxification at high exposure levels. We have also demonstrated that exposure to PFCs increased the aerobic metabolism of zebra mussels.
References
Agra AR, Soares A, Barata C (2011) Life-history consequences of adaptation to pollution. “Daphnia longispina clones historically exposed to copper”. Ecotoxicology 20(3):552–562. doi:10.1007/s10646-011-0621-5
ASTM (1999) Standard methods for measuring the toxicity of sediment-associated contaminants with freshwater invertebrates. E 1706 -95b. In: Annual book of ASTM standards, vol 11.05. ASTM, Philadelphia, pp 65–68
Barber I, Baird DJ, Calow P (1994) Effect of cadmium and ration level on oxygen consuption, RNA concentration and RNA-DNA ratio in two clones of Daphnia magna Dtraus. Aquat Toxicol 30:249–258
Beach SA, Newsted JL, Coady K, Giesy JP (2006) Ecotoxicological evaluation of perfluorooctanesulfonate (PFOS). Rev Environ Contam Toxicol 186:133–174
Calow P (1991) Physiological costs of combating chemical toxicants:ecological implications. Comp Biochem Physiol 100C(1–2):3–6
Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR (1990) Expression of the multidrug resistance gene product (P-Glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 38(9):1277–1287
Coughlan J (1969) The estimation if filtering rate from the clearance of suspensions. Mar Biol 2:356–358
Epel D (1998) Use of multidrug transporters as first lines of defense against toxins in aquatic organisms. Comp Biochem Physiol A Mol Integr Physiol 120(1):23–28
Epel D, Luckenbach T, Stevenson CN, Macmanus-Spencer LA, Hamdoun A, Smital T (2008) Efflux transporters: newly appreciated roles in protection against pollutants. Environ Sci Technol 42(11):3914–3920
Eufemia NA, Epel D (2000) Induction of the multixenobiotic defense mechanism (MXR), P- glycoprotein, in the mussel Mytilus californianus as a general cellular response to environmental stresses. Aquat Toxicol 49(1–2):89–100
Faria M, Carrasco L, Diez S, Riva MC, Bayona JM, Barata C (2009) Multi-biomarker responses in the freshwater mussel Dreissena polymorpha exposed to polychlorobiphenyls and metals. Comp Biochem Physiol C 149:281–288
Faria M, Navarro A, Luckenbach T, Piña B, Barata C (2011) Characterization of the multixenobiotic resistance (MXR) mechanism in embryos and larvae of the zebra mussel (Dreissena polymorpha) and studies on its role in tolerance to single and mixture combinations of toxicants. Aquat Toxicol 101(1):78–87
Farré M, Pérez S, Kantiani L, Barceló D (2008) Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC 27(11):991–1007
Fernández-Sanjuan M, Meyer J, Damásio J, Faria M, Barata C, Lacorte S (2010) Screening of perfluorinated chemicals (PFCs) in various aquatic organisms. Anal Bioanal Chem 398(3):1447–1456
Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds—exposure assessment for the general population in western countries. Int J Hyg Environ Health 212(3):239–270
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7):1339–1342
Hekster FM, Laane RWPM, De Voogt P (2003) Environmental and toxicity effects of perfluoroalkylated substances. Rev Environ Contam Toxicol 179:99–121
Houde M, De Silva AO, Muir DCG, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 45(19):7962–7973
Ji K, Kim Y, Oh S, Ahn B, Jo H, Choi K (2008) Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and moina macrocopa) and fish (Oryzias latipes). Environ Toxicol Chem 27(10):2159–2168
Kannan K, Koistinen J, Beckmen K, Evans T, Gorzelany JF, Hansen KJ, Jones PD, Helle E, Nyman M, Giesy JP (2001) Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol 35(8):1593–1598
Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 48(4):559–566
Kennedy GL Jr, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG (2004) The toxicology of perfluorooctanoate. Crit Rev Toxicol 34(4):351–384
Kontreczky C, Farkas A, Nemcsók J, Salánki J (1997) Short- and long-term effects of deltamethrin on filtering activity of freshwater mussel (Anodonta cygnea L.). Ecotoxicol Environ Saf 38(3):195–199
Kurelec B, Smital T, Pivèeviæ B, Eufemia N, Epel D (2000) Multixenobiotic resistance, P-glycoprotein, and chemosensitizers. Ecotoxicology 9(5):307–327
