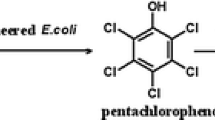
Abstract
It is the first report in which a novel psychrotrophic Pseudomonas putida SKG-1 strain was evaluated for simultaneous bioremediation of pentachlorophenol and Cr6+ under various cultural and nutritional conditions. Pentachlorophenol (PCP) dechlorination products, bacterial structure, and functional groups were characterized by gas chromatography and mass spectrometry (GC–MS), scanning electron microscope and energy dispersive X-ray spectroscopy (SEM–EDS), and Fourier-transform infrared (FTIR) techniques. The strain was extremely tolerant to excessively higher individual concentration of PCP (1,400 mg l−1) and Cr6+ (4,300 mg l−1). Increasing concentration of PCP and Cr6+ exerted inhibitory effect on bacterial growth and toxicants’ removal. The strain exhibited growth, and concomitantly remediated both the pollutants simultaneously over a broad pH (7.0–9.0) and temperature (28–32 °C) range; maximum growth, PCP dechlorination (87.5 %), and Cr6+ removal (80.0 %) occurred at optimum pH 8.0 and 30 °C (from initial PCP 100 mg l−1 and Cr6+ 500 mg l−1) under shaking (150 rpm) within 72 h incubation. Optimization of agitation (125 rpm) and aeration (0.4 vvm) in bioreactor further enhanced PCP dechlorination by ~10 % and Cr6+ removal by 2 %. A direct correlation existed between growth and bioremediation of both the toxicants. Among other heavy metals, mercury exerted maximum and cobalt minimum inhibitory effect on PCP dechlorination and Cr6+ removal. Chromate reductase activity was mainly associated with the supernatant and cytosolic fraction of bacterial cells. GC–MS analysis revealed the formation of tetrachloro-p-hydroquinone, 2,4,6-trichlorophenol, and 2,6-dichlorophenol as PCP dechlorination products. FTIR spectrometry indicated likely involvement of carbonyl and amide groups in Cr3+ adsorption, and SEM–EDS showed the presence of chromium on P. putida surface. Thus, our promising isolate can be ecofriendly employed for biotreatment of various industrial wastes contaminated with high PCP and Cr6+ concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution by toxic metals and phenolics results largely from industrial activities, although agriculture and sewage disposal also contribute to some extent (Nriagu and Pacyna 1989). Toxic metallic species, once mobilized into the environment, tend to persist, circulate, and eventually accumulate at different trophic levels in members of the food chain. Ultimately, metal pollutants pose a serious threat to the environment, and affect plants, animals, and humans (Mance 1987). Metal pollutants eventually affect ecosystem function, and impose an economic and public health burden. Hexavalent chromium and pentachlorophenol discharged from industrial effluents are being listed as priority pollutants by US Environmental Protection Agency.
The chromates are considered carcinogenic and mutagenic, and are discharged as environmental pollutant by metal finishing, petroleum refining, leather tanning, paints and pigments, steel production, textile manufacturing, and pulp production industries (Chirwa and Wang 2000; Mukherjee 1998). Chromium exists in oxidation states ranging +6 to −2; however, the most stable and common are the hexavalent (Cr6+) and trivalent (Cr3+) forms that are present in the environment (Nath et al. 2009). Cr6+, which is considered as the most toxic form, is highly soluble in water and is usually associated with oxygen as chromate (CrO 2−4 ) or dichromate (Cr2O 2−7 ) ions. Chromate (CrO 2−4 ) is a strong oxidizing agent which is reduced intracellularly in the presence of electron donors to Cr5+, which then reacts with nucleic acids and other cell components to produce mutagenic/carcinogenic effects on biological systems (Clark 1994; McLean and Beveridge 2001). Although, reduction to Cr5+ is responsible for chromate toxicity, its further reduction leads to the formation of a stable, less soluble and less toxic Cr3+. Therefore, reduction of Cr6+ to Cr3+ is a potentially beneficial process in Cr6+-affected environments (Michel et al. 2001). Reduction of Cr6+ results in precipitation of chromium as Cr3+, and thus offers a promising bioremediation strategy (Garg et al. 2012). Several bacterial genera, viz., species of Arthrobacter, Bacillus, Brevibacterium, and Pseudomonas are reported to reduce Cr6+ to Cr3+ (Liu et al. 2006; Masood and Malik 2011; Megharaj et al. 2003; Tripathi and Garg 2010; Xu et al. 2009). Various functional groups, such as OH−, COO−, C=O, PO4, SO4, −SH, etc., present on bacterial cell surface remain involved in biosorption of metal ions (Garg et al. 2012), and can be characterized by Fourier-transform infrared (FTIR) analysis. The electron microscopy along with X-ray energy dispersive analyses are performed for the detection of chromium species and its localization on bacterial cell surface (Srivastava and Thakur 2012).
Generally, the environments contaminated by aromatic compounds also receive discharges containing toxic metal pollutants (Alves de Lima e Silva et al. 2007). Among aromatics, the phenolic compounds are major environmental pollutants. As per Indian Standard Institution limits, the permissible level for phenolic compounds in inland surface waters is 0.002 mg l−1, whereas the similar limit in leachates is 1 mg l−1. The European Council Directive has set a limit of 0.5 mg l−1 to regulate phenol concentration in drinking water. Phenolics are easily bioaccumulated in various food chains of biological systems, which can cause profound human health problems. They can damage sensitive cells by permeating cytoplasmic membranes and coagulating cytoplasm. Phenolics are quite inhibitory to environmental microbes as well, which is due to their action on membrane function (Copley 2000) and ability to uncouple oxidative phosphorylation (Ito and Ohnishi 1982). Therefore, wastewater contaminated simultaneously with Cr6+ and phenolics should be treated carefully before being discharged into receiving water bodies (Garg et al. 2012). Despite widespread pollution caused by pentachlorophenol (PCP), only a few bacterial strains, viz., Bacillus sp., Pseudomonas sp., Serratia marcescens, and Sphingomonas chlorophenolicum have been isolated that were capable of degrading PCP (Sharma et al. 2009; Sharma and Thakur 2008; Singh et al. 2007; Tripathi et al. 2011). The pathway for PCP degradation has been elucidated (Orser and Lange 1994; Yang et al. 2006). Different researchers have reported various PCP degradation products through gas chromatography and mass spectrometry (GC–MS) analysis which vary depending upon the organisms employed (Sharma et al. 2009; Singh et al. 2009).
While extensive research has been performed on the uptake of single heavy metal species or toxic organic compounds by microbial cells, little attention has been paid to the bioremediation of binary organic compound–metal ion mixtures. Therefore, treatment strategies that are applicable for a specific metallic pollutant may not work for dual ones, because of the toxicity to the system posed by the organic component(s) or vice versa. Hence, isolation of microbial strains with the potential to degrade PCP and simultaneously bioremediate chromium would be valuable for effecting binary compound bioremediation (Srivastava et al. 2007). During the last decade, microbial studies designed to remove organic compounds and/or Cr6+ were conducted by several researchers (Chirwa and Wang 2005; Srivastava et al. 2007; Tripathi et al. 2011; Tziotzios et al. 2008).
Various factors, viz., pH, temperature, initial Cr6+ concentration, presence of other heavy metals are likely to influence the bioremediation process. In view of the above facts, the effect of some factors on simultaneous bioremoval of PCP and Cr6+ was assessed. Under the flask level-optimized conditions, the bench-scale fermentor trial for remediation of both the environmental toxicants by Pseudomonas putida SKG-1 was also attempted. Chromate reductase activity was examined to evaluate the extent of aerobic enzymatic reduction of Cr6+ to Cr3+. Gas chromatography–mass spectrometry was performed to characterize the dechlorination products during PCP bioremediation process. The characterization of functional groups associated with metal binding and structural changes on P. putida cell surface under metal stress were also attempted by FTIR and scanning electron microscope and energy dispersive X-ray spectroscopy (SEM–EDS) analyses.
Materials and methods
Bacterial culture
A psychrotrophic, metal- and solvent-tolerant P. putida SKG-1 strain (MTCC 10510; GenBank accession number HQ259593), isolated previously in our laboratory from dairy sludge (Singh et al. 2011), was employed in this study. The culture was maintained in glucose yeast extract (GYE) medium (pH 8.0) at 4 °C.
