Abstract
Purpose and aim
In general, direct current (DC) is used in an electrocoagulation processes. In this case, an impermeable oxide layer may form on the cathode as well as corrosion formation on the anode due to oxidation. This prevents the effective current transfer between the anode and cathode, so the efficiency of electrocoagulation processes declines. These disadvantages of DC have been diminished by adopting alternating current (AC) in electrocoagulation processes. The main objective of this study is to investigate the effects of AC and DC on the removal of copper from water using magnesium alloy as anode and cathode.
Materials and methods
Magnesium alloy of size 2.0 dm2 was used as anode and as cathode. To optimize the maximum removal efficiency, different parameters like effect of initial concentration, effect of temperature, pH, and effect of current density were studied. Copper adsorbed magnesium hydroxide coagulant was characterized by SEM, EDAX, XRD, and FTIR.
Results
The results showed that the optimum removal efficiency of copper is 97.8 and 97.2 % with an energy consumption of 0.634 and 0.996 kWh/m3 at a current density of 0.025 A/dm2, pH of 7.0 for AC and DC, respectively. The adsorption of copper is preferably fitting the Langmuir adsorption isotherm for both AC and DC respectively. The adsorption process follows the second-order kinetics model with good correlation. Temperature studies showed that adsorption was endothermic and spontaneous in nature.
Conclusions
The magnesium hydroxide generated in the cell removes the copper present in the water, reducing the copper concentration to less than 1 mg/L, making it safe for drinking. The results of the scale-up study show that the process was technologically feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The presence of heavy metals in the aquatic environment is a source of great environmental alarm. Copper is known to be one of the most toxic heavy metals to living organisms and it is one of the more widespread heavy metal contaminants of the environment (Vinikour et al. 1980; James et al. 2006). Copper, a metal that occurs naturally in rocks, soil, water, and air throughout the environment, is an essential element in plants, animals, and humans (Billon et al. 2006). The adverse health effects caused by copper, mercury, and arsenic poisoning are far more catastrophic than any other natural calamity in the world in recent times (Ozer et al. 2004; Onmez and Aksu 1999; Prasad and Freitas 2000). The potential sources of copper in industrial effluents include metal cleaning and plating baths, pulp, paper board mills, wood pulp production, fertilizer industry, paints and pigments, municipal and storm water run-off, etc. (Boujelben et al. 2009). Common symptoms of copper toxicity are injury to red blood cells and lungs. It also gives damage to liver and pancreatic functions. Long-term exposure to copper may cause irritation to nose, mouth, and eyes; and also cause headaches, stomachaches, dizziness, vomiting, and diarrhea (Yu et al. 2000; Ajmal et al. 1998; Ozcan et al. 2005; Gardea-Torresde et al. 1996; Bailey et al. 1999). As recommended by the World Health Organization, the drinking water guideline value is 2 mg/L. The United States Environmental Protection Agency (USEPA) has set the maximum contaminant level of copper allowed in drinking water is 1.3 mg/L (CPCB 2002). The conventional methods for removing copper from water include ion exchange, reverse osmosis, co-precipitation, coagulation, electrodialysis, and adsorption (Larous et al. 2005; Ozcan et al. 2005; Papandreou et al. 2007; Villaescesa et al. 2004; Saeed et al. 2005; Fiol et al. 2006; Shukla and Sakhardane 1992; Jain et al. 1997, 1995b; Jain et al. 1995a; Gupta et al. 2002; Gupta and Rastogi 2008a; Gupta et al. 2009a, b, c, d; Gupta and Rastogi 2010). The physical methods such as ion exchange, reverse osmosis and electrodialysis have proven to be either too expensive or inefficient to remove copper from water. Nowadays, the chemical treatment methods are not used due to heavy maintenance costs, problems pertaining to sludge handling and its disposal, and neutralization of the effluent.
Recent studies have demonstrated that electrocoagulation method offers an attractive alternative to the traditional methods for treating water (Vasudevan et al. 2008, 2009, 2010a, b, c, d, e; Vasudevan and Lakshmi 2011; Ali et al. 2011, 2012). In this process, anodic dissolution of metal electrode takes place with the evolution of hydrogen gas at the cathode. Electrochemically generated metallic ions from the anode can undergo hydrolysis to produce a series of activated intermediates that are able to adsorb, like chemical adsorption process (Gupta et al. 2006a, b, c, 1997a, b, 2003a, b, 2007a, b, c, d, e, f, 1998, 1999, 2001, 2002; Gupta 1998; Gupta and Sharma 2002; Gupta and Rastogi 2008a, b, 2009; Gupta and Ali 2000; Mittal et al. 2010; Mohan et al. 2001; Srivastava et al. 1995, 1996a, b, 1997; Ali and Gupta 2007; Ali 2010; Goyal et al. 2005, 2007a, b). The advantages of electrocoagulation are high particulate removal efficiency, compact treatment facility and the possibility of complete automation. This method is characterized by reduced sludge production, a minimum requirement of chemicals and ease of operation (Chen 2004; Adhoum and Monser 2004; Mrozowski and Zielinski 1983; Miwa et al. 2006; Onder et al. 2007; Carlesi Jara et al. 2007; Christensen et al. 2006; Carlos et al. 2006; Bailey et al. 1999). The overall reactions are as follows,
Besides, the main disadvantage in case of aluminum electrode is the residual aluminum (The USEPA guidelines suggest maximum contamination is 0.05–0.2 mg/L) present in the treated water due to cathodic dissolution. This will lead to health problems like cancer. There is no such health problem in the case of magnesium electrode, because the USEPA guidelines suggest that the maximum contamination level of magnesium in water is 30 mg/L. Although there are numerous reports dealing with electrochemical coagulation as a means of removal of many pollutants from water and wastewater, there are few studies on copper removal by electrocoagulation method.