Latała A, Nedzi M, Stepnowski P (2009) Acute toxicity assessment of perfluorinated carboxylic acids towards the Baltic microalgae. Environ Toxicol Pharmacol 28(2):167–171
Liu C, Gin KYH, Chang VWC, Goh BPL, Reinhard M (2011) Novel perspectives on the bioaccumulation of PFCs—the concentration dependency. Environ Sci Technol 45(22):9758–9764
Luckenbach T, Epel D (2005) Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ Health Perspect 113(1):17–24
Luckenbach T, Epel D (2008) ABCB- and ABCC-type transporters confer multixenobiotic resistance and form an environment-tissue barrier in bivalve gills. Am J Physiol Regul Integr Comp Physiol 294(6):R1919–R1929
MacDonald MM, Warne AL, Stock NL, Mabury SA, Solomon KR, Sibley PK (2004) Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid to Chironomus tentans. Environ Toxicol Chem 23(9):2116–2123
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA (2004) Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 38(2):373–380
Minier C, Abarnou A, Jaouen-Madoulet A, Le Guellec AM, Tutundjian R, Bocquené G, Leboulenger F (2006) A pollution-monitoring pilot study involving contaminant and biomarker measurements in the Seine Estuary, France, using zebra mussels (Dreissena polymorpha). Environ Toxicol Chem 25(1):112–119
Pain S, Parant M (2007) Identification of multixenobiotic defence mechanism (MXR) background activities in the freshwater bivalve Dreissena polymorpha as reference values for its use as biomarker in contaminated ecosystems. Chemosphere 67(6):1258–1263
Pan K, Wang WX (2009) Biodynamics to explain the difference of copper body concentrations in five marine bivalve species. Environ Sci Technol 43(6):2137–2143
Sánchez-Avila J, Meyer J, Lacorte S (2010) Spatial distribution and sources of perfluorochemicals in the NW Mediterranean coastal waters (Catalonia, Spain). Environ Pollut 158(9):2833–2840
Smital T, Kurelec B (1997) Inhibitors of the multixenobiotic resistance mechanism in natural waters: in vivo demonstration of their effects. Environ Toxicol Chem 16(10):2164–2170
Smital T, Luckenbach T, Sauerborn R, Hamdoun AM, Vega RL, Epel D (2004) Emerging contaminants—pesticides, PPCPs, microbial degradation products and natural substances as inhibitors of multixenobiotic defense in aquatic organisms. Mutat Res Fund Mol Mech Mutagen 552(1–2):101–117
Stevenson CN, Macmanus-Spencer LA, Luckenbach T, Luthy RG, Epel D (2006) New perspectives on perfluorochemical ecotoxicology: inhibition and induction of an efflux transporter in the marine mussel, Mytilus californianus. Environ Sci Technol 40(17):5580–5585
Thompson RJ, Bayne BL (1972) Active metabolism associated with feeding in the mussel Mytilus edulis L. J Exp Mar Biol Ecol 9(1):111–124
Tutundjian R, Minier C (2007) Effect of temperature on the expression of P-glycoprotein in the zebra mussel, Dreissena polymorpha. J Therm Biol 32(3):171–177
Vicente J, Bertolero A, Meyer J, Viana P, Lacorte S (2012) Distribution of perfluorinated compounds in Yellow-legged gull eggs (Larus michahellis) from the Iberian Peninsula. Sci Total Environ 416:468–475
Vosloo D, Sara J, Vosloo A (2012) Acute responses of brown mussel (Perna perna) exposed to sub-lethal copper levels: Integration of physiological and cellular responses. Aquat Toxicol 106–107:1–8
Zar JH (1996) Bioestatistical analysis, 3rd edn. Bioestatistical AnalysisPrentice-Hall International, Inc, New Jersey
Acknowledgements
This study was funded by the Ministerio de Ciencia e Inovación and Ministerio de Medio Ambiente, Medio Rural y Marino projects CTM2011-30471-C02-01, 041/SGTB/2007/1.1 and 042/RN08/03.4 and by the Spanish and German Integrated action project DE2009-0089.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Fernández-Sanjuan, M., Faria, M., Lacorte, S. et al. Bioaccumulation and effects of perfluorinated compounds (PFCs) in zebra mussels (Dreissena polymorpha). Environ Sci Pollut Res 20, 2661–2669 (2013). https://doi.org/10.1007/s11356-012-1158-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1158-8