Abstract
Highly persistent perfluorooctane sulfonate (PFOS) is an industrial fluorinated organic chemical with significant bioaccumulation and biomagnification properties. The purpose of this study was to determine the toxic effects of sublethal PFOS on the aquatic invertebrate organism, narrow-clawed crayfish [Astacus leptodactylus Eschscholtz, 1823]. The 96 h LC50 value was determined as 48.81 mg/L (34.19–63.68 mg/L) with probit analysis. The sublethal experimental design was formed into four groups solvent control (DMSO, dimethyl sulphoxide), non-treated control group, and 1/10 (5 mg/L) and 1/100 (0.5 mg/L) of 96 h LC50 of PFOS, and crayfish were exposed for 48 h, 7 d, and 21 d under laboratory conditions. Total haemocyte counts (THCs) decreased, while the haemolymph total antioxidant status (TAS) values increased (p < 0.05) after exposure to 0.5 and 5 mg/L PFOS for 48 h, 7 d, and 21 d. Haemolymph total oxidative stress (TOS) levels significantly increased at 5 mg/L PFOS concentration (p < 0.05). Catalase (CAT) activities increased at both concentrations after 48 h and 7 d and then returned to control levels after 21 d; whereas superoxide dismutase (SOD) and glutathione peroxidase (GPX) activities did not change in muscle tissue (p > 0.05). GPX and CAT activities decreased, but SOD activity increased in hepatopancreas tissue (p < 0.05). SOD activity at both concentrations and CAT activity at 5 mg/L PFOS exposure decreased in gill tissue, while GPX activity increased at both concentrations of 48 h and 7 d and returned to control values on day 21 of exposure. Histopathological alterations were detected in hepatopancreas and gill tissues. Lamellar deformations, epithelial hyperplasia, and haemocytic infiltrations were observed in the gill tissues, whereas tubular degeneration, tubule loss, necrosis, and lesions in the hepatopancreas tissues were the major recorded alterations. As a result, the sublethal concentrations of PFOS have toxic effects on crayfish and histologically cause tissue damage. Our findings also support a better understanding of the early toxicological effects of PFOS in freshwater ecosystems. Also, it could be concluded that A. leptodactylus is a reliable model for examining histopathological alterations and differences in enzyme activities together with the haemolymph findings in toxicology studies amid aquatic species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent Organic Pollutants (POPs) are toxic chemicals that generally have lipophilic properties adversely affecting human and environmental health by accumulating in tissues, transferring from one species to the next through the food web, and being transported between countries (Sharma et al. 2014; Kissa 2001). Perfluorooctane sulfonate (PFOS), a water-soluble contaminant contrary to the classical apolar POPs, binds protein-rich tissues like the liver, circulates in the blood, and persists for years (Giesy and Kannan 2001; Jones et al. 2003; Olsen et al. 2007). The main causes of water PFOS contamination (surface, ground, and waste) and soil are the discharges from production facilities, firefighting activities, wastewater, sludge of sewage plants, and pesticide applications (Moody et al. 2003; Schultz et al. 2004; Huset et al. 2011; Liu et al. 2017). In addition, waste products containing PFOS are released via leachates from landfills due to their high solubility (Oliaei et al. 2013).
Depending on their accumulation capacity, PFOS is more toxic than the congener perfluorooctanoic acid (PFOA), a fully fluorinated anion (Cui et al. 2009). Generally, it is used as a salt or incorporated into larger polymers. Potassium, diethanolamine, ammonium, and lithium salts of PFOS are considerable examples of commercial use (UNEP 2006). In addition to those, PFOS can also be used in metal plating, photo imaging semi-conductor, medical device industries, sealing materials, aqueous film-forming foams, hydraulic fluids for aviation, and insecticide formulations (Chen et al. 2017; Kannan et al. 2002; Paul et al. 2009; Saikat et al. 2013; Stahl et al. 2011; Huang et al. 2020).
PFOS and its salts were added to Annex B of Persistent Organic Pollutants under the Stockholm Convention in 2009 (UNEP 2009). Even though production has halted, and applications limited to North America and Europe for its global elimination, there has been an increased demand in China (Liu et al. 2017). Although the usage of PFOS and its derivatives has been restricted since 2000, PFOS is still measured in aquatic environments due to its high persistent and lipophilic characteristics. The concentrations of PFOS in surface waters have been stated in the range from high pg/L to high ng/L (Kunacheva et al. 2012; Lindim et al. 2016). It has been measured at the highest levels in predatory and bottom-dwelling species (>3000 ng/g wet weight) due to its biomagnification attribute (Houde et al. 2006; Kannan et al. 2005; Martin et al. 2004). Laboratory and field studies have shown that the concentrations of PFOS ranging from µg/L to mg/L have the potential to induce sublethal toxicity including reproduction, development, and gene expression in various aquatic species (Shi et al. 2008; Jeong et al. 2016). Studies on the acute toxicity of freshwater invertebrates are very limited (Boudreau et al. 2003; Li 2009; Jeong and Simpson 2019; Logeshwaran et al. 2021). Li (2009) reported that PFOS is toxic to aquatic invertebrates, with acute toxicity values ranging from 10 to 300 mg/L and green neon shrimp (Neocaridina denticulate) was found to be the most sensitive freshwater species to PFOS with a 96 h LC50 of 10 mg/L. Hayman et al. (2021) reported the order of species sensitivity to PFOS and PFOA, starting with the most sensitive as Mediterranean mussel Mytilus galloprovincialis, the purple sea urchin Stronglyocentrotus purpuratus, dinoflagellate Pyrocystis lunula, and opossum shrimp Americamysis bahia.
In previous studies on various animal species, immunotoxic and neurotoxic effects, developmental and reproductive toxicity, oxidative stress, endocrine disruption, and genetic toxicity have been reported for PFOS (Austin et al. 2003; Ankley et al. 2005; Abbott 2009; DeWitt et al. 2009; Wan et al. 2012; Chen et al. 2018). Additionally, various other toxic effects of PFOS have been reported in aquatic invertebrates. Logeshwaren et al. (2021) studied 48 h acute and 21 d chronic assays on water fleas (Daphnia carinata) exposure to PFOS and PFOA. Chronic exposure to PFOS exhibited mortality and reproductive defects in D. carinata at 0.001 mg/L. Using the comet assay, DNA damage was detected at 1 and 10 mg/L for 96 h. Seyoum et al. (2020) reported developmental and reproductive toxicity of PFOS on Daphnia magna. Downregulation of related genes and decline in fertility were observed. Also, the lifespan with early mortality after 5 d and hatching decreased after exposure to 25 μM PFOS. Embryotoxic effects of increasing concentrations of PFOS (ranging from 0.5 to 10 mg/L) have been reported for the sea urchin, Paracentrotus lividus by Gunduz et al. (2013). Larval malformations in the skeletal system at low concentration and developmental arrest induced by dose-depended exposure were recorded. The growth of embryos was affected highly by 10 mg/L PFOS in the early life stages. Extensive screening of biota samples worldwide showed PFOS to be a global pollutant, and that it bioaccumulates at higher trophic levels in the food chain (Bossi et al. 2005; Fromme et al. 2009).
Related to their location in the food chain and their sensitivity to environmental stress factors, crustaceans are excellent representatives as biomarkers for monitoring water quality (Issartel et al. 2010). Crayfish are the largest mobile macrobenthic detrivores with a relatively simple anatomy in temperate freshwater ecosystems (Brittle et al. 2016). Astacus leptodactylus is a crayfish species with commercial value, widely distributed in Eurasian fresh waters (Balık et al. 2005; Köksal 1988). Freshwater crayfish are such an ideal model organism for monitoring the ecotoxicological risks of freshwater bodies (Sepici Dinçel et al. 2013).
Previous studies presented some toxic effects of PFOS in different fish species (Oakes et al. 2005; Liu et al. 2007; Hagenaars et al. 2008; Ji et al. 2008; Dorts et al. 2011). Hyperactivity, locomotor activity, developmental toxicity, thyroid disruption, hepatotoxicity, alteration of gene expression, yolk sac utilization, induced cell death in the tail region, brain and eye of embryos, histologically induced hepatic steatosis, and disrupted lipid metabolism caused lesions in the muscle fibers and various malformations in embryos/larvae of zebrafish exposed to PFOS were shown (Shi et al. 2009; Huang et al. 2010; Cheng et al. 2016; Cui et al. 2017; Jantzen et al. 2016; Tse et al. 2016).