MIC determination and culture conditions
The minimum inhibitory concentration (MIC) of PCP and Cr6+, at which no bacterial growth occurred, was determined by broth culture technique (Luli et al. 1983). The bacterial isolate was grown in 100 ml GYE medium containing (g l−1): glucose, 0.5; yeast extract, 0.5; and peptone, 0.5 amended with pentachlorophenol (100–1,500 mg l−1) and Cr6+ (100–4,350 mg l−1) as per the experiment. The medium was sterilized at 10 psi for 20 min. The sterilized medium (pH 8.0) was inoculated with exponentially growing culture (at 4 %, v/v) of 1.12 absorbance (OD620) containing 2.9 × 107 cfu ml−1 and incubated at 30 °C at 150 rpm in an incubator shaker (New Brunswick Scientific C-24). The GYE broth without bacterial culture, but with PCP and Cr6+ as per experimental concentration served as a control. The samples withdrawn periodically at every 24-h intervals up to 96 h were centrifuged at 10,000 rpm (4 °C) for 10 min. The residual Cr6+ and chloride ions released (from PCP) were analyzed in culture supernatant.
Effect of PCP and Cr6+ concentrations
The effect of individual PCP (100–900 mg l−1) and Cr6+ (100–1,200 mg l−1) concentrations on PCP dechlorination and Cr6+ removal by P. putida SKG-1 were evaluated in GYE medium as above.
Combined effect of initial pH and temperature
The initial pH of medium was adjusted to 7.0, 8.0, and 9.0 using 0.1N HCl or 0.1N NaOH, prior to sterilization. The sterilized culture flasks were inoculated as per above culture conditions, and incubated for 96 h at different temperatures in the range of 28–32 °C in an incubator shaker (150 rpm) so as to determine the combined effect of pH and temperature on simultaneous dechlorination of PCP and Cr6+ removal at optimized initial PCP (100 mg l−1) and Cr6+ (500 mg l−1) concentrations.
Effect of other heavy metals
The GYE medium (pH 8.0) was amended with maximum tolerable concentration [as reported for P. putida SKG-1 in earlier study by Singh et al. (2011)] of other individual heavy metals (milligrams per liter) such as Ni (250), Co (100), Pb (800), As (2500), and Hg (25). The sterilized medium was inoculated with P. putida SKG-1 and incubated at optimized 30 °C.
PCP dechlorination studies
The PCP dechlorination products were analyzed by GC–MS (Agilent Technologies 7890A). The dechlorination study was performed in GYE broth containing 100 mg PCP l−1. Control (0 h) and experimental (48 and 72 h) cultured broths (100 ml) were taken and centrifuged at 10,000 rpm (4 °C) for 10 min. The PCP dechlorination metabolites in cell-free supernatant fractions were extracted using equal volume of n-hexane and acetone mixture (1:1 ratio). The organic layer was dried with anhydrous sodium sulfate and solvent evaporated till dryness. The sample residue was diluted with 1 ml n-hexane and analyzed immediately on GC–MS. The MS was operated in the electron ionization mode (70 eV). Helium was used as carrier gas and a solvent delay of 3 min was selected. The MS was operated in the total ion current mode, scanning from 40 to 600 m/z at 70 eV (electron energy).
Scanning electron microscope and energy dispersive X-ray spectroscopy
The bacterial samples (grown without and with 500 mg Cr6+ l−1 GYE broth) were taken, fixed primarily in 3 % glutaraldehyde (pH 7.4) for 4–6 h at 4 °C, washed twice with saline sodium phosphate buffer (0.1 M, pH 6.8), and then postfixation was done with 1 % osmium tetraoxide (OsO4) solution in the same buffer for 2 h. Further, the bacterial specimens were washed twice with above phosphate buffer, dehydrated in graded series by ethanol/acetone solution (30, 50, 70, and 90 % ethanol, 10 min each) and dried under CO2-rich atmosphere (31.1 °C at 1,072 psi). Finally, sputter coating was done using gold–palladium on carbon to increase conductivity and reduce charging of the specimens. The coated specimens were examined with field emission scanning electron microscopy (JEOL JSM-7600F, Japan). Energy dispersive X-ray spectrometry (Oxford Instruments) was performed at 15 kV for analyses of desired elements.
FTIR spectroscopy
The control and experimental (with 500 mg Cr6+ l−1) bacterial culture samples were taken from GYE broth to study the functional groups associated with Cr adsorption using FTIR spectrum. The bacterial culture samples were centrifuged at 10,000 rpm (4 °C) for 10 min. The bacterial pellets were collected, dried overnight in an oven at 50 °C, powdered with the help of pestle–mortar, and mixed with KBr (in a ratio of 2:100) to make KBr disks for FTIR spectrum analysis. A blank disk of pure potassium bromide was taken as a reference. The infrared spectra of control and experimental samples, before and after metal treatment, were recorded on FTIR spectrometer (Bruker, Germany Model: 3000 Hyperion Microscope with Vortex 80) at sophisticated analytical instrumentation facility, Indian Institute of Technology, Bombay, India. The infrared spectra were recorded in the range of 4,000–400 cm−1 (wave number).
Bench-scale bioreactor level bioremediation of PCP and Cr6+
Effect of aeration
Bioremediation of PCP and Cr6+ were performed in a stirred tank bioreactor (Bioflo 110, New Brunswick Scientific Co. Inc. Edison, NJ, USA) of 3 l capacity. The fermentor was equipped with direct drive dual Rushton style impeller, PID temperature, agitation control, probes and controller of pH and DO. The bioremediation study was carried out in 1 l GYE broth amended with PCP (100 mg l−1) and Cr6+ (500 mg l−1) under flask level optimized cultural conditions of pH 8.0, temperature 30 °C and 150 rpm. The medium was inoculated (at 4.0 %, v/v) with the mother culture of strain SKG-1 (OD620 1.12; 1 cm cuvette) containing 2.9 × 107 cfu ml−1. The aeration of culture broth was done at different rates (0.2–0.6 vvm), and samples (5.0 ml) were drawn periodically at 24-h intervals. The bacterial growth, PCP dechlorination and Cr6+ remediation were assessed as per the analytical determinations.
Effect of agitation at optimized aeration
The bacterial growth, PCP dechlorination and Cr6+ removal were further studied by varying the agitation speed from 100 to 150 rpm at optimized aeration rate of 0.4 vvm. Other experimental conditions remained the same as above.
Chromate reductase activity
Preparation of cell-free extract and chromate reductase assay were performed as per the slightly modified method of Ilias et al. (2011). The bacterial cells grown in GYE medium (100 ml) were harvested during the exponential growth phase (48 h) and centrifuged at 10,000 rpm (4 °C) for 10 min. The culture supernatant was collected and assayed for soluble protein and chromate reductase activity. The cells pellet was suspended in 5 ml phosphate buffer (50 mM, pH 7.0), kept in an ice bath (4 °C) and disrupted with an ultrasonicator at 30-sec pulses. The treatment was repeated five times at an interval of 30 sec. After sonication, the cell lysate was centrifuged at 16,000 rpm (4 °C) for 30 min. and cell-free extract was filtered through nitrocellulose membrane (pore size 0.2 μM) to get cytosolic fraction. The cytosolic fraction was transferred in a fresh tube and was kept in an ice bath. Cell debris was resuspended in 5 ml phosphate buffer and kept in an ice bath. Boiled culture supernatant, cell debris and cytosolic fraction were used as controls. The chromate reductase activity was assayed in reaction mixture containing 0.5 ml enzyme solution (culture supernatant, cell debris, and cytosolic fraction), 0.5 ml of 50 mM phosphate buffer and K2Cr2O7 as Cr6+ at 3.4 μM final concentration. The reaction mixture was incubated at 30 °C for 30 min. The reaction was stopped by adding 0.2 ml of 20 % trichloroactetic acid (Horitsu et al. 1987). Further, Cr6+ reduction was measured by estimating the decrease in Cr6+ concentration in the reaction mixture using 1,5 diphenyl carbazide method (APHA 1998). Protein was estimated as per Bradford (1976). One unit of enzyme activity was defined as 1 μM of Cr6+ reduced min−1.
Analytical determinations
Bacterial growth
The growth of strain SKG-1 in terms of absorbance (A620, 1.0 cm cuvette) was analyzed in GYE broth periodically at 24-h intervals up to 96-h incubation.