In general, direct current (DC) is used in an electrocoagulation processes. In this case, an impermeable oxide layer may form on the cathode as well as corrosion formation on the anode due to oxidation. These prevent the effective current transport between the anode and cathode, so the efficiency of electrocoagulation processes declines. These disadvantages of DC have been overcome by adopting alternating current (AC) in electrocoagulation processes. The main objective of this study is to investigate the effect of AC/DC on the removal efficiency of copper from water using magnesium as anode and cathode. To optimize the maximum removal efficiency of copper, the different parameters such as effect of initial concentration, effect of temperature, effect of current density, and pH were studied. In doing so, the equilibrium adsorption behavior is analyzed by fitting models of Langmuir, Freundlich, and Dubinin–Radushkevich (D–R) isotherms. Adsorption kinetics of electrocoagulants is analyzed using first-, second-order, Elovich, and Intraparticle kinetic models. The activation energy is evaluated to study the nature of adsorption.
2 Materials and methods
2.1 Experimental apparatus and procedures
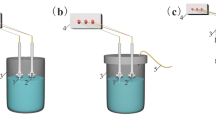
The electrochemical cell (Fig. 1) consists of a 1.0 L glass vessel fitted with a PVC cover having suitable holes to introduce the electrodes, thermometer, pH sensor and electrolyte. Magnesium alloy (Magnesium Elektron Ltd; AZ31 consisting of 3.0 wt % Al, 1.0 wt % Zn) of size 2 dm2 was used both as anode and cathode. The inter electrode gap between anode and cathode was 0.5 cm. The temperature of the electrolyte was controlled to the desired value with variation of ±2 K by adjusting the rate of flow of thermostatically controlled water through an external glass-cooling spiral. A regulated DC was supplied from a rectifier (10 Å, 0–25 V; Aplab model) and regulated AC was supplied from a source (0–5 A, 0–270 V, 50 Hz; AMETEK Model: EC1000S).
Copper nitrate (CuNO3; Analar Reagent, Merck, Germany) was dissolved in de-ionized water for the required concentration. The pH of the electrolyte was adjusted, if required, with 1 M HCl or NaOH (Analar Reagent, Merck, Germany) solutions. Temperature studies were carried at varying temperatures (313–343 K) to determine the type of reaction. To examine the effect of co-existing ions, for the removal of copper, Analar Grade (Merck, Germany) sodium salts of carbonate, phosphate, silicate, and fluoride were added to the electrolyte for required concentrations.
2.2 Analytical procedure
The copper was analyzed using UV–Visible Spectrophotometer (MERCK, Spectroquant Pharo 300, Germany) using MERCK kit. The SEM and EDAX of copper-adsorbed magnesium hydroxide coagulants were analyzed with a scanning electron microscope (SEM) made by Hitachi (model S-3000H). The XRD of electrocoagulation by-products were analyzed by a JEOL X-ray diffractometer (type—JEOL, Japan). The Fourier transform infrared spectrum of magnesium hydroxide was obtained using Nexus 670 FTIR spectrometer (Thermo Electron Corporation, USA). The concentration of carbonate, silicate, fluoride, and phosphate were determined using UV–Visible Spectrophotometer (MERCK, Pharo 300, Germany) with MERCK kit.
3 Results and discussion
3.1 Effect of pH
It has been established that the initial pH of the electrolyte is one of the important factor that affecting the performance of electrochemical processes particularly on the performance of electrocoagulation. To evaluate its effect, a series of experiments were performed, using 10 mg/L copper-containing solutions, with an initial pH varying in the range of 4–12 using the AC and DC source. From Fig. 2, it can be seen that the removal efficiency of copper was increased with increasing the pH and the maximum removal efficiency of 97.8 and 97.2 % was obtained at pH 7.0 for AC and DC, respectively. The percentage of copper removal increased with increasing pH 7, a decreasing trend in adsorption was observed when below and above pH 7 for both AC and DC source. At acidic and alkaline pHs, the oxide surfaces exhibit net positive and negative charges respectively and would tend to repulse the adsorption of copper and resulting the maximum adsorption at pH 7.0.