Touaylia et al. (2019) assessed oxidative stress caused by PFOS on Gammarus insensibilis by measuring malondialdehyde (MDA) and superoxide dismutase (SOD) activities and the activity of acetylcholinesterase (AChE). SOD activity increased at a low concentration of PFOS while MDA increased in all treated groups (1, 1.6, and 3.1 mg/L of PFOS). AChE activity did not exhibit any significant changes, indicating no PFOS neurotoxicity. Immunotoxic effects of PFOS have been shown in Chinese mitten-handed crab (Eriocheir sinensis). Total haemocyte counts (THCs) were found low in PFOS exposed groups (0.1 mg/L, 1.0 mg/L, 10 mg/L), and the lowest count was measured after 21 d in the 10 mg/L treated group. Inhibition of phenoloxidase and superoxide dismutase activities were also significant in crabs exposed to 10 mg/L PFOS. Phenoloxidase and SOD increased initially, then decreased overtime at the other concentrations (Zhang et al. 2015).
In the present study, crayfish, an alternative bioindicator model organism for aquatic toxicology, was selected due to their key role in the freshwater aquatic food webs. The toxicity and the effects of PFOS have been examined on some crustacean species but are not available on crayfish, A. leptodactylus in the open literature. The aim of the present study is to focus on the effects of the persistent organic compound, PFOS in freshwater habitats using the bioindicator organism crayfish. We analyzed the biochemical and physiological responses of A. leptodactylus to the toxic effects of the sublethal PFOS exposure experiments at three time intervals, and at two concentrations. The total haemocyte counts, selected haemolymph parameters, and histopathological changes were evaluated in addition to direct toxicological data. The study also aimed to assess the antioxidant and oxidative status of the crayfish with the haemolymph total antioxidant status (TAS), total oxidative stress (TOS) levels, and the tissue levels of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) activities as biomarkers potentially affected by PFOS.
Materials and methods
Test organism and acclimation process
The freshwater crayfish (Astacus leptodactylus Eschscholtz, 1823), weight 29.10 ± 0.39 g (mean ± SEM) and length 10.27 ± 0.05 cm (mean ± SEM) were obtained from a local breeder (Lake Egirdir, Turkey) during the inter-moult stage. Crayfish were immediately transferred to the laboratory and stocked in glass aquariums during the acclimation period. Aquariums were filled with aerated dechlorinated tap water.
Crayfish were adapted for two weeks under laboratory conditions. Aquariums were constantly aerated with air pumps and regularly cleaned by siphoning. Water temperature was adjusted to 21 °C by thermostatic heaters. Experiments were carried out in the laboratory of the Biology Education Department at Gazi University. Experiments were performed according to the rules of “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication No. 85–23, revised 1996). The crayfish were fed daily with raw trout fish during the adaptation period.
Test chemical and experimental design
Heptadecafluorooctanesulfonic acid potassium salt (PFOS, CAS Number 2795-39-3, purity ≥ 98%, Sigma–Aldrich, USA) was used in the experiments. The substance was stored at +4 °C and brought to room temperature before use. To prepare the stock solution, PFOS in powder form was weighed in a volumetric glass flask, a certain volume was obtained by completing analytical purity with DMSO (dimethyl sulphoxide). Automatic pipettes with polypropylene tips were used for dosing, then glass mixing rods provided homogenization.
An acute toxicity test was carried out to estimate the median lethal concentration of PFOS on the selected organism to decide the experimental sublethal concentrations. After probit analysis (Supplementary Material A), the sublethal concentrations were selected as 1/10 and 1/100 of 96 h LC50 concentrations according to Sprague (1971) and decided as 5 mg/L and 0.5 mg/L PFOS. Applied concentrations in the aquarium water were in line with nominal and remained constant during the study.
Experimental aquariums containing 15 L of dechlorinated tap water were classified as exposure groups (0.5 and 5 mg/L PFOS concentrations) and control groups (DMSO control group and non-treated control group). Semi-static bioassay experimental method was used. Exposure media were renewed every 48 h. In the experiments, exposure results of 48 h, 7 d, and 21 d were investigated. All tests were repeated twice.
The water quality parameters were measured daily during the experiment (Supplementary Material B). The 18 h L: 6 h D cycle photoperiod was maintained during the experiments. Air pumps were on during the experiments and stopped only before the dosing instance. Crayfish were not fed for 48 h before and during the acute toxicity tests. The health status of crayfish was followed throughout the assays. No mortality was observed in the experiments.
Haemolymph sampling and analysis
The haemolymph samples were drawn from the base of the second walking legs of the crayfish using a disposable 2.5 cc syringe after exposure to PFOS for 48 h, 7 d, and 21 d. Prior to the sampling, crayfish were anesthetized on ice to slow down the animal activity. For haemocyte counting, the haemolymph samples were diluted with an equal volume of fixative (4% formalin). The other samples were placed in Eppendorf tubes without anticoagulants and stored at −80 °C until haemolymph analysis.
Total haemocyte counts (THCs)
THCs of crayfish exposed to two different concentrations (0.5 mg/L, 5 mg/L) of PFOS for 48 h, 7 d, and 21 d were determined. The haemocytes were counted with Thoma under a light microscope according to Yavuzcan and Benli (2004).
Total antioxidant status (TAS)
Total antioxidant status was measured using a commercial assay kit (Rel Assay Diagnostics, TR). The principle of the assay is incubation of ABTS [2,2′-azino-di-(3-ethylbenzthiazoline sulphonated)] with a peroxidase (metmyoglobin) and H2O2 to produce the radical cation ABTS+. ABTS+ loses colour based on antioxidant status. The change of colour shows total antioxidant level of the sample, absorbance measured at 660 nm. The antioxidant status of the added samples and the concentration of the colour formed are proportional (Miller et al. 1993).
Total oxidative stress level (TOS)
Total oxidative stress levels were measured using a commercial assay kit (Rel Assay Diagnostics, TR). TOS test principle is based on the oxidative stress level of the environment, the transformation of N, N-dimethyl-p-phenylenediamine (DMPD) into its radical form, and determination of the formed coloured radical product (DMPD+) colorimetrically. After 20 μL DMPD (100 mM) was added to 10 μL haemolymph sample taken into 2 ml acetate buffer (0.1 M, pH: 4.8), the sample was incubated at 37 °C for 75 min. DMPD+ formation was determined spectrophotometrically at 505 nm. The assay is calibrated with hydrogen peroxide prepared in 2.52 mM FeCl2 and PBS as standard (Verde et al. 2002). The results are expressed in micromolar H2O2 equivalents per liter (μM H2O2 Equiv/ L).
Tissue processing for histopathology
After haemolymph sampling, gills, hepatopancreas, nephridium, heart, gonads, and muscle tissues were dissected and removed immediately for histological examination; fixed in Davidson’s fixative (330 mL- 95% ethyl alcohol; 220 mL- formalin; 115 mL glacial acetic acid; 335 mL distilled water) (Bell and Lightner 1988). Tissues were placed in 70% ethyl alcohol after 24 h fixation.
Tissue samples were rinsed in tap water, dehydrated in graded alcohol series and embedded in paraffin. Paraffin blocks of tissue samples were cut at 5–6 µm thickness using rotary ThermoShandon microtome. After deparaffinization, slides were stained with Hematoxylin and Eosin according to routine procedures (Luna 1968). The slides were examined under a light microscope.
Biochemical analyses
For biochemical analyses, dissected gill, hepatopancreas, and muscle tissues were immediately frozen in liquid nitrogen, afterward stored at −80 °C until enzyme activity measurements. Superoxide dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPX) activities were measured using commercial assay kits (Cayman Chemical, USA). The biochemical analysis of the tissues were carried out individually with seven crayfish for each exposure concentration in two replicates.