Chloride ions
The extent of PCP dechlorination was determined by estimation of chloride ions released in the culture supernatant at 460 nm as per the method of Bergmann and Sanik (1957), and was extrapolated against the standard curve of sodium chloride.
Chromium
Total chromium in biomass
The bacterial cells were harvested by centrifugation at 10,000 rpm (4 °C) for 10 min and washed twice with deionized water. The method of American Public Health Association (APHA; 1998) was followed for the digestion of biomass. Briefly, the biomass dried in an oven at 80 °C was acid lysed with 5 ml aquaregia, incinerated on hot plate till the white residue formed and then digested with acid mixture (6:1 perchloric acid and HNO3). The concentration of total chromium in digested sample was analyzed using atomic absorption spectrophotometer at 357.9 nm.
Total chromium in supernatant
The supernatant was dried on hot plate and then digested with acid mixture (6:1 perchloric acid and HNO3) as per the method of APHA (1998). The concentration of total chromium in digested sample was analyzed as described above.
Hexavalent chromium (Cr6+)
1,5 Diphenyl carbazide method was followed for the estimation of Cr6+ in the culture supernatant, and was extrapolated against K2Cr2O7 standard curve at 540 nm (APHA 1998). Trivalent chromium was calculated from total minus hexavalent chromium.
Statistical analysis
All experiments were performed in triplicate. The standard deviation was calculated using Microsoft Excel, and results are presented as mean ± SD values.
Results and discussion
The bioremediation of toxic pollutants is affected by several factors such as nature and concentration of pollutants, pH, temperature, incubation time, presence of other heavy metals, etc. The present investigation is, therefore, an attempt to evaluate the effect of above factors on single and simultaneous (dual) bioremediation of Cr6+ and pentachlorophenol from GYE medium (amended with these pollutants) by P. putida SKG-1 isolate.
Minimum inhibitory concentration determination
P. putida SKG-1 strain isolated and identified by morphological, biochemical, and molecular analyses (16S rDNA and FAME) in our previous study (Singh et al. 2011) was employed in the present investigation for bioremediation of toxic PCP and Cr6+. This strain was extremely tolerant to excessively higher concentration of PCP (1,400 mg l−1) and Cr6+ (4,300 mg l−1) individually. The MIC of PCP and Cr6+, at which no growth of culture occurred were 1,500 and 4,350 mg l−1, respectively (Table 1). No growth of bacterial culture at very high PCP and Cr6+ concentrations can be attributed to the toxic effect of both the pollutants. Higher MIC value also revealed that the organism was highly tolerant to toxic Cr6+ and PCP. Several other researchers also isolated Cr6+ and PCP-tolerant bacteria, and were employed for single and simultaneous bioremediation studies (Srinath et al. 2001; Srivastava et al. 2007; Tripathi et al. 2011). Srinath et al. (2001) isolated species of Micrococcus, Aerococcus, and Aeromonas from tannery effluent which were tolerant to ≥400 μg Cr6+ ml−1 concentration. Srivastava et al. (2007) reported that Acinetobacter sp. isolated from pulp paper mill effluent could tolerate maximum 50 μg PCP ml−1 and 500 μg Cr6+ ml−1. Sharma et al. (2009) isolated Acinetobacter sp. ISTPCP-3 from sediment core of pulp paper mill effluent which tolerated PCP up to 200 mg l−1. Xu et al. (2009) reported Pseudomonas aeruginosa tolerant to 40 mg Cr6+ l−1. Tripathi et al. (2011) reported 38 bacterial isolates from tannery effluent resistant to Cr6+ at >50 μg ml−1, four to PCP at >50 μg ml−1, while one isolate designated as Bacillus cereus RMLAU1 was simultaneously tolerant to both PCP (500 μg ml−1) and Cr6+ (200 μg ml−1). Thus, it can be inferred that tolerance limit of microorganisms to toxic pollutants varies from organism to organism.
Effect of initial PCP concentrations
The highly chlorinated organic compounds are stable and difficult to degrade. However, certain microbes can catalyze biotransformation reactions in which chloride ions (Cl−) of the xenobiotic chlorinated compounds are displaced by protons (H+). The quantum of Cl− ions removed is directly related with the mineralization as well as increased reactivity of resultant organic compounds, thereby making them more susceptible to biodegradation (Karn et al. 2010; Garg et al. 2011; Garg and Tripathi 2011). Figure 1 reveals that increasing concentrations of PCP (100–900 mg l−1) were increasingly inhibitory for bacterial growth as well as PCP dechlorination at each time of incubation up to 96 h. No PCP dechlorination in abiotic (control without bacterium) samples suggests dechlorination in experimental samples by the bacterial culture and not due to any other abiotic reason. The PCP dechlorination was in the range of 30.5–98.0 % (at initial 600–100 mg PCP l−1) within 72-h incubation. The PCP concentrations higher than 600 mg l−1 were highly toxic, and allowed very little bacterial growth (without PCP removal) at 900 mg l−1 throughout the incubation period. Decreasing trend of PCP dechlorination with increasing PCP concentration was possibly due to decreased activity of dechlorinating enzymes (Karn et al. 2010). Figure 1 further depicts continuously increasing growth up to 72 h with concomitant PCP dechlorination up to 48 h at initial 200–700 mg PCP l−1; however, PCP removal decreased (except at 300 mg PCP l−1) during 48–96 h incubation. The maximum growth (OD 0.79) and PCP dechlorination (98.0 %) occurred at 100 mg PCP l−1 at 72 h, which was only slightly higher than the values obtained at 48-h incubation. Therefore, 100 mg PCP l−1 concentration was chosen for further experimentation on simultaneous bioremediation of PCP and Cr6+. In general, a direct correlation existed between growth and PCP dechlorination.
Our findings on concentration dependent bacterial growth and PCP dechlorination are in consonance with the results of other researchers. Gu and Korus (1995) reported rapid PCP degradation at initial 100 mg PCP l−1; nearly maximum degradation rate occurred at 60 mg PCP l−1 by Flavobacterium sp. Further, as the initial PCP concentration increased from 50 to 250 mg l−1, the specific cell growth rate decreased, which directly corresponded with PCP degradation. Sharma et al. (2009) also observed an inhibitory effect of increasing higher concentrations of PCP (>100–250 mg l−1) on its own biodegradation as well as growth of Acinetobacter sp. ISTPCP-3. The authors noted that the isolate remediated PCP completely within 24 and 48 h, respectively at 50 and 100 mg PCP l−1, while no growth and PCP degradation occurred at >250 mg PCP l−1. In accordance with our findings, Karn et al. (2010) also reported growth dependent release of Cl− ions from PCP, which increased with time of incubation. Further, they observed decreased growth associated dechlorination with increasing concentration from 100 to 600 mg PCP l−1 within 72-h incubation; however, increase in PCP concentration up to 200 mg l−1 required extended time of 120 h to degrade > 95 % PCP.
Effect of initial Cr6+ concentrations
Figure 2 depicts increased bacterial growth response and Cr6+ removal in harmony with time up to 72 h at all the concentrations in the range of 200–1,100 mg Cr6+ l−1, followed by slight decrease during 72–96 h incubation. However, at 600−800 mg Cr6+ l–1, the growth increased throughout the incubation period (24–96 h). Megharaj et al. (2003) observed that the time required for Cr6+ reduction by Bacillus and Arthrobacter spp. increased as Cr6+ concentration enhanced, and that Cr6+ reducing abilities of bacteria were growth dependent. Desai et al. (2008) reported that Pseudomonas sp. G1DM21 isolate remediated concentration-dependent Cr6+ (99.7 % of initial 500 μM and 93 % of initial 1,000 μM) within 48-h incubation.
