Abstract
Introduction
The aims of the present study are to investigate the effects of Ce3+ on the growth and some antioxidant metabolisms in rice seedlings (Oryza sativa L. cv Shengdao 16).
Materials and methods
The rice was treated with 0, 0.05, 0.1, 0.5, 1.0, and 1.5 mM Ce3+, respectively. The growth index of rice was measured. The chlorophyll content; catalase, superoxide dismutase, and peroxidase activities; and the level of hydrogen peroxide (H2O2), superoxide anion (O ·−2 ), and malondialdehyde were assayed. The accumulation of Ce3+ and the uptake of mineral nutrition elements were analyzed with ICP-SF-MS.
Results and discussion
Hormetic effects of Ce3+ on the growth and some antioxidant metabolisms were found in the roots and shoots of rice. The roots can accumulate a much higher content of Ce3+ than shoots and Ce3+ mainly located in the cell wall of roots. Moreover, the uptake of K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo in the roots and shoots was affected with the exposure of different Ce3+ treatments, which indicated that Ce3+ affected the nutritional status of roots and shoots and further affected the growth of rice.
Conclusion
The appropriate amount of Ce3+ improved the defense system and growth of rice. The roots can accumulate a much higher content of Ce3+ than shoots. Moreover, the uptake of K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo in the roots and shoots was affected with the exposure of different Ce3+ treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rare earth elements (REEs) comprise a group of 17 trivalent metallic elements with similar chemical properties. REEs have been widely used in a variety of modern industries over the past years and can enter the environment and accumulate in the ecosystem. Recently, the potential adverse effects of REEs on human and environmental health have been given much attention (Zhu et al. 1996; Gu et al. 2001; d’Aquino et al. 2009).
The effects of REEs on physiological responses have been reported in different plant species (Fashui et al. 2000; Chen et al. 2001; Hu et al. 2002, 2004). An appropriate amount of REEs not only promotes seed germination and root development, but also improves harvest quality and plant resistance against stress (He and Xue 2005; d’Aquino et al. 2009). REEs could enhance chlorophyll content, improve photosynthetic rate, and increase plant biomass (Wu et al. 1983; Chang 1991; He and Xue 2005). It has been found that trivalent cerium (Ce3+) stimulates the growth of spinach and increases chlorophyll content and photosynthetic rate (Fashui et al. 2002), and Ce3+ has positive effects on the cell growth of C. deserticola at proper concentrations (Ouyang et al. 2003).
Reactive oxygen species (ROS), which mainly include hydrogen peroxide (H2O2), superoxide anion (O ·−2 ), singlet oxygen (1O2), and hydroxyl radical (OH.), will be generated in plants under biological and physical stimuli (Doke 1983; Jabs et al. 1997; Stennis et al. 1998). Moreover, ROS can act as a signal molecule in plants and trigger a series of cellular responses (Lamb and Dixon 1997; Mittler et al. 2004). ROS will be toxic to plants when ROS levels exceed the detoxification capacity of the plant tissues. Plants have non-enzymatic and enzymatic antioxidants to scavenge ROS. Non-enzymatic antioxidants mainly include α-tocopherol, glutathione, and ascorbate. Enzymatic antioxidants mainly include superoxide dismutase (SOD, EC.1.15.1.1), catalase (CAT, EC.1.11.1.6), ascorbate peroxidase (EC 1.11.1.11), glutathione-S-transferase (EC. 2.5.1.18), and glutathione reductase (EC 1.6.4.2) (Aravind and Prasad 2005). It has been found that the activities of SOD, CAT, and peroxidase (POD) were induced by REEs (Fashui et al. 2002; Nardi et al. 2004). La3+ protects soybean plants from oxidative stress by reacting with ROS directly or by improving the defense system of plants (Wang et al. 2009). La3+ alleviates the oxidative damage induced by UV-B radiation and reduces the content of H2O2, O ·−2 , and malondialdehyde (MDA) (Wang et al. 2009; Peng and Zhou 2009).