Hepatopancreas, gill, and muscle tissue samples were rinsed with phosphate-buffered saline, pH: 7.4 to determine superoxide dismutase activity. Tissues were homogenized in 5–10 mL cold 20 mM HEPES buffer (at pH 7.2 containing 1 mM EDTA, 210 mM mannitol, and 70 mM sucrose) per gram. The homogenates were centrifuged at 4 °C for 5 min at 1500 × g, and the removed supernatants were kept at −80 °C until the assay. SOD assay was performed according to the method of Sun et al. (1988). SOD activity was inhibited by decreasing NBT, with xanthine–xanthine oxidase used as a superoxide generator, and one IU was defined as the quantity of SOD required to produce 50% inhibition. Tissue SOD activity was expressed as U/100 mg tissue.
CAT activity was measured according to the manufacturer’s directions. It was done by the dismutation of H2O2 at 240 nm based on the method of Aebi (1984). The tissues were homogenized on ice with 5–10 mL cold buffer (50 mM phosphate buffer at pH: 7.0 with 1 mM EDTA). Tissues were centrifuged at 4 °C for 15 min at 10,000 × g; the supernatants were removed and kept on ice until the enzyme assay.
GPX activity was measured according to the protocol of the Glutathione Peroxidase Assay Kit (Cayman Chemical, the USA). Tissue samples were homogenized in Tris-HCl containing EDTA (1/10; w/v). Tissue glutathione peroxidase activity results were stated as nmol/min/100 mg.
Statistical analysis
The LC50 value with 95% confidence limits was calculated by a computer program (CEAM 1999) using Finney’s Probit Analysis. The data were tested for normality and homogeneity of variances using the Shapiro–Wilks and Levene test. To compare continuous variables between multiple outcome variables, two-way ANOVA was used. Bonferroni test was used for multiple comparisons of groups. The statistical level of significance for all tests was considered to be 0.05. The results were expressed in the form of mean ± standard error (SEM).
Results
Acute toxicity
The determined 96 h LC50 values with 95% confidence limits of PFOS on crayfish was 48.81 mg/L (34.19–63.68 mg/L). There was no mortality in the control groups. The probit results of PFOS on crayfish are depicted in Supplementary Material A. The toxicity of PFOS is found in the Category: Acute 3 in crayfish. The Category: Acute 3 means 96 hr LC50 values are calculated as >10 – ≤100 mg/L (GHS, 2011).
Total haemocyte counts (THCs)
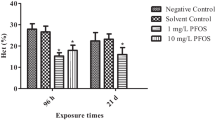
THCs decreased significantly after exposure to two different sublethal PFOS concentrations in groups (p < 0.05). The difference was not statistically significant in groups exposed to PFOS except 5 mg/L for 7 d, and no significant differences were observed between the control groups (p > 0.05). The results are shown in Fig. 1A.
Total antioxidant status (TAS)
Haemolymph TAS levels increased significantly in all groups exposed to PFOS and reached the highest level after 21 d (p < 0.05). There were no significant differences between control groups. The results are shown in Fig. 1B.
Total oxidative stress (TOS)
Haemolymph TOS levels of crayfish were increased after 48 h and 7 d exposure to 5 mg/L PFOS. Although a decrease was observed after 21 d, it was still higher than the control statistically (p < 0.05). There was no significant difference of haemolymph TOS levels between 0.5 mg/L PFOS exposed, and the control groups. The results are shown in Fig. 1C.
Histopathological alterations
After exposure to sublethal PFOS, the muscle, heart, gonads, and nephridium tissues of the crayfish did not reveal any significant histopathological findings compared to the control groups. However, the gill and the hepatopancreas tissues were affected histologically from the sublethal PFOS exposure. The main histopathological lesions were observed in the hepatopancreatic tissues. All results of the histopathological findings are shown in Table 1. Gill tissues of the crayfish following exposure to PFOS exhibited lamellar deformations, epithelial hyperplasia, and haemocytic infiltrations (Fig. 2). Tubular degeneration, tubular loss, necrosis, and lesions were observed in the hepatopancreas tissues depending on the duration and the concentration of the PFOS (Fig. 3).
Gill tissue of crayfish (a) longitudinal section of normal gill lamella (black thick arrow) with epithelial cells (black thin arrow), (b) epithelial hyperplasia (black thin arrow) and hemocytic infiltration (blue arrow) after exposed to 5 mg/L PFOS for 21 days, (c) cross section of the normal gill tissue with afferent and efferent vessels (red arrow), (d) hemocytic infiltration (red arrow) of afferent and efferent vessels after exposed to 5 mg/L PFOS for 21 days
Hepatopancreas tissue of crayfish (a) histological appearance of normal tubules (black thick arrow) with lumens (L) and blister like B cells (black thin arrow), (b) degeneration of tubules (blue arrow) after exposed to 5 mg/L PFOS for 21 days, (c) enlargement of tubule lumen (red arrow), (d) necrosis of tubule (red arrow) with hemocytic infiltration (HI) after exposed to 5 mg/L PFOS for 21 days.
Biochemical analyses
SOD activities decreased in gill tissues at both concentrations. The highest decline was observed in the group exposed to 5 mg/L PFOS for 48 h, but the decrease was not statistically significant (p > 0.05). SOD activities in the gill tissue of crayfish are described in Fig. 4A. In hepatopancreas tissues, SOD activities increased in all groups significantly except the group exposed to 0.5 mg/L for 48 h (p < 0.05) (Fig. 4B). It was detected that there was no significant difference in the SOD activities between the control group and the groups exposed to PFOS in muscle tissue. (p > 0.05) (Fig. 4C).
CAT activities of gill tissues in crayfish exposed to 0.5 mg/L for 21 d and 5 mg/L PFOS during all exposure period decreased significantly (p < 0.05). There were no differences between the control groups and the groups exposed to 0.5 mg/L PFOS considerably for 48 h and 7 d (p > 0.05). The CAT activity results of gill tissues are shown in Fig. 5A. CAT activities of hepatopancreas tissues did not change in the groups exposed to 0.5 mg/L of PFOS following 48 h and 7 d compared to control groups; however, a sharp decrease was observed in the group after 21 d and reached the lowest level compared to other groups (p < 0.05). CAT activity in the groups exposed to 5 mg/L PFOS decreased significantly for all test periods and reached the lowest level after 21 d in hepatopancreas (Fig. 5B). In muscle tissues, CAT activities increased significantly after 48 h and 7 d in both groups exposed to 0.5 mg/L and 5 mg/L PFOS. However, there was no significant difference between the groups after 21 d (p > 0.05). CAT activity results of the muscle are shown in Fig. 5C.
Muscle tissue GPX activities did not alter after PFOS exposure (p > 0.05) (Fig. 6A). GPX activities significantly increased after 48 h and 7 d at both concentrations (p < 0.05), but returned to control groups’ levels after 21 d in gill tissues (Fig. 6B). In hepatopancreas, GPX activities significantly decreased after 48 h and 7 d when compared to control groups at both concentrations (Fig. 6C). Although GPX activity increased in the groups exposed to PFOS after 21 d in hepatopancreas, the results were still statistically lower compared to control groups (p < 0.05).
Discussion
This study presents the toxicological data of persistent organic pollutant PFOS on the crayfish, an aquatic model and bioindicator invertebrate organism. Haemocytes are quite reliable biomarkers for monitoring humoral and cellular immunologic status of invertebrates. Their number would increase or decrease when the species is affected by environmental stress factors including pollutants (Jussila et al. 1997; Perazzolo et al. 2002; Smith and Johnston 1992; Le Moullac and Haffner 2000; Liu et al. 2021). The total haemocyte counts (THCs) of A. leptodactylus decreased significantly after exposure to two different sublethal PFOS concentrations for all time periods. This decrease in the THCs in the freshwater crayfish may be a reflection of the stress on organisms due to the deterioration of the water quality of the pollutant (PFOS). These results may also indicate the immunosuppressive effects of PFOS on freshwater crayfish immunity, which could make them susceptible to disease (Yavuzcan and Benli 2004; Günal et al. 2021; Arslan 2022; Khoei 2022). Decreases in haemocytes have been recorded in various crustaceans under stress conditions caused by environmental contaminants or changes in water parameters. Zhang et al. (2015) indicated similar significant decrease of Eriocheir sinensis THCs after PFOS exposure (0.1, 1.0, and 10 mg/L). Qiu et al. (2011) recorded a remarkable reduction in THCs in the first three and sixth hours when temperature dropped in the shrimp Litopenaeus vannamei. Qin et al. (2012) observed a decrease in THCs of the freshwater crab Sinopotamon henanense after exposure to cadmium (58 and 116 mg/L) for 96 h. Similarly, Mello et al. (2011) reported a decline in THCs after exposure to 250 SC Sirus herbicide of pyrazosulfuron‐ethyl (0.1–1000 µg/L) for 96 h in Litopenaus vannamei. Contrary to our results, Gunal et al. (2021) determined an increase in THCs of A. leptodactylus exposed to 0.09 µg/L permethrin for 48 and 96 h.