The maximum Cr6+ removal (98 %) was evident at 100 mg Cr6+ l−1 within 48 h, followed by slight decrease up to 96 h incubation (Fig. 2). Since the extent of Cr6+ removal (98–84 %) was not much affected by increasing 100–500 mg Cr6+ l−1 concentrations, 500 mg l−1 level was selected for simultaneous bioremediation studies. A significantly increasing inhibitory effect on growth as well as Cr6+ remediation at >500 mg Cr6+ l−1 was evident at each time of incubation. In general, the increasing Cr6+ concentration from 100 to 1,200 mg l−1 was inhibitory not only for bacterial growth but also concomitantly for Cr6+ removal. The results further revealed that the concentration of Cr6+ at tested levels (100–1,100 mg l−1) decreased to variable extent up to 72-h incubation. An extreme toxicity was experienced by the bacterial culture at 1,200 mg Cr6+ l−1, which was evident by insignificant growth with no Cr6+ removal (Fig. 2). Furthermore, no Cr6+ removal was evident in control samples indicating no possibility of abiotic Cr6+ reduction in the absence of bacterial culture under present experimental conditions. The influence of Cr6+ concentration on its own reduction appears to indicate two opposing trends. First, the time required for complete reduction increases progressively with an increase in initial Cr6+ concentration. The longer period taken for Cr6+ reduction may be attributed to the toxic effect of the higher Cr6+ concentration on the growth of the bacterial strain. Second, a slower initial rate of Cr6+ reduction occurs at higher initial Cr6+ concentration.
The results in Fig. 2 further revealed a growth-dependent Cr6+ removal by SKG-1 strain. Xu et al. (2009) also noted that Cr6+ reduction by P. aeruginosa was directly correlated with bacterial growth, wherein Cr6+ concentration gradually decreased as the bacterial growth increased with the time of incubation. Furthermore, a significant increase in bacterial growth was noted as Cr6+ was more efficiently reduced, indicating that Cr6+ reduction and bacterial growth affected each other, i.e., the growth of bacterial cells stimulated Cr6+ removal and in turn, the efficient Cr6+ reduction promoted bacterial growth. Das and Mishra (2010) also reported a bacterial growth dependent reduction of 99 % Cr6+ by Brevibacterium casei (from initial 50 mg l−1) within 12 h.
Combined effect of temperature and pH on simultaneous bioremediation
The SKG-1 strain was able to grow and simultaneously bioremediate PCP and Cr6+ (in GYE medium augmented with 100 mg PCP and 500 mg Cr6+ l−1) throughout the pH (7.0–9.0) and temperature (28–32 °C) range under study. The bioremoval extent of both the toxicants with concomitant growth increased with time up to 72 h irrespective of initial pH and incubation temperature; thereafter, the response either decreased at pH 8.0 or remained variable (at pH 7.0 and 9.0) during 72–96 h incubation. At all the temperatures under study (28–32 °C), the maximum PCP dechlorination and Cr6+ remediation were evident at slightly alkaline pH of 8.0, irrespective of incubation time. The results revealed that the growth and PCP/Cr6+ remediation capacity was dependent on pH and temperature. The optimal pH and temperature for growth corresponded with the maximum removal (percent) of both the pollutants by P. putida SKG-1 isolate (Table 2). The maximum simultaneous PCP dechlorination (87.5 %) and Cr6+ removal (80 %) were evident at optimum pH 8.0, 30 °C, and 72-h incubation. Any deviation in pH and/or temperature from optimum decreased the extent of bioremediation of both the toxicants.
With increase in temperature from 28° to 30 °C, PCP and Cr6+ removal increased with time up to 72 h irrespective of initial pH in the range of 7.0–9.0. Furthermore, the lower pH (7.0) than optimum (8.0) was relatively more inhibitory compared to higher pH (9.0) for the bacterial growth, PCP dechlorination and Cr6+ removal at all the temperatures under study throughout the incubation period. Conversely, slightly higher temperature (32 °C) than optimum (30 °C) was relatively more inhibitory compared to slightly less (28 °C) temperature for growth and simultaneous bioremediation of PCP and Cr6+ during 72-h incubation (Table 2). The decreased Cr6+ removal and PCP dechlorination can be attributed to reduced growth at pH and/or temperature higher or lower than optimum (pH 8.0, temperature 30 °C). Deiana et al. (2007) asserted that decreased redox potential for Cr6+/Cr3+ couple at higher pH may also contribute to overall Cr6+ reduction. The findings in our study also indicate the growth-dependent bioremediation of both the toxicants.
Our findings on pH optimization for PCP and Cr6+ remediation are in agreement with the results of other researchers. Premlatha and Rajkumar (1994) reported optimum pH 7.6–8.0 for the growth and PCP degradation by P. aeruginosa. Camargo et al. (2003) reported maximum Cr6+ reduction by Bacillus sp. strains at pH 7.0–9.0. The optimum pH for growth directly corresponded with the highest rate of Cr6+ reduction in most of these isolates. Ilias et al. (2011) observed pH optima between 7.0 and 8.0 for maximum Cr6+ reduction by Staphylococcus aureus and Pediococcus pentoceous isolated from tannery effluent. Karn et al. (2010) also reported slightly alkaline pH of 7.5 and 8.5, respectively for 96.5 % and 94.5 % Cr6+ reduction by Pseudomonas stutzeri isolate. The hydrogen ion concentration (pH) is an important index reflecting the microbial enzyme activity thereby affecting removal of PCP and Cr6+ by P. putida SKG-1. The pH of solution is known to modulate the speciation of ions, cellular metabolism and sites of interactions leading to changes in accumulation and toxicity of metals (Volesky 1990). Since Cr6+ reduction is enzyme-mediated, changes in pH will affect the degree of ionization of the enzyme(s) leading to alteration in protein conformation thereby affecting the enzyme activity (Farrell and Ranallo 2000).
Similar to our results, the optimum temperature of 30 °C for maximum Cr6+ removal by Micrococcus sp. and B. casei has been reported by other researchers (Congeevaram et al. 2007; Das and Mishra 2010). Camargo et al. (2003) also reported an optimum temperature of 30 °C for the growth of Bacillus spp., which directly correlated with the highest Cr6+ reduction rate. The slight difference in optimal pH and temperature observed by different researchers indicate that specific requirement is important for various cultures to achieve the maximum Cr6+ and PCP removal during their detoxification process. Similar to pH, the temperature is also an important parameter for bacterial growth, and accordingly affects the enzymatic reactions necessary for Cr6+ reduction and PCP dechlorination. Since the processes responsible for bioremoval are largely physicochemical in nature, enzyme-dependent mechanisms are more likely to be affected by temperature. Above the optimum temperature, cell degradation probably becomes dominant over the growth process and with sub-optimal temperature, the regulation of metabolism may fail. Hence, at optimum temperature, the bacterium could utilize the substrate better, in consonance with other optimum cultural and nutritional conditions. Further, the temperature is also known to affect the stability of microbial cell wall, its configuration, and can cause ionization of chemical moieties (Volesky 1990).
Effect of other heavy metals
It is necessary to evaluate the effect of other metal ions while studying the bioremediation strategy for heavy metals and/or organic toxicants. When microbes are used in bioremediation, it is an advantage for them to have tolerance to metals other than the targeted ones, so that they can withstand their presence in effluents as they perform their desired bioremediation activities.
Figure 3 reveals the inhibitory effect of other heavy metals on growth, PCP dechlorination and Cr6+ removal. The respective inhibition order of PCP dechlorination and Cr6+ removal in the presence of other heavy metal was (%): Hg (100) > Pb (97.7) > As (92.6) > Ni (80.6) > Co (66.8) and Hg (100) > As (100) > Pb (100) > Ni (90.0) > Co (85.6) at 72 h, relative to maximum PCP dechlorination (87.5 %) and Cr6+ removal (80.0 %) at initial 100 mg PCP l−1 and 500 mg Cr6+ l−1 concentration. The extent of inhibition in the presence of Pb and As was calculated at 24 and 96 h incubation. Further, mercury exerted the most, while cobalt the least inhibitory effect on PCP dechlorination. Whereas, Cr6+ removal was completely inhibited in the presence of mercury, arsenic, and lead (Fig. 3). The results indicate that other heavy metals have variable effects on the bioremediation of PCP and Cr6+; the extent however, depends upon the type and concentration of other metal ions present. Overall, the coexistence of other heavy metals in the presence of PCP and Cr6+ exerted an inhibitory effect on removal of both the contaminants under study. Conversely, in the absence of PCP and Cr6+, the strain SKG-1 was highly tolerant to other heavy metals (Singh et al. 2011). Tolerance to other heavy metals by this organism has an added advantage of withstanding the presence of different metallic ions while performing the desired activity.