3.2 Effect of initial ion concentration
To study the effect of initial concentration, experiments were conducted at varying initial concentrations from 2 to 10 mg/L using AC and DC sources, and the results are illustrated in Fig. 3. From the results, it can be seen that the adsorption of copper is increased with an increase in copper concentration and it remains constant after equilibrium time. The equilibrium time was 10 min for all of the concentrations studied (2–10 mg/L) for the AC source. The amount of copper adsorption (q e) increased from 1.77 to 8.92 mg/g when the concentration was increased from 2 to 10 mg/L for AC source. The figure also shows that the adsorption was rapid in the initial stages and gradually decreased with progress of adsorption. The plots are single, smooth and continuous curves leading to saturation, suggesting the possible monolayer coverage to copper on the surface of the adsorbent. In the case of DC the equilibrium time was found to be 45 min for all concentration studied (figure not shown).
3.3 Effect of current density
It is well known that the current density is the very important factor for elecrocoagulation process. The amount of copper removal and removal rate has increased by increasing current density. Further, the amount of copper removal depends upon the quantity of adsorbent (magnesium hydroxide) generated, which is related to the time and current density. To investigate the effect of current density on the copper removal, a series of experiments were carried out by solutions containing constant copper loading of 10 mg/L, at a pH 7.0, with current density being varied from 0.01 to 0.1 A/dm2 using both AC and DC current source. The results showed that the optimum removal efficiency of 97.8 and 97.2 % with the energy consumption of 0.634 and 0.996 kWh/kL was achieved at a current density of 0.025 A/dm2, at pH of 7.0 using AC and DC, respectively. The results are presented in Table 1. The results show that the removal efficiency of copper was higher and energy consumption was lower in the case of AC than DC. This may be due to the uniform dissolution of anode and cathode during electrocoagulation in the case of AC. The removal efficiency was found showing the amount of copper adsorption increases with the increase in adsorbent concentration, which indicates that the adsorption depends up on the availability of binding sites for copper. Hence, further studies were carried out at 0.025 A/dm2
3.4 Effect of co-existing ions
To study the effect of co-existing ions, in the removal of copper, sodium salts of carbonate (0–250 mg/L), phosphate (0–50 mg/L), silicate (0–15 mg/L), and arsenate (0 5.0 mg/L) was added to the electrolyte and electrolysis was carried out using AC.
The effect of carbonate on copper removal was evaluated by increasing the carbonate concentration from 0 to 250 mg/L in the electrolyte. The removal efficiencies are 97.8, 97, 79, 67.2, 33, and 24.5 % for the carbonate ion concentration of 0, 2, 5, 65, 150, and 250 mg/L respectively. From the results it is found that the removal efficiency of the copper is not affected by the presence of carbonate below 5 mg/L. A significant reduction in removal efficiency was observed, above 5 mg/L of carbonate concentration is due to the passivation of anode resulting, the hindering of the dissolution process of anode.
The concentration of phosphate ion was increased from 0 to 50 mg/L at the contaminant range of phosphate in the ground water. The removal efficiency for copper was 97.8, 96.9, 77.3, 55.1, and 50 % for 0, 2, 5, 25, and 50 mg/L of phosphate ion, respectively. There is no change in removal efficiency of copper below 5 mg/L of phosphate in the water. At higher concentrations (at and above 5 mg/L) of phosphate, the removal efficiency of copper decreased to 50 %. This is due to the preferential adsorption of phosphate rather than copper as the concentration of phosphate increase.
The effect of silicate on the removal efficiency of copper was investigated. The respective efficiencies for 0, 5, 10, and 15 mg/L of silicate are 97.8, 65, 60.2, and 53 %. The removal of copper decreased with increasing silicate concentration from 0 to 15 mg/L. In addition to preferential adsorption, silicate can interact with magnesium hydroxide to form soluble and highly dispersed colloids that are not removed by normal filtration.
From the results it is found that the efficiency decreased from 97.8, 88.1, 71.5, 52, and 26.7 % by increasing the concentration of fluoride from 0, 0.2, 0.5, 2, and 5 mg/L. This is due to the preferential adsorption of fluoride over copper as the concentration of fluoride increases. So, when fluoride ions are present in the water to be treated, fluoride ions compete greatly with copper ions for the binding sites.
3.5 Kinetic modeling
The dynamics of the adsorption process in terms of the order and the rate constant can be evaluated using the kinetic adsorption data. The process of copper removal from an aqueous phase by any adsorbent can be explained by using kinetic models.
3.5.1 First-order Lagergren model
The variation of the adsorbed copper with time was kinetically characterized using the First and second-order rate equation proposed by Lagergren. The first-order Lagergren model as (Wan Ngah and Hanafiah 2008; Ho and McKay 1998),
where, q t is the amount of copper adsorbed on the adsorbent at time t (in minutes) and k 1 (in per minute) is the rate constant of first-order adsorption. The integrated form of the above equation with the boundary conditions t = 0 to t > 0 (q = 0 to q > 0) and then rearranged to obtain the following time dependence function,
where q e is the amount of copper adsorbed at equilibrium. The q e and rate constant k 1 were calculated from the slope of the plots of log (q e − q t ) versus time (t).