TAS represents the full spectrum of antioxidant activity against various nitrogen and ROS species (Yağan et al. 2014). The measurement of individual antioxidant biomarkers leads to loss of labour time and cost; necessitates qualified staff and complex techniques. Nonetheless, the measurement of the total antioxidant status (TAS) is the preferred, easy to perform and rapid method by researchers. Previous studies have indicated that plasma or haemolymph TAS levels of aquatic animals are reliable biomarkers of immune reactions against stressors (Livingstone 2003; Valavanidis et al. 2006; Sepici Dincel et al. 2009). In the present study, haemolymph TAS levels of PFOS exposed crayfish exhibited significant rise dependent on exposure time for both concentrations. Hoff et al. (2003) did not observe any changes in serum TAS of juvenile carp, Cyprinus carpio exposed to PFOS after 1 and 5 d. Similarly, our findings, Yucel Isıldar et al. (2020) determined increased haemolymph TAS levels of crayfish A. leptodactylus exposed to 0.05 μg/L deltamethrin but the increase was not significant. Franco-Martinez et al. (2016) observed an increase in TAS levels of the gill tissues of mussels Mytilus galloprovincialis exposed to Pb, Cd, and mixture of Cu+Pb+Cd, but results were not statistically significant. The increase in TAS, especially its increase with time, reveals the defensive response of the antioxidant system against increased reactive oxygen species (ROS) (Selvi et al. 2011). Contrary to these, significant reduction in haemolymph TAS of M. galloprovincialis was observed by Kaloyianni et al. (2009). They firstly recorded unaltered haemolymph TAS levels of mussels after 6 d of exposure to Cd and Zn, but a certain decrease revealed after exposure to PAHs mixture and lindane for 12 d. Patetsini et al. (2013) observed a reduction in haemolymph TAS levels of mussels, M. galloprovincialis after exposure to 0.05 μg/L chlorpyrifos and penoxsulam from the initial day to the 30th day. A decrease was reported in plasma TAS of Cyprinus carpio exposed to 24 h and 48 h to esbiothrin (5 and 10 µg/L), but TAS levels increased significantly after 72 h in both exposure doses (Selvi et al. 2011). Low TAS levels mostly indicate oxidative stress or increased susceptibility to oxidative damage (Young 2001).
Haemolymph TOS levels in the present study did not change in groups exposed to 0.5 mg/L PFOS, whereas it was higher in groups exposed to 5 mg/L PFOS compared to controls. Although TOS level decreased after 21 d when compared to 48 h and 7 d in 5 mg/L PFOS exposed groups, it was still higher than that of the control groups. Knowledge gaps exist in evaluation of TOS as biomarker in aquatic invertebrates in the open literature. Our findings are valuable as the first report about TOS levels determination of A. leptodactylus. A similar increase was observed by Franco-Martinez et al. (2016) in TOS and total antioxidant capacity (TAC) in gills of Mytilus galloprovincialis exposed to Pb, but a rise in TAC was not statistically significant.
Crayfish exposed to PFOS revealed evident alterations in the gills and hepatopancreas tissues in the present study. Control groups did not show any histopathological changes in the tissues. In addition, no significant histopathological changes were observed in the muscle, heart, gonads, and nephridium tissues of crayfish exposed to PFOS. Concentrations of 0.5 mg/L and 5 mg/L PFOS caused obvious lamellar deformations and haemocytic infiltration in the gill tissues. Presently no study reporting PFOS toxicity damage in crayfish gill histology is in the open literature. Similar to our results, Desouky et al. (2013) reported haemocytic infiltration in the haemocoelic space and swelling of the gill lamellae after 24 h exposure to 0.36 mg/L of ethion on gills of Procambarus clarkii. Benli et al. (2016) reported hyperemia and melanisation on the gill lamella after being exposed to 9 mg/L (2,4-dichlorophenoxy) acetic acid (2,4-D) for one week on the same crayfish species, narrow-clawed crayfish (A. leptodactylus). Yu et al. (2018) found 44.29% gill cuticula vagueness, degeneration, and 33.27% epithelial cell lesions at 96 h after 0.24 mg/L pymetrozine exposure on gills of the crayfish Procambarus clarkii.
In this study, tubular degeneration, tubular loss, necrosis, and lesions were observed in the hepatopancreas of the crayfish exposed to PFOS. Few studies are showing the hepatotoxic effect of PFOS in aquatic organisms. Tse et al. (2016) indicated that exposure to PFOS can cause liver pathogenesis and non-alcoholic fatty liver disease changes in different metabolic processes in the liver in zebrafish. Fang et al. (2013) determined that the fish larvae of marine medaka (Oryzias melastigma) after exposure to 16 mg/L PFOS at 17 days’ post-hatch showed a marked enlargement on the cell nuclei of the liver. Similar to histopathological findings in the present study, histological alterations were observed in hepatopancreas tissues of crayfish exposed to various types of toxicants. Benli (2015) determined necrosis on tubule cells in the hepatopancreas of A. leptodactylus in the treated groups with etofenprox (0.04 and 0.1 µg/L) for 96 h. The lesions in hepatopancreas tissue were more severe at the 0.1 µg/L etofenprox exposed group. Gunal et al. (2021) reported degenerative tubules in hepatopancreas tissues and haemocytic infiltrations in the afferent and efferent vessels in gills of A. leptodactylus after being exposed to 0.09 μg/L permethrin for 48 and 96 h. Zhang et al. (2019) found tubule lumen dilatation in the hepatopancreas tissue of adult male P. clarkii after exposure to Cd. The hepatopancreas exposed to 10 mg/L Cd for 72 h showed evident epithelium vacuolization. Chabera et al. (2021) observed focal dilatation of tubules, increased number of fibrillar cells, and haemocyte infiltration in the interstitium of Pacifastacus leniusculus exposed to 0.45 µg/L and 2.7 µg/L chloridazon-desphenyl (Ch metabolite) and 2.7 µg/L chloridazon (Ch) for 30 days.
ROS are produced as a normal product of cellular metabolism in healthy organisms. The level of ROS can be stabilized by an antioxidant defense system including SOD, CAT and GPX. However, if the balance is broken and shifts in the direction of oxidants, that is, if the cell is exposed to more ROS, it results in oxidative stress (Halliwell 1999). ROS are produced in mitochondria by leakage electrons in the complex I and III enzyme systems in the electron transport chain. Due to leakage of electrons into the cell, the concentration of molecular oxygen is reduced. Therefore, the cell protects itself via antioxidant enzyme systems like SOD, CAT, GPX, and small non-protein antioxidants such as reduced glutathione (Bal et al. 2021). Some environmental pollutants may induce ROS and cause oxidative stress, thus altering antioxidant activities (Stara et al. 2012). Several studies have reported oxidative stress responses to PFOS in aquatic organisms (Shi and Zhou 2010; Feng et al. 2015; Lu et al. 2015). In previous studies, antioxidant enzyme activities have been observed as biochemical biomarkers for environmental monitoring in crustacean species (Hossain et al. 2021; Kim et al. 2018; Pan and Zhang 2006). However, data on the antioxidant enzyme activities of A. leptodactylus exposed to PFOS are still lacking.