Although the physiological and biochemical responses of plants to REEs have widely been reported, the results of field trials and laboratory studies on REEs are still contradictory (Diatloff et al. 1995a, b, c; 1999; He and Loh 2000; von Tucher and Schmidhalter 2005; Wang et al. 2005, 2007). In addition, there are fewer reports about the toxic effects of REEs on plant mineral nutrition and developmental characters of plants, and the mechanisms of REE toxicity are still unclear (Dai et al. 2011; Wang et al. 2011). Furthermore, the abundance of Ce3+ in soil is almost equal to that of copper and zinc. Ce3+ is one of the main ingredients in REE-based fertilizers though Ce3+ has not been characterized as an essential plant nutrient until recent years. The aims of the present study are to investigate the effects of Ce3+ on the growth and some antioxidant metabolisms in rice seedlings (Oryza sativa L. cv Shengdao 16) and explore the potential mechanism involved in the effect of Ce3+ on growth and antioxidant metabolism of rice.
2 Materials and methods
2.1 Plant material and plant growth
Seeds of rice (O. sativa L. cv Shengdao 16) were surface sterilized by soaking in 75 % alcohol for 60 s, 0.1 % mercury chloride for 15 min, and followed by 1.0 % sodium hypochlorite for 20 min. The seeds were then rinsed five times in sterilized water prior to culture. The 1/2 modified Murashige and Skoog medium (containing 0.75 mM MgSO4, 10.0 mM NH4NO3, 9.4 mM KNO3, 0.625 mM KCl, 1.5 mM CaCl2, 2.5 μM KI, 50 μM H3BO3, 50 μM FeSO4, 50 μM MnSO4, 15 μM ZnSO4, 0.05 μM CuSO4, 0.05 μM CoCl2, 0.5 μM Na2MoO4, 50 μM Na2H2EDTA, 0.15 μM thiamine, 1.2 μM pyridoxine, 2.0 μM nicotinic acid, 275 μM inositol, 0.56 % agar, 3.0 % sucrose, 0.05 % Mes) was used for seed germination and as basal medium, and the pH of the medium was adjusted to 5.8 before agar was added. Plants were grown at 25.0 ± 2°C using a 14/10-h light/dark cycle in a growth chamber under a light intensity of 6,000 lx. Subsequently, the different concentrations of Ce(NO3)3 were added to the basal medium, and the concentration of Ce3+ in the basal medium was as follows: 0, 0.05, 0.1, 0.5, 1.0, and 1.5 mM.
2.2 Root and shoot growth measurements
The rice plants were grown for 13 days. The lengths of seminal roots, total nodal roots, and shoot heights (20 plants) were directly measured with a ruler. Then the number of nodal roots and the number of lateral roots (LRs) on seminal or total nodal roots were determined respectively by counting all LRs that had emerged from the seminal or nodal roots of 20 seedlings. In addition, the fresh weights (FW) and dry weights (DW) of roots and shoots (20 plants) were measured, respectively.
2.3 Assay of SOD, POD, and CAT activities and the levels of H2O2, O ·−2 , and MDA
Plants were collected at the 13th day after seed germination. Root and shoot samples were harvested and the FW recorded. Root and shoot samples were collected and homogenized in cold phosphate buffer (10.0 mmol/L mercaptoethanol, 1.0 % polyvinylpyrrolidone, pH 7.8). After homogenization in cold phosphate buffer, homogenates were centrifuged at 10,000×g for 20 min at 4°C to remove the precipitated debris. The supernatant was assayed for SOD, POD, CAT, MDA, H2O2, and O ·−2 analysis.
The SOD activity was determined at 550 nm according to the procedures described by Oyanagui (1984). One unit of SOD activity is defined as the amount of enzyme that inhibits by 50 % nitrite formation. The CAT activity was measured at 405 nm by assaying H2O2 forming stable complex with ammonium molybdate under the assay conditions specified by Góth (1991), and one unit CAT decomposes 1.0 μM H2O2 per minute. The POD activity was determined by an increase in absorbance at 470 nm during the oxidation of guaiacol according to procedures described by Maehly (1955).
The level of H2O2 was determined at 405 nm according to procedures described by Góth (1991), which was based on the formation of its stable complex with ammonium molybdate. The level of O ·−2 was determined at 530 nm according to procedures described by Wang and Luo (1990). Lipid peroxidation was measured by the level of MDA using a reaction with thiobarbituric acid as described by Heath and Parker (1968).
2.4 Assay of chlorophyll content
Leaf samples were harvested and the FWs recorded. Then leaf samples were treated with 3.0 ml dimethyl sulfoxide in the presence of polyvinylpolypyrrolidone at 60°C for 2 h in the dark. Photosynthetic pigments of all of the samples were extracted, and chlorophyll concentrations were calculated using the extinction coefficients and equation given by Barnes et al. (1992). A spectrophotometer is used at two wavelengths (648.2 and 664.9 nm) for maximum absorption of chlorophyll a and b, respectively.