3.5.2 Second-order Lagergren model
The second-order kinetic model is expressed as (Benaissa and Elouchd 2007; Wu et al. 2005),
Equation (5) can be rearranged and linearized as,
where q e and q t are the amount of copper adsorbed on Mg(OH)2 (in milligrams per gram) at equilibrium and at time t (in minutes), respectively, and k 2 is the rate constant for the second-order kinetic model. The equilibrium adsorption capacity, q e (in calories) and k 2 were determined from the slope and intercept of plot of t/q t versus t (Fig. 4). Table 2 depicts the computed results obtained from first- and second-order kinetic model for AC and DC source. The calculated q e values well agree with the experimental q e values for second-order kinetics model better than the first-order kinetics model for both AC and DC. This implies that the second-order model is in good agreement with experimental data and can be used to favorably explain the copper adsorption on Mg(OH)2.
3.5.3 Elovich model
The simplified form of Elovich model is (Oke et al. 2008),
where α is the initial adsorption rate (in milligrams per gram per hour) and, β is the desorption constant (in grams per milligram). If copper adsorption fits the Elovich model, a plot of q t versus loge(t) should yield a linear relationship with the slope of (1/β) and an intercept of 1/β loge (α β). Table 3 depicts the results obtained from the Elovich equation. Lower regression value shows the inapplicability of this model.
3.5.4 Intraparticle diffusion model
Intraparticle diffusion is expressed as (Weber and Morris 1963; Allen et al. 1989),
A linearized form of Eq. (8) is followed by
in which a depicts the adsorption mechanism and k id may be taken as the rate factor (percent of copper adsorbed per unit time). Lower and higher value of k id illustrates an enhancement in the rate of adsorption and better adsorption with improved bonding.
Tables 2 and 3 depict the computed results obtained from first-order, second-order, Elovich, and Intraparticle diffusion models for AC and DC, respectively. The correlation coefficient values decreases from second-order, first-order, and Intraparticle diffusion to Elovich model. This indicates that the adsorption follows the second-order than the other models for both AC and DC. Further, the calculated q e values well agrees with the experimental q e values for second-order kinetics model concluding that the second-order kinetics equation is the best-fitting kinetic model.
3.6 Isotherm modeling
The adsorption capacity of the adsorbent has been tested using Freundlich, Langmuir and Dubinin–Redushkevich isotherms. These models have been widely used to describe the behavior of adsorbent–adsorbate couples. To determine the isotherms, the initial pH was kept at 7 and the concentration of copper used was in the range of 2–10 mg/L using AC and DC.
3.6.1 Freundlich isotherm
The Freundlich isotherm is an empirical model relates the adsorption intensity of the sorbent towards adsorbent. The isotherm is adopted to describe reversible adsorption and not restricted to monolayer formation. The mathematical expression of the Freundlich model can be written as (Leea et al. 2004; Prasanna Kumar et al. 2006),
According to Freundlich isotherm model, initially amount of adsorbed compounds increases rapidly, this increase slow down with the increasing surface coverage. Equation (10) can be linearized in logarithmic form and the Freundlich constants can be determined as follows,
where, k f is the Freundlich constant related to adsorption capacity, n is the energy or intensity of adsorption; C e is the equilibrium concentration of copper (in milligrams per liter). In testing the isotherm, the copper concentration used was 2–10 mg/L and at an initial pH 7, the adsorption data is plotted as logq e versus logC e and should result in a straight line with slope n and intercept k f. The intercept and the slope are indicators of adsorption capacity and adsorption intensity, respectively. The value of n falling in the range of 1–10 indicates favorable sorption. The Freundlich constants k f and n values were shown in Table 4 for AC and DC sources. From the analysis of the results, it is found that the Freundlich plots fit satisfactorily with the experimental data obtained in the present study.
3.6.2 Langmuir isotherm
The linearized form of Langmuir adsorption isotherm model is (Bouzid et al. 2008; Sarioglu et al. 2005),
where, q e is amount adsorbed at equilibrium concentration C e, q m is the Langmuir constant representing maximum monolayer adsorption capacity and b is the Langmuir constant related to energy of adsorption. The plots of 1/q e as a function of 1/C e for the adsorption of copper on Mg(OH)2 are shown in Fig. 5. The plots were found linear with good correlation coefficients (>0.99) indicating the applicability of Langmuir model in the present study. The values of monolayer capacity (q m) is found to be 277.21 and 264.55 mg/g for AC and DC (Table 4). The values of q m calculated by the Langmuir isotherm were all close to experimental values at given experimental conditions. These facts suggest that copper is adsorbed in the form of monolayer coverage on the surface of the adsorbent.
The essential characteristics of the Langmuir isotherm can be expressed as the dimensionless constant R L.
where R L is the equilibrium constant it indicates the type of adsorption, b, is the Langmuir constant. c o represents the various concentrations of copper solution. The R L values between 0 and 1 indicate the favorable adsorption. The R L values were found to be between 0 and 1 for all the concentration of copper studied.