The results of the present study revealed different responses of enzyme activities in selected tissues of crayfish. This might be explained as the different metabolic activities of the examined tissues and their different responses to environmental conditions (Borkovic et al. 2008). The SOD activity showed a tissue-dependent response in the present study after exposure to PFOS. The SOD activity decreased in gill tissues after exposure to all concentrations of PFOS whereas it significantly increased in hepatopancreas. There was no significant change recorded in muscle tissues. Similar to our results, Amraoui et al. (2018) observed a decrease in SOD activity in the gill tissues after 7 d of exposure to 10 mg/L PFOS in Unio ravoisieri. Lu et al. (2015) reported that SOD activity reduced by 0.2, 1, and 5 mg/L PFOS exposure in Daphnia magna. Liu et al. (2007) studied SOD activities in the hepatocytes of Oreochromis niloticus. SOD activity did not change in 1, 5, and 30 mg/L PFOS treated groups whereas there was an increase in the 15 mg/L treated group after 24 h. Contrary to our results, increased SOD activity in the gill tissues was determined in A. leptodactylus after exposure to carbaryl for 48 h and 7 d (Benli et al. 2012).
CAT activity decreased in the gill and hepatopancreas tissues at 0.5 and 5 mg/L PFOS for 21 d. In particular, a marked decline was observed in hepatopancreas tissues at both concentrations at high exposure time (21 d). In contrast, an increase was seen in muscle tissues after 48 h and 7 d at both concentrations, and no change was observed between control after 21 d in muscle tissues. The decreased CAT activity induces the accumulation of hydrogen peroxide and increases lipid peroxidation (Halliwell 1999). Similar to our findings, Lu et al. (2015) found decreased CAT activity in all PFOS treated groups; furthermore, maximum reduction was recorded in the 5 mg/L PFOS exposed group after 7 d. Kim et al. (2010) reported a decrease in serum of C. carpio 864 ng/g treated group, but it was not significant. Contrarily, a concentration-dependent increase in CAT activity (0.2, 0.4, 1 mg/L) was observed in zebrafish embryos (96 hpf) by Shi and Zhou (2010). Amraoui et al. (2018) determined high CAT activity in the gill and digestive gland after exposure to 10 mg/L PFOS. CAT activity increase in the gill and hepatopancreas tissues was also determined in different pesticide exposure of A. leptodactylus (Benli et al. 2012; Yucel Isildar et al. 2020). Contrasting results of the CAT activities in the literature may be explained with the changes in potential antioxidants depending on the habitat, the species, the tissues, and chemicals in contaminated environments (Pala 2019).
GPX activities of gill tissues significantly increased in both concentrations of PFOS after 48 h and 7 d, but it returns to control group levels after 21 d. On the other hand, GPX decreased in hepatopancreas tissues. Despite the increase after 21 d in both concentrations, GPX activity was still statistically lower than in the control groups. Also, there was no alteration observed in muscle tissues. Feng et al. (2015) reported a decline in GPX levels in the liver of Carassius auratus exposed to 10 µmol/L PFOS after 4 d. Liu et al. (2007) reported a dose-dependent reduction of GPX activity in primary cultured hepatocytes of Oreochromis niloticus. Antioxidant enzyme activities facilitate organisms’ partial or complete stress defense induced under unsafe environmental conditions. However, high level toxicity may have inhibited these enzymes by causing excessive ROS production (Xu et al. 2013a), which leads to oxidative cell damage in tissues (Xu et al. 2013b).
In the 0.5 mg/L PFOS exposed group, the TAS in the haemolymph, SOD activities in the hepatopancreas and GPX activities in the gill tissues were higher, while the TOS in the haemolymph, SOD activities in the gill, GPx activities in the hepatopancreas, and CAT activities in the gill tissues decreased. Compared to the 0.5 mg/L PFOS exposed group, the SOD activities in the gill tissues, GPX activities in the hepatopancreas and CAT activities in the gill and hepatopancreas tissues were lower, the TAS and TOS in the haemolymph, SOD activities in the hepatopancreas, GPX activities in the gill tissues and CAT activities in the muscle tissues increased in the 5 mg/L PFOS exposed group.
Conclusion
The current study clearly showed that the persistent organic pollutant PFOS reduces the THCs, alters the antioxidant enzyme activities, and produces histopathological alterations in gill and hepatopancreas tissues of the freshwater crayfish even at low doses. While total antioxidant status levels increased depending on the doses and exposure time, total oxidant status levels increased only 5 mg/L at 48 h and 7 d but decreased at 21 d. While catalase and glutathione peroxidase activities decreased, superoxide dismutase activity increased in hepatopancreatic tissues. The superoxide dismutase activity decreased in the gill tissues. The obtained results of this study indicated that the sublethal concentrations of PFOS are toxic even at the cellular, biochemical and histopathological levels for aquatic organisms. The biomarkers used in the current study are proper for determining the PFOS toxicity, and the freshwater crayfish can be used as a bioindicator species to exhibit early PFOS toxicity.
References
Abbott BD (2009) Review of the expression of peroxisome proliferator-activated receptors alpha (PPARα), beta (PPARβ), and gamma (PPARγ) in rodent and human development. Reproduc Tox 27(3–4):246–257. https://doi.org/10.1016/j.reprotox.2008.10.001
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Amraoui I, Khalloufi N, Touaylia S (2018) Effects to perfluorooctane sulfonate (PFOS) on the mollusk Unio ravoisieri under laboratory exposure. Chem Ecol 34:324–339. https://doi.org/10.1080/02757540.2018.1433168
Ankley GT, Kuehl DW, Kahl MD, Jensen KM, Linnum A, Leino RL, Villeneuve DA (2005) Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ Toxicol Chem 24(9):2316–2324. https://doi.org/10.1897/04-634R.1
Arslan, P (2022) Determinations of the effects of cyfluthrin on the hemocytes parameters of freshwater mussel (Unio delicatus). Ege J Fish Aquat Sci 39(1):39–45. Retrieved from http://www.egejfas.org/tr/pub/issue/68550/988408
Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SMJ (2003) Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect 111(12):1485–1489. https://doi.org/10.1289/ehp.6128
Bal A, Panda F, Pati SG, Das K, Agrawal PK, Paital B (2021) Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp Biochem Physiol Part C: Toxicol Pharmacol 241:108971. https://doi.org/10.1016/j.cbpc.2020.108971
Balık S, Ustaoglu MR, Sarı HM, Berber S (2005) Determination of traits some growth and morphometric of crayfish (Astacus leptodactylus Eschscholtz, 1823) at Demirkopru Dam Lake (Manisa). Ege J Fish Aquat Sci 22:83–89
Bell TA, Lightner DV (1988) A handbook of normal penaeid shrimp histology. Baton Rouge, Louisiana
Benli ACK, Şahin D, Koçak B, Sepici Dinçel A (2012) Determination of antioxidant enzyme levels of narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823) exposed to carbaryl. Turk J Bioch 37(2):162–166
Benli ACK (2015) The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): acute toxicity and sublethal effects on histology, hemolymph parameters, and total hemocyte counts. Environ Toxicol 30:887–894. https://doi.org/10.1002/tox.21963