2.5 Ce and mineral nutrition elements analysis
The root and shoot samples were washed thoroughly with deionized water, and a microwave-assisted digestion procedure was applied. Approximately 0.1 g of sample (DW) was weighed into Teflon bombs. Then 10.0 cm3 of HNO3 was added, and the samples were digested. After digestion, samples were transferred quantitatively into polypropylene tubes and filled up to 10.0 cm3. Subsequently, Ce, K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo concentrations in the digest were investigated using inductively coupled plasma sector field mass spectrometry (ICP-SF-MS) (Agilent, Japan) with external calibration using 114.8In and 102.9Rh as an internal standard.
2.6 TEM analysis
The samples of leaves and roots (the control and the group exposed to 1.0 mM Ce3+) were fixed for 2 h at 4°C in 2.5 % (v/v) glutaraldehyde in 0.1 M phosphate buffer solution (pH 7.3) and postfixed in 1 % (w/v) aqueous osmium tetroxide for 2 h. Samples were dehydrated in a 50–100 % ethanol series and finally embedded in Epon 812 resin. Ultrathin sections of 70 nm thickness were cut using an Ultracut Eultramicrotome (Leica, Germany) with a diamond knife, and the subcellular distribution of Ce was studied using a Hitachi H-600 transmission electron microscope (TEM). The ultrathin sections were then stained with uranyl acetate and lead citrate, and the changes of the ultrastructure were examined using TEM.
2.7 Statistical analyses
All assays for SOD, POD, CAT, MDA, H2O2, O ·−2 , and chlorophyll content, and element analysis were carried out in triplicate, and results are expressed as mean ± standard deviation (SD). Statistical comparisons were done with one-way ANOVA in SPSS 16.0 for Windows (SPSS Inc., Chicago, USA). When the differences were significant (P < 0.05), Tukey’s tests were performed for post hoc comparisons.
3 Results
3.1 Effect of Ce3+ on the root length and shoot height
The root length and shoot height of rice under the effect of different Ce3+ concentrations were shown in Figs. 1 and 2. Compared with the control group, the seminal root, total nodal root length, and shoot height decreased significantly at 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 2). The total nodal root length increased significantly at 0.05 and 0.1 mM Ce3+ (Fig. 2). However, the seminal root length was not significantly more than the control at 0.05 and 0.1 mM Ce3+ (Fig. 2). The shoot height increased significantly at 0.1 mM Ce3+ (Fig. 2).
3.2 Effect of Ce3+ on the root number of rice
The root number of rice under the effect of different Ce3+ concentrations was shown in Fig. 3. Compared with the control group, the lateral root number of seminal roots decreased significantly at 0.1, 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 3a). However, the lateral root number of seminal roots was significantly more than the control at 0.05 mM Ce3+ (Fig. 3a).
When exposed to 0.1 mM Ce3+, the number of nodal root was increased significantly (Fig. 3b). However, the number of nodal roots was not significantly more than the controls at 0.05 mM Ce3+, and the number of nodal roots was not significantly less than the controls at 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 3b). In addition, the total lateral root number of nodal roots was significant more than the controls at 0.05, 0.1, and 0.5 mM Ce3+ (Fig. 3c).
3.3 Effect of Ce3+ on the fresh and dry weights of roots and shoots
As shown in Fig. 3, the fresh and dry weights of roots and shoots were decreased significantly when the rice was exposed to 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 4a and b). The fresh weight of roots was increased significantly at 0.05 and 0.1 mM Ce3+, and the fresh weight of shoots was increased significantly at 0.1 mM Ce3+ (Fig. 4a). Moreover, the dry weight of shoots was increased significantly at 0.1 mM Ce3+ (Fig. 4b).
3.4 Effect of Ce3+ on the chlorophyll content
Compared with the controls, the content of chlorophyll a and total chlorophyll was increased significantly at 0.05 and 0.1 mM Ce3+, while the content of chlorophyll b was increased significantly at 0.1 mM Ce3+ (Fig. 5). However, the content of chlorophyll a, chlorophyll b, and total chlorophyll was not decreased significantly when exposed to 0.5 and 1.0 mM Ce3+ (Fig. 5). The content of chlorophyll a and total chlorophyll were all decreased significantly at 1.5 mM Ce3+ (Fig. 5).