Effect of other heavy metals on simultaneous remediation of PCP (P) and Cr6+ (C) and growth of P. putida SKG-1 isolate at optimized initial concentrations (milligrams per liter) of PCP (100) and Cr6+ (500) in GYE medium (pH 8.0), temperature 30 °C under shake flask (150 rpm) conditions (error bars standard deviation)
Several researchers have reported the effect of other heavy metals on Cr6+ removal (McLean and Beveridge 2001; Srinath et al. 2002). Faisal and Hasnain (2006) reported inhibitory effect of cobalt on Cr6+ reduction by B. cereus S-6. Similar to our findings, Ilias et al. (2011) also reported that the growth of S. aureus and Pediococcus pentosaceous, and hence Cr6+ removal were almost completely inhibited in the presence of Hg2+. Srinath et al. (2002) noted an inhibitory effect of Ni2+ on Cr6+ reduction by Bacillus sp. Conversely, Tripathi et al. (2011), while studying the effect of Pb, As, Hg, Zn, Co, and Ni on growth of B. cereus isolate, observed that the strain was resistant to these multi heavy metals in the presence of Cr6+ and PCP.
Characterization of pentachlorophenol dechlorination products
The GC chromatogram (Fig. 4) shows a major peak of PCP (RT = 10.3 min) in control sample. Whereas, new peaks appeared in the experimental samples, which revealed the formation of tetrachloro-p-hydroquinone (RT = 11.5 min), 2,4,6-trichlorophenol (RT = 8.7), and 2,6-dichlorophenol (RT = 8.0) using documented data from National Institute of Standards and Technology mass database. In addition to PCP dechlorination products, few additional peaks of other compounds, viz., dibutyl phthalate (RT = 13.0), benzenedicarboxylic acid, bis (2-methylpropyl) ester (RT = 19.2) and phthalates (RT = 20.4) also appeared both in control and PCP degraded samples, which are due to contamination of GC column and sample bottle packing. Figure 4 further depicts the disappearance of PCP peak in degraded sample as compared to control, thereby indicating decreased PCP concentration in experimental sample. Our results are in agreement with the findings of other researchers. Singh et al. (2009) also studied PCP degradation by B. cereus, and reported tetrachloro-p-hydroquinone, 2,3,4,6-tetrachlorophenol, 6-chlorohydroxyquinol, benzo (F) quinoline, 2(1 H)quinolinone and 2 H-1-benzopyran-2-one in the extract of degraded samples. Our results indicated conversion of PCP into dechlorination products through oxidative route into tetrachloro-p-hydroquinone which further converted to tri- and dichlorophenols. However, dechlorination to monochlorophenol was not detected. Further detailed study is required to establish the exact pathway for PCP degradation in SKG-1 strain. Sharma et al. (2009) reported that Acinetobacter sp. ISTPCP-3 was able to utilize PCP through an oxidative route with the formation of 2,3,5,6-tetrachlorohydroquinone and 2-chloro-1,4-benzenediol.
In our study, the –Cl bond from PCP was dechlorinated at 3, 4, and 5 positions, and formed partially chlorine-free end products. Our results corroborate with the findings of Homada et al. (1987) and Kennes et al. (1996), who also reported mineralization of chlorinated phenols to partially chlorine-free end products. Suegara et al. (2005) calculated the C–Cl bond dissociation energies in each of the PCP molecule substitutes. The C–Cl bond dissociation energy was found to be lowest at position 4, followed by positions 3 and 5. It was, therefore, affirmed that the dissociation of C–Cl bond at position 4 was easiest, followed by bonds at 3 and 5 positions. The hardest to disassociate appeared to be the bonds at 2 and 6 positions.
SEM–EDS analysis
The bacterial cell morphological changes and chromium detection studies were performed with and without stress of 500 mg Cr6+ l−1 by SEM–EDS analysis. The Cr6+ unexposed P. putida cells are smooth surfaced rods with an average diameter of 0.81 × 1.03 μm without any peak of chromium. Whereas, in Cr6+ exposed culture, few cells are distorted with broken cell wall, and a peak of Cr appeared which provides evidence for the likely presence of Cr3+ (reduced) species on the cell surface (Fig. 5). The bacterial cells at 72-h incubation were in the stationary phase of growth. If the microbial population enters the stationary phase as a result of the exhaustion of an essential nutrient (e.g., the carbon and nitrogen source), the viable count, the total count, and the cell mass all become stationary at approximately the same moment, and the population may remain unchanged in size. But, when growth is limited by toxic materials, the transition to the stationary phase is much less sharp. As a result of slight differences in the susceptibility of the cells, the population may have reached the stationary phase as judged by viable count, but still show a slow increase of cell mass (estimated spectrophotometrically through absorbance measurement; Stanier et al. 1972), as reported in our study in the presence of Cr6+ (at initial 500 mg l−1 concentration; Fig. 2). The toxic effect of Cr6+ on cell morphology was also reported by other researchers (Ackerley et al. 2006; Li et al. 2008). Dhal et al. (2010) noted that the SEM micrographs of Bacillus sp. CSB-4 strain treated with Cr6+ were coated either with the precipitate of reduced or adsorbed Cr3+. The corresponding EDS with small chromium signals provided further evidence in favor of reduced Cr3+ on the cell surface. Thakur and Srivastava (2011) also reported that biosorbed chromate was assumed to be Cr3+, as Cr6+ is reduced to Cr3+ in the living cells due to reducing environment and enzymes present inside the cell. Polti et al. (2011) also observed morphological difference between Cr6+ exposed and unexposed Streptomyces sp. MC1 cells by SEM; EDXA showed the presence of Cr peak in exposed, while absent in unexposed control cells. Rida et al. (2012) also observed morphological changes in Ochromobactrum intermedium cell surface by SEM, and reported chromium precipitates on bacterial surface. Likewise, Naik et al. (2012) reported morphological changes in treated B. cereus cells (small, round-shaped with uneven edges on cell wall), and there was a peak of chromium observed in EDX. They asserted that the change in cell shape due to heavy metal exposure is an adopted mechanism to resist the toxicity of heavy metals. The stress-induced morphological changes might have an important role in cell survival/metabolic activity, and hence absorption of chromium from the effluent. The SEM-EDX and elemental analysis of chromate-containing samples confirmed the binding of chromium with Serratia sp. (Srivastava and Thakur 2012).
Characterization of functional groups by FTIR
FTIR technique can be employed to determine the presence of certain functional groups which absorb at definite frequencies. Absorption in the infrared region is due to changes in the vibrational and rotational levels of molecules. The absorption of infrared radiations causes various bands in a molecule to stretch and bend with respect to each other. The characterization of bacterial surface functional groups with and without Cr6+ exposure (as performed by FTIR technique) is depicted in Fig. 6. The spectra of metal unexposed bacterial cells showed several distinct and sharp bands of different wavelength. The FTIR spectra of Cr6+ exposed cells revealed shifting of some characteristic bands, which depict changes in functional groups after metal exposure. In both Cr6+ exposed and unexposed P. putida cells, the characteristic infrared spectra between 3,700 and 3,300 cm−1 depicts the presence of –OH, –NH2, and –NH groups. The amide bands arise due to symmetrical and asymmetrical N–H stretching vibration. The adsorption bands between 2,800 and 2,700 cm−1 corresponds the –CHO group. After the treatment of bacterial cells with Cr6+, there were changes in FTIR spectra with appearance of new peaks, which might be due to Cr3+ coordination with surface functional groups of bacterial cell. A strong adsorption band around 1,650 cm−1 in Cr6+ exposed cells may be attributed to the presence of carbonyl group. Low intensity new peaks between 800 and 950 cm−1, characteristic of Cr–O vibration, were also present; the same were also reported earlier by Chhikara et al. (2010) at 950 cm−1 and Dhal et al. (2010) at 840–725 cm−1. In Fig. 6, the IR peaks near 600 cm−1 representing C=0 bending vibrations were due to lipids. Chhikara et al. (2010) also made similar observation at 579 cm−1.
Surface functional groups, viz., carboxyl, phosphoryl, and hydroxyl play a major role in bioaccumulation of metals thereby significantly removing toxic chromate (Beveridge 1988; Leusch et al. 1995). Our results revealed that the functional groups like carbonyl and amide of bacterial cells might be involved in adsorption of reduced Cr3+ on P. putida surface. Kang et al. (2006) reported that chromium binding sites on the bacterial cell surface were most likely carboxyl and amide groups. Similarly, Naik et al. (2012) also reported the role of carboxyl, phosphate, and amide groups for the adsorption of chromium.