Benli ACK, Sahin D, Selvi M, Sarıkaya R, Memmi BK, Dinçel AS (2016) The sublethal effects of (2,4-dichlorophenoxy) acetic acid (2,4-D) on narrow-clawed crayfish (Astacus leptodactylus Eschscholtz, 1823). Arch Ind Hy. Toxicol 67:289–296
Borkovic SS, Pavlovic SZ, Kovacevic TB, Stajn AS, Petrovic VM, Saicic ZS (2008) Antioxidant defence enzyme activities in hepatopancreas, gills and muscle of Spiny cheek crayfish (Orconectes limosus) from the River Danube. Comp Biochem Phys C 147:122–128. https://doi.org/10.1016/j.cbpc.2007.08.006
Bossi R, Riget FF, Dietz R, Sonne C, Fauser P, Dam M, Vorkamp K (2005) Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ Pollut 136(2):323–329. https://doi.org/10.1016/j.envpol.2004.12.020
Boudreau TM, Sibley PK, Mabury SA, Muir DG, Solomon KR (2003) Laboratory evaluation of the toxicity of perfluorooctane sulfonate (PFOS) on Selenastrum capricornutum, Chlorella vulgaris, Lemna gibba, Daphnia magna, and Daphnia pulicaria. Arch Environ Contam Toxicol 44:0307–0313
Brittle SW, Paluri SLA, Foose DP, Ruis MT, Amato MT, Lam NH, Buttigieg B, Gagnon ZE, Sizemore IE (2016) Freshwater crayfish: a potential benthic-zone indicator of nanosilver and ionic silver pollution. Environ Sci Technol 50:7056–7065. https://doi.org/10.1021/acs.est.6b00511
CEAM (Center for Exposure Assessment Modeling) (1999) U.S.E.P.A. LC50 Software Program, Version 1.00. CEAM Distribution Center, Athens, GA
Chabera J, Stara A, Kubec J, Buric M, Zuskova E, Kouba A, Velisek J (2021) The effect of chronic exposure to chloridazon and its degradation product chloridazon-desphenyl on signal crayfish Pacifastacus leniusculus. Ecotoxicol and Environ Saf 208:111645. https://doi.org/10.1016/j.ecoenv.2020.111645
Chen H, Han J, Zhang C, Cheng J, Sun R, Wang X, Han G, Yang W, He X (2017) Occurrence and seasonal variations of per- and polyfluoroalkyl substances (PFASs) including fluorinated alternatives in rivers, drain outlets and the receiving Bohai Sea of China. Environ Pollut 231:1223–1231. https://doi.org/10.1016/j.envpol.2017.08.068
Chen J, Zheng L, Tian L, Wang N, Lei L, Wang Y, Dong Q, Huang C, Yang D (2018) Chronic PFOS exposure disrupts thyroid structure and function in zebrafish. Bull Environ Contam Toxicol 101:75–79. https://doi.org/10.1007/s00128-018-2359-8
Cheng J, Lv S, Nie S, Liu J, Tong S, Kang N, Xiao Y, Dong Q, Huang C, Yang D (2016) Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquat Toxicol 176:45–52. https://doi.org/10.1016/j.aquatox.2016.04.013
Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB (2009) Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 56:338. https://doi.org/10.1007/s00244-008-9194-6
Cui Y, Lv S, Liu J, Noe S, Chen J, Dong Q, Huang C, Yang D (2017) Chronic perfluorooctanesulphonic acid (PFOS) exposure disrupts lipid metabolism in zebrafish model. Hum Exp Toxicol 36:207–217. https://doi.org/10.1177/0960327116646615
Desouky MMA, Abdel Gawad H, Hegazi B (2013) Distribution, fate and histopathological effects of ethion insecticide on selected organs of the crayfish, Procambarus clarkii. Food Chem Toxicol 52:42–52. https://doi.org/10.1016/j.fct.2012.10.029
DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden Adams MM, Luebke RW, Luster MI (2009) Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Critical Rev Toxicol 39:76–94. https://doi.org/10.1080/10408440802209804
Dorts J, Kestemont P, Marchand PA, D’Hollander W, Thezenas ML, Raes M, Silvestre F (2011) Ecotoxicoproteomics in gills of the sentinel fish species, Cottus gobio, exposed to perfluorooctane sulfonate (PFOS). Aquat Toxicol 103:1–8. https://doi.org/10.1016/j.aquatox.2011.01.015
Fang C, Huang Q, Ye T, Chen Y, Liu L, Kang M, Lin Y, Shen H, Dong S (2013) Embryonic exposure to PFOS induces immunosuppression in the fish larvae of marine medaka. Ecotoxicol Environ Saf 92:104–111. https://doi.org/10.1016/j.ecoenv.2013.03.005
Feng M, He Q, Meng L, Zhang X, Sun P, Wang Z (2015) Evaluation of single and joint toxicity of perfluorooctane sulfonate, perfluorooctanoic acid, and copper to Carassius auratus using oxidative stress biomarkers. Aquat Toxicol 161:108–116. https://doi.org/10.1016/j.aquatox.2015.01.025
Franco-Martinez L, Romero D, García Navarro JA, Teles M, Tvarijonaviciute A (2016) Esterase activity (EA), total oxidant status (TOS) and total antioxidant capacity (TAC) in gills of Mytilus galloprovincialis exposed to pollutants: analytical validation and effects evaluation by single and mixed heavy metal exposure. Mar Pollut Bull 102:30–35. https://doi.org/10.1016/j.marpolbul.2015.12.010
Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds – Exposure assessment for the general population in western countries. Int J Hyg Environ Health 212:239–270. https://doi.org/10.1016/j.ijheh.2008.04.007
GHS, 2011. Globally Harmonized System for the classification and labelling of chemicals. (accessed 22.05.2021). https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev04/English/ST-SG-AC10-30-Rev4e.pdf
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7):339–1342. https://doi.org/10.1021/es001834k
Gunal AC, Tunca SK, Arslan P, Gül G, Sepici Dinçel A (2021) How does sublethal permethrin effect non-target aquatic organisms? Environ Sci Pollut Res 28:52405–52417. https://doi.org/10.1007/s11356-021-14475-4
Gunduz G, Parlak H, Arslan OÇ, Boyacioglu M, Karaaslan MA (2013) Embryotoxic effects of perfluorooctane sulfonate compounds in Sea Urchin Paracentrotus lividus. Fresenius Environ Bull 22:171–177
Hagenaars A, Knapen D, Meyer IJ, van der Ven K, Hoff P, De Coen W (2008) Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio). Aquat Toxicol 88(3):155–163. https://doi.org/10.1016/j.aquatox.2008.04.002
Halliwell B (1999) Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res 31:261–272. https://doi.org/10.1080/10715769900300841
Hayman NT, Rosen G, Colvin MA, Conder J, Arblaster JA (2021) Aquatic toxicity evaluations of PFOS and PFOA for five standard marine endpoints. Chemosphere 273:129699
Hoff PT, Dongen WV, Esmans EL, Blust R, De Coen WM (2003) Evaluation of the toxicological effects of perfluorooctane sulfonic acid in the common carp (Cyprinus carpio). Aquat. Toxicol. 62:349–359. https://doi.org/10.1016/S0166-445X(02)00145-5
Hossain MM, Huang H, Yuan Y, Wan T, Jiang C, Dai Z, Xiong S, Cao M, Tu S (2021) Silicone stressed response of crayfish (Procambarus clarkii) in antioxidant enzyme activity and related gene expression. Environ Pollut 274:115836. https://doi.org/10.1016/j.envpol.2020.115836
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG (2006) Biological monitoring of polyfluoroalky substances: a review. Environ Sci Technol 40(11):3463–3473. https://doi.org/10.1021/es052580b
Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, Tanguay RL, Dong Q (2010) Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat Toxicol 98:139–147. https://doi.org/10.1016/j.aquatox.2010.02.003
Huang J, Sun L, Mennigen JA, Liu Y, Liu S, Zhang M, Wang Q, Tu W (2020) Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: an emphasis on cilia disruption. J Hazardous Mater 409:124491. https://doi.org/10.1016/j.jhazmat.2020.124491
Huset CA, Barlaz MA, Barofsky DF, Field JA (2011) Quantitative determination of fluorochemicals in municipal landfill leachates. Chemosphere 82:1380–1386. https://doi.org/10.1016/j.chemosphere.2010.11.072
Issartel J, Boulo V, Wallon S, Geffard O, Charmantier G (2010) Cellular and molecular osmoregulatory responses to cadmium exposure in Gammarus fossarum (Crustacea, Amphipoda). Chemosphere 81:701–710. https://doi.org/10.1016/j.chemosphere.2010.07.063
Jantzen CE, Annunziato KM, Cooper KR (2016) Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat Toxicol 180:123–130. https://doi.org/10.1016/j.aquatox.2016.09.011