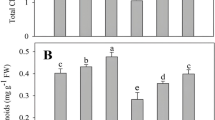
3.5 Effect of Ce3+ on the antioxidant metabolism in the roots and shoots
The effect of Ce3+ on the antioxidant metabolism in the roots and shoots was shown in Fig. 6. Compared with the controls, the content of MDA in the roots and shoots was increased significantly at 1.0 and 1.5 mM Ce3+ (Fig. 6a). The content of H2O2 in the shoots was increased significantly at 1.0 and 1.5 mM Ce3+ (Fig. 6b), and the content of H2O2 in the roots was increased significantly at 1.5 mM Ce3+ (Fig. 6b). To MDA and H2O2 content in the roots and shoots, there was no significant difference between the controls and the groups of 0.05, 0.1, and 0.5 mM Ce3+ (Fig. 6a, b). The level of O ·−2 in the roots and shoots was increased significantly at 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 6c). However, there was no significant difference to the level of O ·−2 in the roots and shoots between the controls and the groups of 0.05 and 0.1 mM Ce3+ (Fig. 6c).
The activity of CAT in the roots was increased significantly at 0.05, 0.1, and 0.5 mM Ce3+ (Fig. 6d), and CAT activity in the shoots was increased significantly at 0.5 and 1.0 mM Ce3+ (Fig. 6d). There was no significant difference to the CAT activity in the roots and shoots between the controls and the group of 1.5 mM Ce3+ (Fig. 6d). Moreover, CAT activity in the shoots was not significantly higher than the controls at 0.05 and 0.1 mM Ce3+ (Fig. 6d). The activity of SOD in the roots was increased significantly at 0.1, 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 6e), but SOD activity in the shoots was decreased significantly at 0.5 and 1.5 mM Ce3+ (Fig. 6e). The activity of POD in the roots was increased significantly at 0.1 and 0.5 mM Ce3+ (Fig. 6f), but POD activity in the shoots was increased significantly at 0.5, 1.0, and 1.5 mM Ce3+ (Fig. 6f). The activity of SOD and POD in the roots and shoots was not increased significantly at 0.05 mM Ce3+ (Fig. 6e, f).
3.6 Bioaccumulation of Ce in the roots and shoots
Bioaccumulation of Ce in the roots and shoots was investigated with the technique of ICP-SF-MS. The bioaccumulation of Ce in the roots and shoots of rice was observed to be increased with the increase of the concentration of Ce3+ (Tables 1 and 2). Maximum accumulation of Ce was recorded in the roots and shoots exposed to 1.5 mM Ce3+ for 13 days (Tables 1 and 2).
3.7 Effect of Ce3+ on the uptake of mineral nutrition elements in the roots and shoots
The effect of Ce3+ on the uptake of K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo in the roots and shoots was investigated with the technique of ICP-SF-MS. The concentration of various nutrients was affected markedly in response to the different concentration of Ce3+.
The concentration of K and Ca in the roots was higher than the controls at 0.05 and 0.1 mM Ce3+ and lower than the controls at 0.5 and 1.0 mM Ce3+ (Table 1). However, the concentrations of Na, Zn, Cu, and Mo in the roots were decreased, while the concentration of Mn was increased with the exposure of different concentration of Ce3+ (Table 1). The concentration of Fe in the roots was higher than the controls with the exposure of different concentration of Ce3+ (Table 1), but there was no significant difference to the Mg content between the controls and the groups treated with the different concentration of Ce3+ (Table 1).
The concentrations of Cu and Mn in the shoots were decreased with the exposure of different concentration of Ce3+ (Table 2). The concentration of K, Mg, Ca, Na, Zn, and Mo in the shoots was higher than the controls at 0.05 and 0.1 mM Ce3+ and lower than the controls at 0.5 and 1.0 mM Ce3+ (Table 2). Moreover, the concentration of Fe in the shoots was lower than the controls with the exposure of different concentration of Ce3+ (Table 2).
3.8 Ce subcellular distribution in the roots and the effect of Ce on the chloroplast
The distribution of Ce in the root cells and the effect of Ce on the chloroplast were shown in Fig. 6. There was no Ce deposit in the cell wall in the control (Fig. 7a), and Ce was mainly deposited in the cell wall when the roots were treated with 1.0 mM Ce3+ (Fig. 7b). The chloroplast was intact with an orderly arrangement of the grana and stroma thylakoids in the control (Fig. 7c). By contrast, the chloroplast became more rounded instead of ellipsoidal in shape, and the thylakoids were disorderly arranged in the rice leaves when exposed to 1.0 mM Ce3+ (Fig. 7d). In addition, some large starch grains were observed in the chloroplast (Fig. 7d).