Bench-scale bioreactor level bioremediation of PCP and Cr6+
Effect of aeration
In this set of experiment, the bacterial growth and PCP/Cr6+ remediation were studied at an agitation speed of 150 rpm and variable aeration rates of 0.2–0.6 vvm (Fig. 7). The maximum PCP dechlorination of 94 % and Cr6+ removal of ~81.5 % was noted at 0.4 vvm within 72-h incubation. Other aeration rates of 0.2 and 0.6 vvm were not so effective and resulted in reduced bioremediation of both the toxicants. The decreased extent of bioremediation was more pronounced at lower aeration rate of 0.2 vvm (79 % PCP and 68.5 % Cr6+ removal) than at higher aeration rate of 0.6 vvm (90.5 % PCP and 76 % Cr6+ removal; Fig. 7). Like PCP and Cr6+ removal, bacterial growth was also slow at lower aeration rate and increased with increasing rate of aeration approaching maximum at 0.4 vvm. Further increase in aeration rate caused reduction in bacterial growth (Fig. 7).
Optimization of aeration rate at fixed agitation speed of 150 rpm indicated that oxygen supply to bacterial cell mass is the most important parameter for any fermentation process. This can be achieved by optimizing the agitation speed and by maintaining proper aeration; however, an appropriate balance of agitation and aeration is imperative to avoid any mechanical damage to bacterial cells which might reduce the bioremediation efficiency of the test culture. It can be deduced from the aeration optimization results that a direct correlation existed between bacterial growth and bioremediation of PCP as well as Cr6+.
Effect of agitation at optimized aeration
After optimization of suitable aeration rate (0.4 vvm), we attempted to optimize the agitation rates (100–150 rpm) to study its effect on bacterial growth and bioremediation of PCP and Cr6+. The results are depicted in Fig. 8. The bioremediation of PCP and Cr6+ enhanced with increase in agitation speed, and was maximum (PCP 97 % and Cr6+ 82 %) at 125 rpm within 72 h of incubation. Further increase in agitation speed to 150 rpm caused marginal decrease in PCP dechlorination (3.0 %) and almost no change in the extent of Cr6+ remediation. Hence, an aeration rate of 0.4 vvm and agitation speed of 125 rpm were optimum for maximum growth and PCP/Cr6+ bioremediation by P. putida SKG-1 isolate during 72-h incubation. The results depict an overall increase in PCP dechlorination (~10 %) and Cr6+ remediation (2.0 %, Fig. 8) as compared to maximum PCP (87.5 %) and Cr6+ (80 %) removal at optimized flask level trials (Table 2).
It is evident from the findings that an optimum agitation of fermenting medium is essential for proper oxygen supply to bacterial cells. However, above the optimum agitation speed it may damage the cells or change the cell morphology, which leads to reduced metabolic activities, and hence bioremediation efficiency. Thus, a proper agitation to aeration ratio is mandatory for appropriate oxygen transfer to cell mass, and also to minimize the shearing effect for maximum growth and bioremediation activities.
Song et al. (2009) attempted simultaneous Cr6+ reduction and phenol degradation in a bioreactor by a pure culture of P. aeruginosa CCTCC AB91095. The organism utilized phenol as a sole carbon source, possibly through organic acid production. Cr6+ inhibited its own reduction as well as phenol degradation at a concentration of >20 mg Cr6+ l−1. In contrast, phenol enhanced both the reduction of Cr6+ as well as its own degradation at <100 mg phenol l−1. The concentrations of Cr6+ and phenol declined in 12 h, respectively from 20 to 3.36 mg Cr6+ l−1 and 100 to 29.51 mg phenol l−1 in cultures containing 5 % (v/v) inoculum of P. aeruginosa. Whereas in our study, an enhanced level of bioremediation at much higher initial concentrations of Cr6+ and PCP was achieved by P. putida isolate. Chirwa and Wang (2000) reported that phenol (at a concentration exceeding the optimum level) inhibited the reduction of Cr6+ and its own degradation. They reported nearly 100 % remediation of Cr6+ and phenol at initial 4.41 mg Cr6+ l−1 and 253 mg phenol l−1 during 67 h incubation by Escherichia coli ATCC 33456 and consortium of phenol degraders. Mathew et al. (2010) studied simultaneous bioremediation of Cr6+ (at initial levels of 10–50 ppm) and phenol (at initial levels of 50 and 100 ppm) by five individual bacterial cultures, viz., species of Pseudomonas, Amphibacillus, Micrococcus, Moraxella, and E. coli. Contrary to the findings of Shen and Wang (1995), these authors observed no inhibitory effect of Cr6+ on phenol degradation for any of the test cultures. Moreover, the extent of Cr6+ reduction was similar at both concentrations of phenol tested. Shen and Wang (1995) tested cocultures of E. coli and P. putida, and reported that the degradation of phenol by P. putida was inhibited by Cr6+, which increased as the Cr6+ concentration elevated. Tripathi and Garg (2010) and Tripathi et al. (2011) isolated, for the first time, a native bacterial strain RMLAU1 of B. cereus from treated tannery effluent that was tolerant to 500 mg PCP l−1 and 200 mg Cr6+ l−1. The isolate degraded 56.5 % PCP (from initial 500 mg l−1 level) and simultaneously bioremediated 74.5 % chromium from initial 200 mg l−1 minimal salt medium.
Chromate reductase activity and distribution of reduced chromium (Cr3+)
Table 3 shows that chromate reductase activity was present in culture supernatant, cytosolic fraction, as well as in cell debris of P. putida SKG-1 strain. The maximum enzyme activity in cytosolic fraction (55.7 %) was followed by culture supernatant (40.6 %) and cell debris (3.6 %). This reveals that chromate reductase activity was mainly associated with culture supernatant and cytosolic fraction of bacterial cells. No activity was observed in boiled control samples thereby indicating denaturation, and hence inactivation of enzyme.
Other researchers also reported the chromate reductase activity in cell-free extract during aerobic Cr6+ reduction (McLean and Beveridge 2001; Pal et al. 2005). Similar to our findings, Camargo et al. (2003) found chromate reductase activity mainly in the soluble cell-free extract, and a very low activity in the membrane fraction of Bacillus sp. ES29. Desai et al. (2008) also observed chromate reductase activity in cytosolic fraction of Pseudomonas sp. G1DM21 isolate. Whereas, Ilias et al. (2011) found chromate reductase activity in the culture supernatant and cell lysate (debris) only, but no activity in the cell extract supernatant (cytosolic fraction) of S. aureus and P. pentosaceous isolates. They reported lesser specific enzyme activity of 0.033 and 0.053 μM Cr6+ reduced min−1 mg−1 protein in cell lysate and culture supernatant, respectively. In contrast, we observed very high specific chromate reductase activity of 1.62 and 2.72 μM Cr6+ reduced min−1 mg−1 protein in cell debris and culture supernatant, respectively. Additionally, we noted highest specific enzyme activity of 10.69 μM Cr6+ reduced min−1 mg−1 protein in cytosolic fraction of P. putida cells (Table 3).
Table 4 reveals the extent of reduced chromium (Cr3+) distribution in culture supernatant and bacterial biomass of P. putida SKG-1 strain. The reduced chromium (Cr3+) was present both in culture supernatant and bacterial biomass. The results further reveal that out of total Cr6+ remediated (410 mg of initial 500 mg l−1; 82 %) from GYE broth at 72 h, 97.6 % Cr6+ was reduced to Cr3+, of which 47.6 % was detected in culture supernatant and 51.3 % in the bacterial biomass (Table 4). Presence of Cr3+ in bacterial biomass represents the fraction of Cr3+ attached to cells and/or precipitate as chromium hydroxide. The distribution of Cr3+ in culture supernatant and bacterial biomass (Table 4) correlates directly with the extent of chromate reductase activity in culture supernatant and cytosolic fraction (Table 3).
Our results are in agreement with the findings of other researchers (Shen and Wang 1993; McLean and Beveridge 2001), who also reported significant amount of Cr3+ in the cell-free extract. Li et al. (2008) reported that organic components of LB medium such as amino acids and organic acids could compete with bacteria for Cr3+ coordination; thus, soluble Cr3+ complexes could be formed and the immobilization ratio of chromium decreased correspondingly. Xu et al. (2009) demonstrated that there was only a slight decrease in the concentration of total chromium, and more than 85 % was detected in the culture (P. aeruginosa) supernatant. Thus, only a small amount of chromium (15 %) was adsorbed on the cells, and the remaining reduced chromium was soluble in the supernatant. The medium used by above authors for Cr6+ reduction contained nutrients such as beef extract, peptone and glucose which might have been the source of amino acids and organic acids for Cr3+ coordination.