Jeong TY, Yuk MS, Jeon J, Kim SD (2016) Multigenerational effect of perfluorooctane sulfonate (PFOS) on the individual fitness and population growth of Daphnia magna. Sci Total Environ 569–570:1553–1560. https://doi.org/10.1016/j.scitotenv.2016.06.249
Jeong TY, Simpson MJ (2019) Reproduction stage differentiates the time-course regulation of metabolites in Daphnia magna. Environ Sci Technol 53:12764–12773. https://doi.org/10.1016/j.cbd.2020.100701
Ji K, Kim Y, Oh S, Ahn B, Jo H, Choi K (2008) Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid on freshwater macroinvertebrates (Daphnia magna and Moina macrocopa) and fish (Oryzias latipes). Environ Toxicol Chem 27:2159–2168. https://doi.org/10.1897/07-523.1
Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP (2003) Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol and Chem. https://doi.org/10.1897/02-553
Jussila J, Jago J, Tsvetnenko E, Dunstan B, Evans LH (1997) Total and differential hemocyte counts in western rock lobsters (Panulirus cygnus George) under postharvest stress. Mar Freshwater Res 48:863–868. https://doi.org/10.1071/MF97216
Kaloyianni M, Dailianis S, Chrisikopoulou E, Zannou A, Koutsogiannaki S, Alamdari DH, Koliakos G, Dimitriadis VK (2009) Oxidative effects of inorganic and organic contaminants on haemolymph of mussels. Comparative Biochem Physiol C Toxico Pharma 149:631–639. https://doi.org/10.1016/j.cbpc.2009.01.006
Kannan K, Hansen KJ, Wade TL, Giesy JP (2002) Perfluorooctane Sulfonate in Oysters, Crassostrea virginica, from the Gulf of Mexico and the Chesapeake Bay, USA. Arch Environ Contam Toxicol 42:313–318. https://doi.org/10.1007/s00244-001-0003-8
Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated Compounds in Aquatic Organisms at Various Trophic Levels in a Great Lakes Food Chain. Arch Environ Contam Toxicol 48:559–566. https://doi.org/10.1007/s00244-004-0133-x
Kim H, Kim JS, Kim PJ, Won EJ, Lee YM (2018) Response of antioxidant enzymes to Cd and Pb exposure in water flea Daphnia magna: differential metal and age-specific patterns. Comp Biochem Physiology C Toxico Pharma 209:28–36. https://doi.org/10.1016/j.cbpc.2018.03.010
Kim WK, Lee SK, Jung J (2010) Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. J Hazard Mater 180:395–400. https://doi.org/10.1016/j.jhazmat.2010.04.044
Kissa E (2001) Fluorinated Surfactants and Repellants, 2nd ed. Marcel Decker, New York, p 1–21
Khoei AJ (2022) Seasonal heavy metal accumulations in the bivalve Barbatia decussate and their relationships with water quality and the metal-induced biochemical biomarkers. Environ Sci Pollut Res 29:16103–16112. https://doi.org/10.1007/s11356-021-16893-w
Köksal G (1988) Astacus leptodactylus in Europe. In: Holdich DM, Lowery R eds Freshwater crayfish biology, management and exploitation. Croom Helm, London, UK, p 365–400
Kunacheva C, Fujii S, Tanaka S, Seneviratne STMLD, Lien NPH, Nozoe M, Kimura K, Shivakoti BR, Harada H (2012) Worldwide surveys of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in water environment in recent years. Water Sci Technol 66:2764–2771
Le Moullac G, Haffner P (2000) Environmental factors affecting immune responses in Crustacea. Aquaculture 191:121–13. https://doi.org/10.1016/S0044-8486(00)00422-1
Lindim C, Van Gils J, Cousins IT (2016) Europe-wide estuarine export and surface water concentrations of PFOS and PFOA. Water Res 103:124–132
Li MH (2009) Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to plants and aquatic invertebrates. Environ Toxicol 24:95–101
Liu C, Yu K, Shi X, Wang J, Lam PKS, Wu RSS, Zhou B (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat Toxicol 82:135–143. https://doi.org/10.1016/j.aquatox.2007.02.006
Liu X, Xu Z, Chang X, Fang JKH, Song J, He J, Tai Z, Zhu Q, Hu M (2021) Enhanced immunity and hemocytes proliferation by three immunostimulants in tri-spine horseshoe crab Tachypleus tridentatus. Fish Shellfish Immunol 115:112–123. https://doi.org/10.1016/j.fsi.2021.06.001
Liu Z, Lu Y, Wang P, Wang T, Liu S, Johnson AC, Sweetman AJ, Baninla Y (2017) Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci Total Environ 580:1247–1256. https://doi.org/10.1016/j.scitotenv.2016.12.085
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev. Méd. Vét. Toulouse. 154:427–430
Logeshwaran P, Sivaram AK, Surapaneni A, Kannan K, Naidu R, Megharaj M (2021) Exposure to perfluorooctanesulfonate (PFOS) but not perflurorooctanoic acid (PFOA) at ppb concentration induces chronic toxicity in Daphnia carinata. Sci Total Environ 769:144577
Lu GH, Liu JC, Sun LS, Yuan LJ (2015) Toxicity of perfluorononanoic acid and perfluorooctane sulfonate to Daphnia magna. Water Sci Eng 8:40–48. https://doi.org/10.1016/j.wse.2015.01.001
Luna LG (1968) Manual of histological staining methods of the Armed Forces Institute of Pathology, Third Edition. McGraw Hill, New York
Martin JW, Whittle DM, Muir DC, Mabury SA (2004) Perfluoroalkyl contaminants in a food web from Lake Ontario. Environ Sci Technol 38(20):5379–5385. https://doi.org/10.1021/es049331s
Mello GL, Júnior HA, Garcia S, Vinatea L (2011) Acute toxicity of pyrazosulfuron‐ethyl and permethrin to juvenile Litopenaeus vannamei Acta Scientiarum. Biol Sci Maringá 338:1–6. https://doi.org/10.4025/actascibiolsci.v33i1.6974
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84(4):407–412
Moody CA, Hebert GN, Strauss SH, Field JA (2003) Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J Environ Monit 5:341–345. https://doi.org/10.1039/B212497A
Oakes KD, Sibley PK, Martin JW, MacLean DD, Solomon KR, Mabury SA, Van Der Kraak GJ (2005) Short-term exposures of fish to perfluorooctane sulfonate: acute effects on fatty acyl-CoA oxidase activity, oxidative stress and circulating sex steroids. Environ Toxicol Chem 24:1172–1181. https://doi.org/10.1897/04-419.1
Oliaei F, Kriens D, Weber R, Watson A (2013) PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res 20:1977–1992. https://doi.org/10.1007/s11356-012-1275-4
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:9. https://doi.org/10.1289/ehp.10009
Pala A (2019) The effect of a glyphosate-based herbicide on acetylcholinesterase (AChE) activity, oxidative stress, and antioxidant status in freshwater amphipod: Gammarus pulex (Crustacean). Environ Sci Pollut Res 26:36869–6877. https://doi.org/10.1007/s11356-019-06804-5
Pan L, Zhang H (2006) Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comp Biochem Physiol C 144:67–75. https://doi.org/10.1016/j.cbpc.2006.06.001
Patetsini E, Dimitriadis VK, Kaloyianni M (2013) Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat Toxicol 126:338–345. https://doi.org/10.1016/j.aquatox.2012.09.009
Paul AG, Jones KC, Sweetman AJ (2009) First global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43(2):386–392. https://doi.org/10.1021/es802216n
Perazzolo LM, Gargioni R, Ogliari P, Barracco MAA (2002) Evaluation of some hemato‐immunological parameters in the shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. Aquaculture 214:19–33. https://doi.org/10.1016/S0044-8486(02)00137-0
Qiu J, Wang W-N, Wang L-J, Liu Y-F, Wang A-L (2011) Oxidative stress, DNA damage and osmolality in the Pacific white shrimp, Litopenaeus vannamei exposed to acute low temperature stress. Comp Biochem Physiol Part C Toxicol Pharmacol 154:36–41. https://doi.org/10.1016/j.cbpc.2011.02.007
Qin Q, Qin S, Wang L, Lei W (2012) Immune responses and ultrastructural changes of haemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat Toxicol 106–107:140–6. https://doi.org/10.1016/j.aquatox.2011.08.013