Ce subcellular distribution in the root and the effect of Ce on the chloroplast. a Root cell of control, showing no Ce particle deposited on the cell wall (CW). b Root cell treated with 1.0 mM Ce3+, showing Ce particles located in the CW (the arrow indicates Ce particles). c Chloroplast of control leaf cell. d Chloroplast treated with 1.0 mM Ce3+, the global chloroplast with two large starch grains (the arrow indicates the starch grain)
4 Discussion
The root system of rice, which consists of a seminal (primary) root and numerous nodal (adventitious or crown) roots and lateral roots, plays an important role in the uptake of water and nutrients from soil. The data of the present studies indicated that the height of the shoot and the length of the seminal root and total nodal root were induced at 0.05 and 0.1 mM Ce3+ and inhibited at 0.5, 1.0, and 1.5 mM Ce3+. The lateral root number of seminal roots, the number of nodal roots, and the total lateral root number of nodal roots were increased at lower concentrations of Ce3+, but decreased at higher concentrations of Ce3+. Furthermore, it was found that the fresh and dry weights of roots and shoots were increased at 0.05 and 0.1 mM Ce3+ and decreased at 0.5, 1.0, and 1.5 mM Ce3+. Previous studies indicate that an appropriate amount of REEs promotes seed germination and root development (He and Xue 2005; d’Aquino et al. 2009; Ma et al. 2010). However, negative physiological effects of REEs on sea urchin embryos and duckweed (Lemna minor L.) were also observed (Ippolito et al. 2007, 2010; Oral et al. 2010). The results of the present studies also indicated that the appropriate amount of Ce3+ induced the growth of shoots and roots, while high doses of Ce3+ evidently inhibited their growth, which were consistent with these reports.
As one of the most important plant organs, leaves play a significant role in capturing light and making food via photosynthesis. Photosynthesis is one of the most sensitive processes, and chlorophylls are the most important pigments for photosynthesis. The content of chlorophyll is usually used to highlight stress due to the different environmental factors, and chlorophyll content constitutes potential biomarkers of anthropogenic stress and biotic stress (Ferrat et al. 2003; Xu et al. 2010). Measurement of chlorophyll activity is a method usually used to monitor oxidative stress in green plants by direct measurement of chlorophyll or chlorophyll fluorescence (KrishnaRaj et al. 2000; MacFarlane 2003). In the present studies, it was found that the content of chlorophyll a, b, and total chlorophyll was increased at 0.05 and 0.1 mM Ce3+, but decreased at 0.5, 1.0, and 1.5 mM Ce3+. The results indicate that the lower concentrations of Ce3+ have positive effects, and higher concentrations of Ce3+ have negative effects on photosynthesis. In addition, the higher concentration of Ce3+ affected the structure of chloroplast in the rice leaves.
The generation of ROS is a common event in both abiotic and biotic stresses, which can attack polyunsaturated fatty acids and result in lipid peroxidation (Shah et al. 2001). MDA is a major cytotoxic product of lipid peroxidation. In the studies, Ce3+ slightly decreased the contents of H2O2 and MDA in the roots and shoots at 0.05 and 0.1 mM and significantly increased the contents of H2O2 and MDA in the roots and shoots at 1.0 and 1.5 mM. However, the level of O ·−2 in the roots and shoots was induced when treated with the different concentration of Ce3+. It has been found that La3+ alleviated the oxidative damage induced by UV-B radiation and reduced the contents of H2O2, O ·−2 and MDA (Peng and Zhou 2009; Wang et al. 2009). The results of Wang et al. (2009) indicated that La3+ protected soybean plants from oxidative stress by reacting with ROS directly or by improving the defense system of plants. Our results also indicated that Ce3+ decreased the content of H2O2 and MDA at a lower concentration. Ce3+ can also react with ROS directly and further improve the defense system of rice as La3+.