Conclusions
-
It is the first report on simultaneous bioremediation of PCP and Cr6+ by a psychrotrophic P. putida isolate.
-
P. putida SKG-1 is tolerant to high concentrations of PCP (1,400 mg l−1) and Cr6+ (4,300 mg l−1). This strain is able to grow under wide range of pH and temperature, and remediate high extent of 87.5 % PCP and 80 % Cr6+ (at initial 100 mg PCP and 500 mg Cr6+ l−1) under shaking (150 rpm) at optimum pH 8.0 and 30 °C. Mercury exerted maximum and cobalt minimum inhibitory effect on bioremoval of PCP and Cr6+.
-
In bench-scale bioreactor level remediation study, PCP and Cr6+ removal enhanced by ~10 and 2 %, respectively, at optimized aeration rate of 0.4 vvm and agitation speed of 125 rpm. Out of 82 % Cr6+ remediated, 98.9 % was reduced to Cr3+, of which 47.6 % was present in culture supernatant and 51.3 % was associated with bacterial biomass, which directly corresponded with chromate reductase activity.
-
GC–MS analyses revealed the formation of tetrachloro-p-hydroquinone, tri- and di-chlorophenols as biodechlorination products in PCP remediation experiments. The results can be helpful in elucidation of the PCP dechlorination pathway. Further detailed study is required to elucidate the exact pathway for PCP remediation.
-
FTIR spectroscopy characterized the carbonyl and amide functional groups on P. putida cell surface, which are most likely involved in adsorption of reduced chromium. The SEM study revealed a changed bacterial cell morphology under Cr6+ stress and EDS analysis showed a chromium peak in the experimental sample, which was absent in the control.
-
The results are encouraging for the development of an efficient in situ bioremediation strategy for removal of toxic Cr6+ and PCP pollutants from various contaminated sites.
References
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381. doi:10.1128/JB.188.9.3371-3381
Alves de Lima e Silva A, Pereira MP, Geraldo Silva Filho R, Hofer E (2007) Utilization of phenol in the presence of heavy metals by metal-tolerant nonfermentative gram-negative bacteria isolated from wastewater. Microbiologia 49(3-4):68–73
APHA (1998) Standard methods for the examination of water and wastewaters, 20th ed., Washington, DC
Bergmann JG, Sanik J (1957) Determination of trace amounts of chlorine in naphtha. Anal Chem 29:241–243. doi:10.1021/ac60122a018
Beveridge TJ (1988) The bacterial surface: general considerations towards design and function. Can J Microbiol 34:363–372. doi:10.1139/m88-067
Bradford MM (1976) A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Camargo FAO, Okeke BC, Bento FM, Frankenberger WT (2003) In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu2+. Appl Microbiol Biotechnol 62:569–573. doi:10.1007/s00253-003-1291-x
Chhikara S, Hooda A, Rana L, Dhankhar R (2010) Chromium (VI) biosorption by immobilized Aspergillus niger in continuous flow system with special reference to FTIR analysis. J Environ Biol 31(5):561–566
Chirwa EMN, Wang YT (2000) Simultaneous chromium (VI) reduction and phenol degradation in an anaerobic consortium of bacteria. Water Res 33:2376–2384. doi:10.1016/S0043-1354(99)00363-2
Chirwa EMN, Wang YT (2005) Modeling hexavalent chromium reduction and phenol degradation in a coculture biofilm reactor. ASCE J Environ Eng 131:1495–1506. doi:10.1061/(ASCE)0733-9372
Clark DP (1994) Chromate reductase activity of Enterobacter aerogenes is induced by nitrite. FEMS Microbiol Lett 122:233–238
Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K (2007) Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mat 146:270–277
Copley SD (2000) Evolution of metabolic pathway for degradation of a toxic xenobiotic: the patchwork approach. Trends Biochem Sci 25:261–265. doi:10.1016/S0968-0004(00)01562-0
Das AP, Mishra S (2010) Biodegradation of the metallic carcinogen hexavalent chromium Cr (VI) by an indigenously isolated bacterial strain. J Carcinogen 9:6. doi:10.4103/1477-3163.63584
Deiana S, Premoli A, Senette C (2007) Reduction of Cr (VI) by caffeic acid. Chemosphere 67:1919–1926. doi:10.1016/j.chemosphere.2006.12.003
Desai C, Jain K, Madamwar D (2008) Hexavalent chromate reductase activity in cytosolic fractions of Pseudomonas sp. G1DM21 isolated from Cr (VI) contaminated industrial landfill. Process Biochem 43:713–721. doi:10.1016/j.procbio.2008.02.015
Dhal B, Thatoi H, Das N, Pandey BD (2010) Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. J Chem Technol Biotechnol 85(11):1471–1479. doi:10.1002/jctb.2451
Faisal M, Hasnain S (2006) Detoxification of Cr (VI) by Bacillus cereus S-6. Res J Microbiol 1:45–50. doi:10.3923/jm.2006.45.50
Farrell SO, Ranallo RT (2000) Experiments in biochemistry: a hands-on approach. Saunders, Orlando
Garg SK, Tripathi M (2011) Strategies for decolorization and detoxification of pulp and paper mill effluent. Rev Environ Contam Toxicol 212:213–236. doi:10.1007/978-1-4419-8453-1_4
Garg SK, Tripathi M, Kumar SK, Singh SK, Singh SK (2011) Microbial dechlorination of chloroorganics and simultaneous decolorization of pulp-paper mill effluent by Pseudomonas putida MTCC 10510 augmentation. Environ Monit Assess. doi:10.1007/s10661-011-2359-1
Garg SK, Tripathi M, Srinath T (2012) Strategies for chromium bioremediation from tannery effluent. Rev Environ Contam Toxicol 217:75–140. doi:10.1007/978-1-4614-2329-4_2
Gu Y, Korus RA (1995) Kinetics of pentachlorophenol degradation by Flavobacterium species. Appl Biochem Biotechnol 43:374–378. doi:10.1007/BF00172842
Homada MF, Haddad A, Abd-El-Bary MF (1987) Treatment of phenolics wastes in an aerated submerged fixed-film (ASFF) bioreactor. J Biotechnol 5:279–292. doi:10.1016/0168-1656(87)90025-3
Horitsu H, Futo S, Miyazawa Y, Ogai S, Kawai K (1987) Enzymatic reduction of hexavalent chromium by hexavalent chromium tolerant Pseudomonas ambigua G-1. J Agri Biol Chem 51:2417–2420
Ilias M, Rafiqullah IM, Debnath BC, Mannan KSB, Hoq MM (2011) Isolation and characterization of chromium (VI)-reducing bacteria from tannery effluents. Indian J Microbiol 51(1):76–81. doi:10.1007/s12088-011-0095-4
Ito M, Ohnishi Y (1982) Escherichia coli mutants which are resistant to uncouplers of oxidative phosphorylation. J Microbiol Immunol 26(11):1079–1084. doi:10.1099/00221287-133-10-2759
Kang SY, Bremer PJ, Kim KW, McQuillan AJ (2006) Monitoring metal ion binding in single-layer Pseudomonas aeruginosa biofilms using ATR-IR spectroscopy. Langmuir 22:286–291
Karn SK, Chakrabarti SK, Reddy MS (2010) Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation. doi:10.1007/s10532-010-9376-6
Kennes C, Wu WM, Bhatnagar L, Zeikus JG (1996) Anaerobic dechlorination and mineralization of pentachlorophenol and 2,4,6-trichlorophenol by methanogenic pentachlorophenol degrading granules. Appl Microbiol Biotechnol 44:801–806
Leusch A, Holan ZR, Volesky B (1995) Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically reinforced biomass of marine algae. J Chem Technol Biotechnol 62:279–288. doi:10.1002/jctb.280620311
Li B, Pan D, Zheng J, Cheng Y, Ma X, Huang F, Lin Z (2008) Microscopic investigations of the Cr (VI) uptake mechanism of living Ochrobactrum anthropi. Langmuir 24:9630–9635
Liu YG, Xu WH, Zeng GM, Li X, Gao H (2006) Cr (VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem 41:1981–1986. doi:10.1016/j.procbio.2006.04.020