3.6.3 Dubinin–Radushkevich isotherm
Dubinin and Radushkevich have proposed another isotherm which can be used to analyze the equilibrium data. It is not based on the assumption of homogeneous surface or constant adsorption potential, but it is applied to estimate the mean free energy of adsorption (E). This model is represented by (Tan et al. 2007),
where ε = RT ln [1 + 1/C e], B is related to the free energy of sorption per mole of the adsorbate as it migrates to the surface of the electrocoagulant from infinite distance in the solution and q s is the D–R isotherm constant related to the degree of adsorbate adsorption by the adsorbent surface. The linearized form of Eq. (14):
The isotherm constants of q s and B are obtained from the intercept and slope of the plot of lnq e versus ε 2, respectively (Demiral et al. 2008). The constant B gives the mean free energy E, of adsorption per molecule of the adsorbate when it is transferred to the surface of the solid from infinity in the solution and the relation is given as,
The magnitude of E is useful for estimating the type of adsorption process. It was found to be 10.21 and 12.36 kJ/mol for AC and DC, respectively, which is bigger than the energy range of adsorption reaction, 8–16 kJ/mol. So the type of adsorption of copper on magnesium hydroxide was defined as chemical adsorption.
The correlation coefficient values of different isotherm models are listed in Table 4. The Langmuir isotherm model has a higher regression coefficient (R 2 = 0.999) when compared to the other models for both AC and DC source. The value of R L for the Langmuir isotherm was calculated from 0 to 1, indicating the favorable adsorption of copper.
3.7 Thermodynamic parameters
The amount of copper adsorbed on the adsorbent increases by increasing the temperature indicating the process to be endothermic. The diffusion coefficient (D) for intraparticle transport of copper species into the adsorbent particles has been calculated at different temperature by,
where t 1/2 is the time of half adsorption (s), r o is the radius of the adsorbent particle (in centimeters), D is the diffusion coefficient in square centimeters per second. For all chemisorption systems, the diffusivity coefficient should be 10−5 to 10−13 cm2/s (Yang and Al-Duri 2001). In the present work, D is found to be in the range of 10−10 cm2/s. The pore diffusion coefficient (D) values for various temperatures and different initial concentrations of copper are presented in Table 5.
To find out the energy of activation for adsorption of copper, the second-order rate constant is expressed in Arrhenius form (Golder et al. 2006),
where k o is the constant of the equation (in grams per milligram per minute), E is the energy of activation (in joules per mole), R is the gas constant (8.314 J/mol/K) and T is the temperature in kelvin. Figure 6 shows that the rate constants vary with temperature according to Eq. (18). The activation energy (0.445 and 0.336 kJ/mol for AC and DC, respectively) is calculated from slope of the fitted equation. The free energy change is obtained using the following relationship
where ∆G is the free energy (in kilojoules per mole), K c is the equilibrium constant, R is the gas constant and T is the temperature in K. The K c and ∆G values are presented in Table 6. From the table, it is found that the negative value of ∆G indicates the spontaneous nature of adsorption. Other thermodynamic parameters such as entropy change (∆S) and enthalpy change (∆H) were determined using the van’t Hoff equation,
The enthalpy change and entropy change were obtained from the slope and intercept of the van’t Hoff linear plots of lnK c versus 1/T (Fig. 7). Positive value of enthalpy change (∆H) indicates that the adsorption process is endothermic in nature, and the negative value of change in internal energy (∆G) show the spontaneous adsorption of copper on the adsorbent. Positive values of entropy change show the increased randomness of the solution interface during the adsorption of copper on the adsorbent (Table 6). Enhancement of adsorption capacity of electrocoagulant (magnesium hydroxide) at higher temperatures may be attributed to the enlargement of pore size and or activation of the adsorbent surface (Vasudevan et al. 2010d). Using the Lagergren rate equation, first-order rate constants and correlation coefficients were calculated for different temperatures (305–343 K). The calculated q e values obtained from the second-order kinetics agrees with the experimental q e values better than the first-order kinetics model. Table 7 depicts the computed results obtained for the first- and second-order kinetic models using AC and DC. These results indicate that the adsorption follows second-order kinetic model at different temperatures used in this study.
3.8 Pilot scale study
A bench scale capacity cell (Fig. 8) was designed, fabricated and operated for the removal of copper from water. The system consists of AC/DC power supply, an electrochemical reactor, a water tank, a feed pump, a flow control valve, a flow measuring unit, a circulation pump, settling tank, sludge collection tank, filtration unit provisions for gas outlet and treated water outlet. The reactor is made of PVC with an active volume of 2,000 L. The magnesium anode and cathode each consist of five pieces situated approximately 5 mm apart from each other and submerged in the solution. The total electrode surface area is 1,500 cm2 for both cathodes and anodes. The cell was operated at a current density of 0.025 A/dm2 and the electrolyte pH of 7.0. The results showed that the removal efficiency of 97.8 and 97.2 % with the energy consumption of 0.634 and 0.996 kWh/kL was achieved at a current density of 0.025 A/dm2 and pH of 7.0 using magnesium as electrodes using AC and DC, respectively. The results were consistent with the results obtained from the laboratory scale, showing that the process was technologically feasible.