To protect cells from the cytotoxicity of ROS, cells have an effective ROS-scavenging system, in which SOD, POD, and CAT play a significant role. In the studies, it was found that a significant change in the activity of antioxidative enzymes was noticed in the roots and shoots of rice exposed to the different concentration of Ce3+. CAT and SOD activity of roots and CAT and POD activity of shoots were induced when treated with the different concentration of Ce3+. POD activity in the roots was induced at 0.1 and 0.5 mM Ce3+, but SOD activity in the shoots was inhibited when treated with the different concentration of Ce3+. The results indicated that some protective enzymes were activated in plants when the production of ROS was stimulated by stresses. Furthermore, SOD activity in the roots was enhanced with Ce3+ treatments, which was a circumstantial evidence for the production of O ·−2 and H2O2. In the studies, the increased CAT and POD activity might be related to the lowered H2O2 production observed at 0.1 and 0.5 mM Ce3+, which indicated that the role of CAT and POD might be critical to the removal of H2O2 induced by Ce3+. Previous studies indicated that the activities of SOD, CAT, and POD can be induced by REEs (Fashui et al. 2002; Nardi et al. 2004). The results of the present studies also indicated that an appropriate amount of Ce3+ induced the activities of SOD, CAT, and POD.
The results of present studies indicated that the concentration of Ce in the roots and shoots was significantly increasing with the increase of Ce3+ concentrations. Moreover, the concentrations of Ce in the roots were significantly higher than that in the shoots. It has been found that the concentrations of Ce in the roots of winter wheat were significantly higher than that in stems and leaves (Li et al. 2001), and roots can accumulate a much higher content of REEs than germs and the resting seeds (Yuan et al. 2001). Our results also indicated that roots accumulated a much higher content of Ce than shoots, with Ce mainly located in the cell wall of the rice roots.
Mineral elements are essential nutrients for the healthy growth of plants. Absence or the low level of mineral elements could result in the death of the plant or poor plant growth (Buchanan et al. 2000). Previous studies have demonstrated that REEs regulate the growth of plants by affecting the content of mineral elements (Xu 1997; Hu et al. 2004). Our results also showed that Ce3+ treatments affected the uptake of mineral nutrition elements in the roots and shoots. The concentrations of Na, Zn, Cu, and Mo in the roots were decreased, while Mn and Fe in the roots were increased with the exposure of different concentration of Ce3+. However, the concentration of K and Ca in the roots was higher than the controls at 0.05 and 0.1 mM Ce3+ and lower than the controls at 0.5 and 1.0 mM Ce3+. Moreover, the concentrations of Cu, Mn, and Fe in the shoots were decreased with the exposure of different concentration of Ce3+. The concentration of K, Mg, Ca, Na, Zn, and Mo in the shoots was higher than the controls at 0.05 and 0.1 mM Ce3+ and lower than the controls at 0.5 and 1.0 mM Ce3+. The concentration of various elements changed in response to the different concentration of Ce3+, which indicated that Ce3+ affected the uptake of mineral elements in the rice. It appears that the uptake of mineral elements was related with the concentration of Ce3+.
In the studies, it was found that the different concentration of Ce3+ had different effects on the growth of and some antioxidant metabolisms in the roots and shoots of rice. Calabrese and Baldwin (2003a, b) think that the dose–response relationship may not be linear at a suitable spacing of doses, which shows a biphasic curve and is usually called hormetic effect or hormesis. The hormesis shows two types of curves, which are U-shaped and inverted U-shaped curves (Calabrese and Baldwin 2003a, b). Hormetic effects on the growth and antioxidant metabolism were also found in the roots and shoots of rice treated with low and high concentrations of Ce3+. The inverted U-shaped curves were found in the effects of Ce3+ on the root length, shoot height, root number, fresh and dry weight of root and shoot, chlorophyll content, and the activities of CAT, SOD, and POD. The U-shaped dose–response curves of MDA and H2O2 level indicated that oxidative stress and toxic effect were inhibited in the roots and shoots of rice at low doses of Ce3+. The results indicated that Ce3+ affected the nutritional status of roots and shoots and further affected the growth of rice, and Ce3+ would be toxic to rice if the concentration was higher than 0.05 mM. Although the lower concentration of Ce3+ induced the positive effects on rice, it was difficult to maintain a “safe” concentration on cropping. Since the soil environment is a complicated matrix, the application of REEs in the field should be observed with caution.