Saikat S, Kreis I, Davies B, Bridgman S, Kamanyire R (2013) The impact of PFOS on health in the general population: a review. Environ Sci Process Impacts 15:329–335. https://doi.org/10.1039/C2EM30698K
Schultz MM, Barofsky DF, Field JA (2004) Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol 38:1828–1835. https://doi.org/10.1021/es035031j
Selvi M, Cavaş T, Karasu Benli AC, Koçak Memmi B, Cinkılıç N, Sepici Dinçel A, Vatan O, Yılmaz D, Sarıkaya R, Zorlu T, Erkoç F (2011) Sublethal toxicity of esbiothrin relationship with total antioxidant status and in vivo genotoxicity assessment in fish (Cyprinus carpio L., 1758) using the micronucleus test and comet assay. Environ Toxicol 28:644–651. https://doi.org/10.1002/tox.20760
Sepici Dinçel A, Alparslan N, Karasu Benli AC, Selvi M, Sarıkaya R, Özkul İA, Erkoc F (2013) Haemolymph biochemical parameters reference intervals and total haemocyte counts of narrow clawed crayfish Astacus leptodactylus (Eschscholtz, 1823). Ecol Indicat 24:305–9. https://doi.org/10.1016/j.ecolind.2012.07.002
Sepici Dincel A, Karasu Benli AC, Selvi M, Sarikaya R, Sahin D, Ozkul IA, Erkoc F (2009) Sublethal cyfluthrin toxicity to carp (Cyprinus carpio L.) fingerlings: Biochemical, hematological, histopathological alterations. Ecotoxicol Environ Saf 72:1433–1439. https://doi.org/10.1016/j.ecoenv.2009.01.008
Seyoum A, Pradhan A, Jass J, Olsson PE (2020) Perfluorinated alkyl substances impede growth, reproduction, lipid metabolism and lifespan in Daphnia magna. Sci Total Environ 737:139682. https://doi.org/10.1016/j.scitotenv.2020.139682
Sharma BM, Bharat GB, Tayal S, Nizzetto L, Čupr P, Larssen T (2014) Environment and human exposure to persistent organic pollutants (POPs) in India: a systematic review of recent and historical data. Environ Int 66:48–64. https://doi.org/10.1016/j.envint.2014.01.022
Shi X, Liu C, Wu G, Zhou B (2009) Waterborne exposure to PFOS causes disruption of the hypothalamus–pituitary–thyroid axis in zebrafish larvae. Chemosphere 77:1010–1018. https://doi.org/10.1016/j.chemosphere.2009.07.074
Shi X, Du Y, Lam PK, Wu RS, Zhou B (2008) Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol 230:23–32
Shi X, Zhou B (2010) The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxic Sci 115:391–400. https://doi.org/10.1093/toxsci/kfq066
Smith VJ, Johnston PA (1992) Differential haemotoxic effect of PCB congeners in the common shrimp, Crangon crangon. Comp Biochem Physiol Part C Com Pharm 101:641–649. https://doi.org/10.1016/0742-8413(92)90099-S
Sprague JB (1971) Measurement of pollutant toxicity to fish–III: Sublethal effects and “safe” concentrations. Water Res 5:245–266
Stahl T, Mattern D, Brunn H (2011) Toxicology of perfluorinated compounds. Environ Sci Eur 23:38. https://doi.org/10.1186/2190-4715-23-38
Stara A, Machova J, Velisek J (2012) Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Environ Toxicol Pharm 33:334–343. https://doi.org/10.1016/j.etap.2011.12.019
Sun Y, Oberley LW, Li Y (1988) A Simple Method for Clinical Assay of Superoxide Dismutase. Clin Chem 34(3):497–500. https://doi.org/10.1093/clinchem/34.3.497
Touaylia S, Khazri A, Mezni A, Bejaoui M (2019) Effects of emerging persistent organic pollutant perfluorooctane sulfonate (PFOS) on the Crustacean Gammarus insensibilis. Hum Ecol Risk Assess 25:1–9. https://doi.org/10.1080/10807039.2018.1489717
Tse WKF, Li JW, Tse ACK, Chan TF, Ho JCH, Wu RSS, Wong CKC, Lai KP (2016) Fatty liver disease induced by perfluorooctane sulfonate: novel insight from transcriptome analysis. Chemosphere 159:166–177. https://doi.org/10.1016/j.chemosphere.2016.05.060
UNEP (2006) In: Draft Risk Profile: Perflorooctane Sulfonate (PFOS), Programme U.N.E., Geneva
UNEP (2009) The conference of the parties 4 of the Stockholm Convention (COP-4) in Geneva placed perfluorooctane sulfonate and perfluorooctane sulfonyl fluoride (PFOS and PFOSF) in Annex B. http://chm.pops.int/Convention/Pressrelease/COP4Geneva9May2009/tabid/542/language/en-US/Default.aspx
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox Environ Saf 64(2):178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Verde V, Fogliano V, Ritieni A, Maiani G, Morisco F, Caporaso N (2002) Use of N, N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free Radic Res 36:869–873. https://doi.org/10.1080/1071576021000005302
Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CKC (2012) PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochimica et Biophysica Acta 1820(7):1092–1101. https://doi.org/10.1016/j.bbagen.2012.03.010
Xu D, Li C, Wen Y, Liu W (2013a) Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ Pollut 174:121–127. https://doi.org/10.1016/j.envpol.2012.10.030
Xu Z, Wang Z, Li J-J, Chen C, Zhang PC, Dong L, Chen JH, Chen Q, Zhang X-T, Wang ZL (2013b) Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem Toxicol 58:1–7. https://doi.org/10.1016/j.fct.2013.03.048
Yağan A, Kesim S, Liman N (2014) Effect of low‐dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J Periodontol 85:478–489. https://doi.org/10.1902/jop.2013.130138
Yavuzcan HY, Benli AÇK (2004) Nitrite toxicity to crayfish, Astacus leptodactylus, the effects of sublethal nitrite exposure on hemolymph nitrite, total hemocyte counts, and hemolymph glucose. Ecotoxicol Environ Saf 59:370–375. https://doi.org/10.1016/j.ecoenv.2003.07.007
Young IS (2001) Measurement of total antioxidant capacity. J Clin Pathol 54:339. https://doi.org/10.1136/jcp.54.5.339
Yu JX, Xu EG, Li W, Jin S, Yuan T, Liu J, Li Z, Zhang T (2018) Acute toxicity of an emerging insecticide pymetrozine to Procambarus clarkii associated with rice-crayfish culture (RCIS). Int J Environ Res Public Health 15:984. https://doi.org/10.3390/ijerph15050984
Yucel Isıldar G, Gunal AC, Şahin D, Kocak Memmi B, Sepici Dinçel A (2020) How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms. Turk J Biochem 45:415–421. https://doi.org/10.1515/tjb-2019-0382
Zhang F, Wei J, Li Q, Jiang R, Yu N, Qin J, Chen L (2015) Effects of perfluorooctane sulfonate on the immune responses and expression of immune-related genes in Chinese mitten-handed crab Eriocheir sinensis. Comp Biochem Physiol C Toxicol Pharmacol 172–173:13–18. https://doi.org/10.1016/j.cbpc.2015.04.002
Zhang Y, Li Z, Kholodkevich S, Sharov A, Feng Y, Ren N, Sun K (2019) Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci Total Environ 666:944–955. https://doi.org/10.1016/j.scitotenv.2019.02.159
Acknowledgements
This study was financially supported by the Gazi University Scientific Research Projects Unit, grant number 18/2018-01. The authors would like to thank Betul Dağoğlu Hark (PhD; Fırat University, Faculty of Medicine, Department of Biostatistics and Medical Informatics) for biostatistical analyses, support, advice and Gazi University Academic Writing Application and Research Center for proof reading of the manuscript.
Funding
This study was financially supported by the Gazi University Scientific Research Projects Unit with grant number 18/2018-01.
Author information
Authors and Affiliations
Contributions
NB: methodology, investigation, and writing; BE: methodology, investigation; ASD: methodology; AÇG: conceptualization, methodology, supervision, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belek, N., Erkmen, B., Dinçel, A.S. et al. Does persistent organic pollutant PFOS (perfluorooctane sulfonate) negative impacts on the aquatic invertebrate organism, Astacus leptodactylus [Eschscholtz, 1823]. Ecotoxicology 31, 1217–1230 (2022). https://doi.org/10.1007/s10646-022-02579-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02579-7