3.9 Surface morphology
3.9.1 SEM and EDAX studies
In order to gain more insight into the effect of alternating current, the morphology of the electrode surface after two kinds of electrolysis (AC and DC) was characterized by SEM as shown in Fig. 9a and b. It can be observed that when the AC was fed, less disordered pores formed and a smooth microstructure of magnesium suggesting the magnesium electrodes were dissolved uniformly during the electrolysis. While for the electrodes fed with DC, the electrode surface is found to be rough, with a number of dents. These dents are formed around the nucleus of the active sites where the electrode dissolution results in the production of magnesium hydroxides. The formation of a large number of dents may be attributed to the anode material consumption at active sites due to the generation of oxygen at its surface (Li et al. 2009). Energy-dispersive analysis of X-rays was used to analyze the elemental constituents of copper-adsorbed magnesium hydroxide shown in Fig. 10. It shows that the presence of Cu, Mg, and O appears in the spectrum. EDAX analysis provides direct evidence that copper is adsorbed on magnesium hydroxide.
3.9.2 FTIR studies
FTIR spectrum (Fig. 11) of copper–magnesium hydroxide shows the sharp and strong peak at 3,452.84 cm−1 implies the transformation from free protons into a proton-conductive state in brucite. The 1,640.43 cm−1 peak indicates the bent vibration of H–O–H. The band at 669.93 cm−1 corresponds to Mg–O stretching vibration. Cu–O vibration at 875.21 cm−1 also observed.
3.9.3 XRD studies
X-ray diffraction spectrum of magnesium electrode coagulant showed very broad and sallow diffraction peaks (Fig. 12). This broad humps and low intensity indicate that the coagulant is amorphous or very poor crystalline in nature. This may be due to the crystallization of magnesium hydroxide is a very slow process resulting all magnesium hydroxides found to be either amorphous or very poorly crystalline.
4 Conclusion
The results showed that the optimized removal efficiency of 97.8 and 97.2 % was achieved for AC and DC source at a current density of 0.025 A/dm2 and pH of 7.0 using magnesium as both anode and cathode. The magnesium hydroxide generated in the cell removes the copper present in the water, reducing the copper concentration to less than 1 mg/L, making it safe for drinking. The results of the scale-up study show that the process was technologically feasible. Langmuir adsorption isotherm was found to fit the equilibrium data for copper adsorption for both AC and DC. The adsorption process follows second-order kinetics. Temperature studies showed that adsorption was endothermic and spontaneous in nature.
References
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process 43:1281–1287
Ajmal M, Khan A, Ahmad S, Ahmad A (1998) Role of sawdust in the removal of copper(II) from industrial wastes. Water Res 32:3085–3091
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purf Rev 39:95–171
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sepn Purfn Rev 40:25–42
Ali I, Khan TA, Asim M (2012) Removal of arsenate from ground water by electro-coagulation method. Environ Sci Polln Res. doi:10.1007/s11356-011-0681-3
Allen SJ, Mckay G, Khader KHY (1989) Intraparticle diffusion of basic dye during adsorption onto Sphagnum peat. Environ Pollut 56:39–50
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Benaissa H, Elouchd MA (2007) Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem Eng Process 46:614–622
Billon L, Meric V, Castetbon A, Francois J, Desbrieres J (2006) Removal of copper ions from water of boilers by a modified natural based corncobs. J Appl Polym Sci 102:4637–4645
Boujelben N, Bouzid J, Elouear Z (2009) Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: study in single and binary systems. J Hazard Mater 163:376–382
Bouzid J, Elouear Z, Ksibi M, Feki M, Montiel A (2008) A study on removal characteristics of copper from aqueous solution by sewage sludge and pomace ashes. J Hazard Mater 152:838–845
Carlesi Jara C, Fino D, Specchia V, Saracco G, Spinelli P (2007) Electrochemical removal of antibiotics from wastewaters. Appl CatalsB: Environ 70:479–487
Carlos A, Huitle M, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Christensen PA, Egerton TA, Lin WF, Meynet P, Shaoa ZG, Wright NG (2006) A novel electrochemical device for the disinfection of fluids by OH radicals. Chem Commun 38:4022–4023
CPCB,Central Pollution Control Board (2002) Government of India, Delhi
Demiral H, Demiral I, Tumsek F, Karacbacakoglu B (2008) Adsorption of chromium (Vl) from aqueous solution by activated solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J 144:188–195
Fiol N, Villaescesa I, Martinez M, Miralles N, Poch J, Serarols J (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solutions by olive stone waste. Sep Purif Technol 50:132–140
Gardea-Torresde JL, Tang L, Salvador JM (1996) Copper adsorption by esterified and unesterified fractions of Sphagnum peat moss and its different humic substances. J Hazard Mater 48:191–206
Golder AK, Samantha AN, Ray S (2006) Removal of phosphate from aqueous solution using calcined metal hydroxides sludge waste generated from electrocoagulation. Sep Purif Technol 52:102–109