In summary, the hormetic effects of Ce3+ on the growth and some antioxidant metabolisms were found in the roots and shoots of rice. The appropriate amount of Ce3+ improved the defense system in the roots and shoots and further increased the root length, shoot height, root number, fresh and dry weight of root and shoot, and chlorophyll content. In addition, our data indicated that the concentration of Ce3+ in the roots and shoots was significantly increasing with the increase of Ce3+ concentration treatments with the technique of ICP-SF-MS. The bioaccumulation of Ce in the roots and shoots of rice was observed to be increased with the increase of the concentration of Ce3+. The roots can accumulate a much higher content of Ce3+ than shoots, with Ce3+ mainly located in the cell wall of the rice roots. Moreover, the uptake of K, Mg, Ca, Na, Fe, Mn, Zn, Cu, and Mo in the roots and shoots was affected with the exposure of different Ce3+ treatments, which indicated that Ce3+ affected the nutritional status of roots and shoots and further affected the growth of rice.
References
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Barnes JD, Balaguer L, Manrique E, Elvira S, Davison AW (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophyll a and b in lichens and higher plants. Environ Exp Bot 32:85–100
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. The American Society of Plant Physiologists, Rockville
Calabrese EJ, Baldwin LA (2003a) Toxicology rethinks its central belief: hormesis demands a reappraisal of the way risks are assessed. Nature 421:691–692
Calabrese EJ, Baldwin LA (2003b) Hormesis: the hormetic dose response model is more common than threshold model in toxicology. Toxicol Sci 71:246–250
Chang J (1991) Effects of lanthanum on the permeability of root plasmalemma and the absorption and accumulation of nutrients in rice and wheat. Plant Physiol Commun 27:17–21
Chen WJ, Tao Y, Gu YH, Zhao GW (2001) Effect of lanthanide chloride on photosynthesis and dry matter accumulation in tobacco seedlings. Biol Trace Elem Res 79:169–176
Dai J, Liu JJ, Zhou GQ, Zhang YZ, Liu Y (2011) Effect of Ce(III) on heat production of mitochondria isolated from hybrid rice. Biol Trace Elem Res 143:1142–1148
d’Aquino L, Massimo M, Carboni MA, Staiano M, Antisari MV, Re M, Lorito M, Vinale F, Abadi KM, Woo SL (2009) Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol Biochem 41:2406–2413
Diatloff E, Smith FW, Asher CJ (1995a) Rare earth elements and plant growth: I. Effects of lanthanum and cerium on root elongation of corn and mungbean. J Plant Nutr 18:1963–1976
Diatloff E, Smith FW, Asher CJ (1995b) Rare earth elements and plant growth: II. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continuously flowing nutrient solutions. J Plant Nutr 18:1977–1989
Diatloff E, Smith FW, Asher CJ (1995c) Rare earth elements and plant growth: III. Responses of corn and mungbean to low concentrations of cerium in dilute, continuously flowing nutrient solutions. J Plant Nutr 18:1991–2003
Diatloff E, Asher CJ, Smith FW (1999) Foliar application of rare earth elements to maize and mungbean. Aust J Exp Agric 39:189–194
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
Fashui H, Zhenggui W, Guiwen Z (2000) Effect of lanthanum on aged seed germination of rice. Biol Trace Elem Res 75:205–213
Fashui H, Ling W, Xiangxuan M, Zheng W, Guiwen Z (2002) The effect of cerium (III) on the chlorophyll formation in spinach. Biol Trace Elem Res 89:263–276
Ferrat L, Pergent-Martini C, Roméo M (2003) Assessment of the use of biomarkers in aquatic plants for the evaluation of environmental quality: application to sea grasses. Aquat Toxicol 65:187–204
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–152
Gu Z, Wang X, Gu X, Cheng J, Wang L, Dai L, Chen Y (2001) Effects of fulvic acid on the bioavailability of rare earth elements and GOT enzyme activity in wheat (Triticum aestivum). Chemosphere 44:545–551
He Y, Loh C (2000) Cerium and lanthanum promote floral initiation and reproductive growth of Arabidopsis thaliana. Plant Sci 159:117–124
He Y, Xue L (2005) Biological effects of rare earth elements and their action mechanisms. Ying Yong Sheng Tai Xue Bao 16:1983–1989
Heath RL, Parker L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 25:189–198
Hu X, Ding Z, Wang X, Chen Y, Dai L (2002) Effects of lanthanum and cerium on vegetable growth of wheat (Triticum aestivum L.) seedlings. Bull Environ Contam Toxicol 69:727–733