Luli GW, Talnagi JW, Strohl WR, Pfister RM (1983) Hexavalent chromium resistant bacteria isolated from river sediments. Appl Environ Microbiol 46:846–854
Mance G (1987) Pollution threat of heavy metals in aquatic environments. Elsevier, New York, pp 31–60, 134-135
Masood F, Malik A (2011) Hexavalent chromium reduction by Bacillus sp. strain FM1 isolated from heavy-metal contaminated soil. Bull Environ Contam Toxicol 86:114–119. doi:10.1007/s00128-010-0181-z
Mathew A, Thanga V, Salom G, Reshma JK (2010) Simultaneous phenol degradation and chromium (VI) reduction by bacterial isolates. Res J Biotechnol 5:46–49
McLean J, Beveridge TJ (2001) Chromate reduction by a Pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54. doi:10.1007/s00284-002-3889-0
Michel C, Brugna M, Aubert C, Bernadac A, Bruschi M (2001) Enzymatic reduction of chromate: comparative studies using sulfate-reducing bacteria. Appl Microbiol Biotechnol 55:95–100
Mukherjee AB (1998) Chromium in the environment of Finland. Sci Total Environ 217:9–19. doi:10.1016/S0048-9697(98)00163-6
Naik UC, Srivastava S, Thakur IS (2012) Isolation and characterization of Bacillus cereus IST105 from electroplating effluent for detoxification of hexavalent chromium. Environ Sci Pollut Res. doi:10.1007/s11356-012-0811-6
Nath K, Singh D, Shyam S, Sharma YK (2009) Phytotoxic effects of chromium and tannery effluent on growth and metabolism of Phaseolus mungo Roxb. J Environ Biol 30:227–234
Nriagu JO, Pacyna JM (1989) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139. doi:10.1038/333134a0
Orser CS, Lange CC (1994) Molecular analysis of pentachlorophenol degradation. Biodegradation 5(3–4):277–288
Pal A, Dutta S, Paul AK (2005) Reduction of hexavalent chromium by cell-free extract of Bacillus sphaericus AND 303 isolated from serpentine soil. Curr Microbiol 66:327–330. doi:10.1007/s00284-005-0048-4
Polti MA, Amoroso MJ, Abate CM (2011) Intracellular chromium accumulation by Streptomyces sp. MC1. Water Air Soil Pollut 214:49–57. doi:10.1007/s11270-010-0401-5
Premlatha A, Rajkumar GS (1994) Pentachlorophenol degradation by Pseudomonas aeruginosa. World J Microbiol Biotechnol 10:334–337
Rida B, Yrjala K, Hasnain S (2012) Hexavalent chromium reduction by bacteria from tannery effluent. J Microbiol Biotechnol 22(4):547–554
Sharma A, Thakur IS (2008) Characterization of pentachlorophenol degrading bacterial consortium from chemostat. Bull Environ Contam Toxicol 81:12–18. doi:10.1007/s00128-008-9437-2
Sharma A, Thakur IS, Dureja P (2009) Enrichment, isolation and characterization of pentachlorophenol degrading bacterium Acinetobacter sp. ISTPCP-3 from effluent discharge site. Biodegradation 20:643–650. doi:10.1007/s10532-009-9251-5
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol 59:3771–3777
Shen H, Wang YT (1995) Modeling simultaneous hexavalent chromium reduction and phenol degradation by a defined coculture of bacteria. Biotechnol Bioeng 48:606–616. doi:10.1002/bit.260480608
Singh S, Chandra R, Patel DK, Rai V (2007) Isolation and characterization of novel Serratia marcescens (AY927692) for pentachlorophenol degradation from pulp and paper mill waste. World J Microbiol Biotechnol 23:1747–1754. doi:10.1007/s11274-007-9424-5
Singh S, Singh BB, Chandra R, Patel DK, Rai V (2009) Synergistic biodegradation of pentachlorophenol by Bacillus cereus (DQ002384), Serratia marcescens (AY927692) and Serratia marcescens (DQ002385). World J Microbiol Biotechnol 25:1821–1828. doi:10.1007/s11274-009-0083-6
Singh SK, Singh SK, Tripathi VR, Khare SK, Garg SK (2011) A novel psychrotrophic, solvent tolerant Pseudomonas putida SKG-1 and solvent stability of its psychro-thermoalkalistable protease. Process Biochem 46:1430–1435. doi:10.1016/j.procbio.2011.03.012
Song H, Liu Y, Xu W, Zeng G, Aibibu N, Xu L, Chen B (2009) Simultaneous Cr (VI) reduction and phenol degradation in pure cultures of Pseudomonas aeruginosa CCTCC AB91095. Biores Technol 100:5079–5084. doi:10.1016/j.biortech.2009.05.060
Srinath T, Khare S, Ramteke PW (2001) Isolation of hexavalent chromium-reducing Cr-tolerant facultative anaerobes from tannery effluent. J Gen Appl Microbiol 47:307–312
Srinath T, Garg SK, Ramteke PW (2002) Cr (VI) accumulation by Bacillus circulans: effect of various growth conditions. Indian J Microbiol 42:141–146
Srivastava S, Thakur IS (2012) Biosorption and biotransformation of chromium by Serratia sp. isolated from tannery effluent. Environ Technol 33(1):113–122. doi:10.1080/09593330.2011.551842
Srivastava S, Ahmad AH, Thakur IS (2007) Removal of chromium and pentachlorophenol from tannery effluent. Biores Technol 98:1128–1132. doi:10.1016/j.biortech.2006.04.011
Stanier RY, Doudoroff M, Adelberg EA (1972) Microbial growth. In: General microbiology, 3rd edn. Macmillon Press: London. pp 310–311
Suegara J, Lee BD, Espino MP (2005) Photodegradation of pentachlorophenol and its degradation pathways predicted using density functional theory. Chemosphere 61:341–346. doi:10.1016/j.chemosphere.2005.02.090
Thakur IS, Srivastava S (2011) Biodegradation and bioconversion of chromium and pentachlorophenol in tannery effluent by microorganisms. Int J Technol 3:224–233
Tripathi M, Garg SK (2010) Studies on selection of efficient bacterial strain simultaneously tolerant to hexavalent chromium and pentachlorophenol isolated from treated tannery effluent. Res J Microbiol 5:707–716. doi:10.3923/jm.2010.707.716
Tripathi M, Vikram S, Jain RK, Garg SK (2011) Isolation and growth characteristics of chromium (VI) and pentachlorophenol tolerant bacterial isolate from treated tannery effluent for its possible use in simultaneous bioremediation. Indian J Microbiol 51:61–69. doi:10.1007/s12088-011-0089-2
Tziotzios G, Dermou E, Eftychia P, Dorothea V, Dimitris V (2008) Simultaneous phenol removal and biological reduction of hexavalent chromium in a packed-bed reactor. J Chem Technol Biotechnol 83:829–835. doi:10.1002/jctb.1876
Volesky B (1990) Biosorption and biosorbents. In: Volesky B (ed) Biosorption of heavy metals. CRC, Boca Raton
Xu WH, Liu YG, Zeng GM, Li X, Song HX, Peng QQ (2009) Characterization of Cr (VI) resistance and reduction by Pseudomonas aeruginosa. Trans Nonf Met Soc China 19:1336–1341. doi:10.1016/S1003-6326(08)60446-X
Yang CF, Lee CM, Wang CC (2006) Isolation and physiological characterization of the pentachlorophenol degrading bacterium Sphingomonas chlorophenolicum. Chemosphere 69:709–714. doi:10.1016/j.chemosphere.2005.05.012
Acknowledgments
The authors express sincere thanks to Sophisticated Advanced Instrumentation Facilities, Indian Institute of Technology, Bombay, India for GC–MS, FTIR and SEM–EDS facilities. Facilities provided by Government of Uttar Pradesh under Centre of Excellence and Government of India’s DST-FIST schemes are duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Garg, S.K., Tripathi, M., Singh, S.K. et al. Pentachlorophenol dechlorination and simultaneous Cr6+ reduction by Pseudomonas putida SKG-1 MTCC (10510): characterization of PCP dechlorination products, bacterial structure, and functional groups. Environ Sci Pollut Res 20, 2288–2304 (2013). https://doi.org/10.1007/s11356-012-1101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1101-z