Goyal RN, Gupta VK, Sangal A, Bachheti N (2005) Voltammetric determination of uric acid at a fullerene-C60-modified glassy carbon electrode. Electroanalysis 17:2217–2223
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007a) Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: application in pharmaceutical formulations and biological fluids. Talanta 72:976–983
Goyal RN, Gupta VK, Bachheti N (2007b) Voltammetric determination of adenosine and guanosine using fullerene-C60-modified glassy carbon electrode. Talanta 71:1110–1117
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag-a low cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2000) Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18:131–140
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: equilibrium and adsorption kinetics. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modeling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Rastogi A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Sharma S (2002) Removal of cadmium and zinc from aqueous solutions using red mud. Environ Sci Technol 36:3612–3617
Gupta VK, Ali I, Saini VK (1997a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997b) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste material for the removal of some heavy metal ions. Waste Manage 17:517–522
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium (VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud-an aluminum industry waste. Water Res 35:1125–1134
Gupta VK, Mangla R, Agarwal S (2002) Pb (II) selective potentiometric sensor based on 4-tert-Butylcalix [4] arene in PVC matrix. Electroanalysis 14:1127–1132
Gupta VK, Mohan D, Sharma S (2003a) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003b) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res 37:4038–4044
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Kurup L, Mittal J (2006b) Adsorption of a hazardous dye—erythrosine over hen feathers. J Colloid Interface Sci 304:52–57
Gupta VK, Jain AK, Kumar P, Agarwal S, Maheshwari G (2006c) Chromium(III)-selective sensor based on tri-o-thymotide in PVC matrix. Sens Actuat B: Chem 113:182–186
Gupta VK, Jain R, Mittal A, Mathur M, Shalini S (2007a) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007b) Removal of Reactofix golden yellow 3RFN from aqueous solution using wheat husk- an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2007c) Photochemical degradation of hazardous dye—Safaranin-T Using TiO2 Catalyst. J Colloid Interface Sci 309:460–465
Gupta VK, Singh AK, Gupta B (2007d) Schiff bases as cadmium (II) selective ionophores in polymeric membrane electrodes. Anal Chim Acta 583:340–348
Gupta VK, Jain R, Varshney S (2007e) Electrochemical removal of hazardous dye Reactofix Red 3 BFN from industrial effluents. J Colloid Interface Sci 312:292–296
Gupta VK, Ali I, Saini VK (2007f) Defluoridation of wastewaters using waste carbon slurry. Water Res 41:3307–3316
Gupta VK, Goyal RN, Sharma RA (2009a) Novel PVC membrane based alizarin sensor and its application; determination of vanadium, zirconium and molybdenum. Int J Electrochem Sci 4:156–172
Gupta VK, Al Khayat M, Singh AK, Pal MK (2009b) Nano level detection of Cd (II) using poly (vinyl chloride) based membranes of Schiff bases. Anal Chim Acta 634:36–43
Gupta VK, Goyal RN, Sharma RA (2009c) Comparative studies of neodymium (III)-selective PVC membrane sensors. Analytica Chim Acta 647:66–71
Gupta VK, Carrott PJM, Ribeiro Carrott MML (2009d) Low cost adsorbents: growing approach to wastewater treatment—a review. Critical Rev Environ Sci Technol 39:783–842
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340
Jain AK, Gupta VK, Singh LP (1995a) Neutral carrier and organic resin based membranes as sensors for uranyl ions. Anal Proc Anal Commun (RSC) 32:263–265
Jain AK, Gupta VK, Sahoo BB, Singh LP (1995b) Copper (II)-selective electrodes based on macrocyclic compounds. Anal Proc Anal Commun(RSC) 32:99–101
Jain AK, Gupta VK, Khurana U, Singh LP (1997) A new membrane sensor for UO2+, based on 2-hydroxyacetophenoneoxime—thioureatrioxane resin. Electroanalysis 9:857–860
James R, Sampath K, Selvamani P (2006) Effect of ion-exchanging agent, zeolite on removal of copper in water and improvement of growth in Oreochromis mossambicus (Peters). Asian Fisheries Sci 13:317–325
Larous S, Meniai H, Bencheikh Lehocine M (2005) Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185:483–490
Leea C, Yanga W, Hsiehb C (2004) Removal of copper (II) by manganese-coated sandin a liquid fluidized-bed reactor. J Hazard Mater B114:45–51
Li X, Ma GB, Liu YY (2009) Synthesis and characterization of magnesium hydroxide using a bubbling setup. Ind Eng Chem Res 48:763–768
Mittal A, Jain R, Mittal J, Varshney S, Sirkarwar S (2010) Removal of Yellow ME 7 GL from industrial effluent using electrochemical and sorption technique. Int J Environ Pollution 43:308–323
Miwa DW, Malpass GRP, Machado SAS, Motheo AJ (2006) Electrochemical degradation of carbaryl on oxide electrodes. Water Res 40:281–289
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids and Surfaces A 177:169–181