Hu Z, Richter H, Sparovek G, Schnug E (2004) Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J Plant Nutr 27:183–220
Ippolito MP, Paciolla C, d’Aquino L, Morgana M, Tommasi F (2007) Effect of rare earth elements on growth and antioxidant metabolism in Lemna minor L. Caryologia 60:125–128
Ippolito MP, Fasciano C, d’Aquino L, Morgana M, Tommasi F (2010) Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L.): a defensive weapon or a boomerang? Arch Environ Contam Toxicol 58:42–52
Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor stimulated ion fluxes and O −2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA 94:4800–4805
KrishnaRaj S, Dan TV, Saxena PK (2000) A fragrant solution to soil remediation. Int J Phytorem 2:117–132
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Li D, Huang S, Peng A (2001) The absorbed rate and distribution rule of rare earth elements in wheat by 141Ce as tracer. Huan Jing Ke Xue 22:74–77
Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279
MacFarlane GR (2003) Chlorophyll a fluorescence as a potential biomarker of zinc stress in the grey mangrove, Avicennia marina. Bull Environ Contam Toxicol 70:90–96
Maehly AC (1955) Plant peroxidase. Methods Enzymol 2:801–813
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nardi L, d’Aquino L, De Pinto MC, Carboni MA, Morgana M, Tommasi F (2004) Effect of rare earth elements on seed germination and antioxidant metabolism in Triticum durum. Proceedings of the SIFV-SIGA Joint Congress Lecce 15–18:138
Oral R, Bustamante P, Warnau M, D’Ambra A, Guida M, Pagano G (2010) Cytogenetic and developmental toxicity of cerium and lanthanum to sea urchin embryos. Chemosphere 81:194–198
Ouyang J, Wang X, Zhao B, Yuan X, Wang Y (2003) Effects of rare earth elements on the growth of Cistanche deserticola cells and the production of phenylethanoid glycosides. J Biotechnol 102:129–134
Oyanagui Y (1984) Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem 142:290–296
Peng Q, Zhou Q (2009) Antioxidant capacity of flavonoid in soybean seedlings under the joint actions of rare earth element La(III) and ultraviolet-B stress. Biol Trace Elem Res 127:69–80
Shah J, Kachroo PK, Nandi A, Klessig DF (2001) A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J 25:563–574
Stennis MJ, Chandra S, Ryan CA, Low PS (1998) Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol 117:1031–1036
von Tucher S, Schmidhalter U (2005) Lanthanum uptake from soil and nutrient solution and its effects on plant growth. J Plant Nutr Soil Sci 168:574–580
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 26:55–57
Wang X, Shi GX, Xu QS, Wang CT (2005) Toxic effects of lanthanum, cerium, chromium and zinc on Potamogeton malaianus. J Rare Earth 23:367–371
Wang X, Shi GX, Xu QS, Xu BJ, Zhao J (2007) Lanthanum- and cerium-induced oxidative stress in a submerged Hydrilla verticillata plants. Russ J Plant Physiol 54:693–697
Wang L, Huang X, Zhou Q (2009) Protective effect of rare earth against oxidative stress under ultraviolet-B radiation. Biol Trace Elem Res 128:82–93
Wang C, Lu X, Tian Y, Cheng T, Hu L, Chen F, Jiang C, Wang X (2011) Lanthanum resulted in unbalance of nutrient elements and disturbance of cell proliferation cycles in V. faba L. seedlings. Biol Trace Elem Res 143:1174–1181
Wu Z, Tang X, Tsui C (1983) Studies on the effect of rare earth elements on the increasement of yield in agriculture. J Chin Rare Earth Soc 1:70–75
Xu XK (1997) Application of the rare-earth fertilizers for agricultural sustainable development. Res Dev Resour Environ Ecol Netw 8:23–26
Xu QS, Hu JZ, Xie KB, Yang HY, Du KH, Shi GX (2010) Accumulation and acute toxicity of silver in Potamogeton crispus L. J Hazard Mater 173:186–193
Yuan D, Shan X, Huai Q, Wen B, Zhu X (2001) Uptake and distribution of rare earth elements in rice seeds cultured in fertilizer solution of rare earth elements. Chemosphere 43:327–337
Zhu WF, Xu SQ, Zhang H, Shao PP, Wu DS, Yang WJ, Feng J (1996) Investigation on the intelligence quotient of children in the areas with high REE background (I)—REE bioeffects in the REE-high areas of southern Jiangxi Province. Chin Sci Bull 41:1977–1981
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 30900071).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Liu, D., Wang, X., Lin, Y. et al. The effects of cerium on the growth and some antioxidant metabolisms in rice seedlings. Environ Sci Pollut Res 19, 3282–3291 (2012). https://doi.org/10.1007/s11356-012-0844-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0844-x