Mrozowski J, Zielinski J (1983) Studies of zinc and lead removal from industrial wastes by electrocoagulation. Environ Prot Eng 9:77–85
Oke IA, Olarinoye NO, Adewusi SRA (2008) Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorption 14:73–83
Onder E, Koparal AS, Ogutveren UB (2007) An alternative method for the removal of surfactants from water: electrochemical coagulation. Sep Purif Technol 52:527–532
Onmez G, Aksu Z (1999) The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem 35:35–142
Ozcan A, Ozcan AS, Tunali S, Akar T, Kiran I (2005) Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper (II) ions onto seeds of Capsicum annuum. J Hazard Mater B124:200–208
Ozer A, Ozer D, Ozer A (2004) The adsorption of copper(II) ions onto dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters. Process Biochem 39:2183–2191
Papandreou A, Stournaras CJ, Panias D (2007) Copper and cadmium adsorption on pellets made from fired coal fly ash. J Hazard Mater 148:538–547
Prasad MNV, Freitas H (2000) Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. (holly oak). Environ Pollut 110:277–283
Prasanna Kumar Y, King P, Prasad VSRK (2006) Removal of copper from aqueous solution using Ulva fasciata sp.—a marine green algae. J Hazard Mater B137:367–373
Saeed A, Iqbal M, Akhtar MW (2005) Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater B117:65–73
Sarioglu M, May A, Cebeci Y (2005) Removal of copper from aqueous solutions by phosphate rock. Desalination 181:303–311
Shukla SP, Sakhardane VD (1992) Column studies on metal ion removal by dyed cellulosic materials. J Appl Poly Sci 44:903–910
Srivastava SK, Gupta VK, Dwivedi MK, Jain S (1995) Caesium PVC-Crown (dibenzo-24-crown-8) based membrane sensor. Anal Proc Anal Commun (RSC) 32:21–23
Srivastava SK, Gupta VK, Mohan D (1996a) Kinetic parameters for the removal of lead and chromium from wastewater using activated Carbon developed from fertilizer waste material. Environ Modell Assessment 1:281–290
Srivastava SK, Gupta VK, Jain S (1996b) A PVC-based benzo-15-crown-5 membrane sensor for cadmium. Electroanalysis 8:938–940
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Envorn Engg 123:461–468
Tan IAW, Hameed BH, Ahmed AL (2007) Equilibrium and kinetics studies on the basic dye adsorption by palm fibre activated carbon. Chem Eng J 127:111–119
Vasudevan S, Lakshmi J (2011) Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. Sep Purifi Technol 80:643–651
Vasudevan S, Sozhan G, Ravichandran S, Jayaraj J, Lakshmi J, Margrat Sheela S (2008) Studies on the removal of phosphate from drinking water by electrocoagulation process. Ind Eng Chem Res 47:2018–2023
Vasudevan S, Lakshmi J, Sozhan G (2009) Studies on the removal of iron from drinking water by electrocoagulation—a clean process. Clean 37:45–51
Vasudevan S, Lakshmi J, Vanathi R (2010a) Electrochemical coagulation for chromium removal: process optimization, kinetics, isotherm and sludge characterization. Clean 38:9–16
Vasudevan S, Margrat Sheel S, Lakshmi J, Sozhan G (2010b) Optimization of the process parameters for the removal of boron from drinking water by electrocoagulation–a clean technology. J Chem Technol Biotech 85:926–933
Vasudevan S, Lakshmi J, Sozhan G (2010c) Studies relating to removal of arsenate by electrochemical coagulation optimization, kinetics, coagulant characterisation. Sep Sci Technol 45:1313–1325
Vasudevan S, Lakshmi J, Sozhan G (2010d) Studies on the removal of arsenate by electrochemical coagulation using aluminium alloy anode. Clean 38:506–515
Vasudevan S, Lakshmi J, Packiyam M (2010e) Electrocoagulation studies on removal of cadmium using magnesium electrode. J Appl Electrochem 40:2023–2032
Villaescesa I, Fiol N, Martinez M, Miralles N, Poch J, Serarols J (2004) Removal of copper and nickel ions from aqueous solutions by grape stalk wastes. Water Res 38:992–1002
Vinikour WS, Goldstein RM, Anderson RV (1980) Bioaccumulation patterns of zinc, copper, cadmium and lead in selected fish species from the Fox River, Illinois. Bull Environ Contam Toxicol 24:727–734
Wan Ngah WS, Hanafiah MAKM (2008) Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: kinetic, equilibrium and thermodynamic studies. Biochemical En J 39:521–530
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J SanitDiv Am Soc Civ Eng 89:31–59
Wu Z, Joo H, Lee K (2005) Kinetics and thermodynamics of the organic dye adsorption on the mesoporous hybrid xerogel. Chem Eng J 112:227–236
Yang XY, Al-Duri B (2001) Application of branched pore diffusion model in the adsorption of reactive dyes on activated carbon. Chem Eng J 83:15–23
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80:33–42
Acknowledgment
The authors wish to express their gratitude to the Director, Central Electrochemical Research Institute, Karaikudi, for aid in publishing this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Kamaraj, R., Ganesan, P., Lakshmi, J. et al. Removal of copper from water by electrocoagulation process—effect of alternating current (AC) and direct current (DC). Environ Sci Pollut Res 20, 399–412 (2013). https://doi.org/10.1007/s11356-012-0855-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0855-7