Abstract
Introduction and methods
This study investigated the remediation of cadmium-polluted soil using a combination of stainless steel slag and ammonium humate. These remedial agents were added to an artificially polluted garden soil to inhabit cadmium toxicity in soil by changing the physical and chemical properties of soil in a pot experiment.
Results and conclusions
The results showed that the co-application of ammonium humate and stainless steel slag significantly decreased the total and available soil cadmium concentrations, with maximum decreases of 16.30% and 58.04%, respectively. The co-application of an adequate dose of these remedial agents can significantly increase soil pH. The soil organic matter and cation exchange capacity, as well as the amount of soil aggregates, were also significantly increased by the addition ammonium humate, but not stainless steel slag.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium is a high-toxicity soil pollutant that originates mainly from mining and smelting activities (Tang et al. 2006). The chemical properties of Cd are similar to zinc, and both are stable, not readily eliminated from the environment and accumulate in soil (Gupta et al. 2004). Cadmium is absorbed from soil by crop roots, transferred to seeds, and may enter the human body through the food chain, potentially threatening human health. Cadmium tends to accumulate in the liver, kidney, pancreas, thyroid, and bones, causing kidney lesions, nerve pain, endocrine disorders, and other illnesses (Hrudey et al. 1995). The earliest example of Cd poisoning is the "pain disease event" in Toyama Prefecture, Japan, in the 1960s, where Cd containing wastewater was discharged from a lead and zinc smelter into rice paddies (Gupta and Sharma 2003; Gupta and Ali 2004). The local residents routinely consumed the contaminated rice and drinking water, and exhibited severe symptoms of Cd poisoning. Cadmium damages victims’ skeletal systems, and bones become brittle and easily broken, causing dozens of people to die from the pain (Moreno et al. 2002). Soil microorganisms and enzyme activities are also affected by toxicity caused by high levels of soil cadmium (Karaca et al. 2002; Moreno et al. 2006). A number of physicochemical and biological remediation methods have been developed for reclaiming metal-contaminated soils, including soil washing, soil flushing, phytoremediation, and electrokinetics (Giannis et al. 2007; Gupta et al. 2007a,b).
Ammonium humate is used as a humic acid fertilizer. This complex organic substance includes various functional groups such as carboxyl, carbonyl, amino, aldehyde, phenolic hydroxyl, and sulfur-containing groups (Pehlivan and Arslan 2006). These groups can undergo complex reactions with heavy metals, altering the metal ions’ mobility, migration, adsorption, and bioavailability (Gupta and Rastogi 2008a, b, c). The ammonium nitrogen present in ammonium humate also improves soil fertility by replenishing nitrogen (Gupta et al. 1999, 2000, 2010).
Stainless steel slag is generated in large quantities during the steel refining process and is commonly used in road paving, as a building material and for cement production. Stainless steel slag is rich in Ca, Mg, Si, Fe, and other elements and has been used for soil improvement and as a fertilizer in agriculture (Wang and Cai 2006). Stainless steel slag is also potentially useful for remediation of heavy metal pollution due to its porosity, large surface area, and good adsorptivity. Some studies have shown that stainless steel slag is a good adsorbent of heavy metals, mostly via precipitation and adsorption of the metal oxide on the slag surface (Gupta et al. 2006; Kim et al. 2008). Granulated steel slag has also been tested and found to be effective at adsorbing dyes and metal ions (Liu et al. 2010). Heavy metal leaching experiments have confirmed the safety of stainless steel slag in the environment, so environmental pollution control is an effective use for stainless steel slag.
Various amendment materials have been used to inhibit the absorption of cadmium by plants, including zeolite (Li et al. 2009), calcite (Thakur et al. 2006), humic acid (Ghabbour et al. 2006; Gupta et al. 2001), and sewage sludge (Moreno et al. 2003). However, the combined effect of stainless steel slag and ammonium humate to remediate heavy-metal-contaminated soil has not been investigated. The objective of this study was to evaluate the effectiveness of co-remediation of Cd-polluted soil with stainless steel slag and ammonium humate.
2 Materials and methods
2.1 Stainless steel slag and ammonium humate properties
The stainless steel slag was obtained from Taiyuan Iron and Steel (Group) Co., Ltd., Taiyuan, Shanxi. It was crushed to <0.178 mm for chemical analysis and used in the remediation trials. The stainless steel slag was alkaline (pH = 13.96), with high concentrations of CaO (51.84%), FeO (6.17%), Al2O3 (4.39%), MgO (9.74%), MnO (0.29%), S (0.18%), SiO2 (28.2%), and TiO2 (0.35%); the apparent density, porosity, surface area, and most probable pore size of stainless steel slag were 2.88 g cm−1, 16.20%, 2.61 m2 g−1, and 1.72 μm−1, which were analyzed by Mercury Porosimeter (PoreMaster-60).
The ammonium humate is a traditional chemical organic fertilizer that was obtained from Yongfeng chemical fertilizer plant, Taiyuan, Shanxi. That was sieved to <0.02 mm and alkaline (9.21). The effective components are humic acid (52.22%) and total N (5.60%).
2.2 Soil properties
The soil pH was determined potentiometrically in water. The organic matter was determined using Tiurin’s method with dichromate oxidation. The soil cation exchange capacity (CEC) was determined using the NH4Cl–NH4COOH method; and the amount of soil aggregates was measured by the dry sieving method.
2.3 Pot experiments
An uncontaminated loess-derived, calcareous cinnamon soil was obtained, finely ground, and then sieved to <2 mm. The soil properties are shown in Table 1.
The soil (250 g) was placed in polyethylene pots and mixed with 3CdSO4·8H2O to give a soil Cd concentration of 150 mg kg−1. Various amounts (0, 100, 200, and 300 mg g−1) of both the stainless steel slag and ammonium humate were blended into the treated soil, as shown in Table 2. Each treatment was performed in triplicate and incubated for 60 days. The moisture content of each pot was monitored by weighing the pot every 3 days. The experiment was laid out in a random block design.
2.4 Analysis of total and available Cd in soils
For the available Cd analysis, an extractant, consisting of 5 mmol L−1 DTPA, 10 mmol L−1 CaCl2, and 0.1 mol L−1 TEA (triethanolamine), was prepared in deionized water, and the pH was adjusted to 7.3 with 1:1 HCl. Triplicate extractions were carried out using 5 g soil and 25 mL of extractant in 60 mL HDPE sample bottles. The samples were shaken for 2 h (120 cycles/min) in a water bath at 20°C after which the extract was filtered (Whatman no.42 filer paper) and the supernatant was acidified for analysis prior to analysis by atomic adsorption spectrophotometer (Shimadzu-AA6800).
For the total Cd analysis, soil samples were digested in an HNO3–HF–HClO4–HCl mixture. The total Cd concentration in the extract was determined by atomic absorption spectrophotometer (Shimadzu-AA6800).
2.5 Statistical analysis
A one-way variance analysis (ANOVA) was performed on all data, with different concentrations of Cd in soils for different amounts of stainless steel slag and ammonium humate determined using Duncan’s test. Statistical significance was defined as p < 0.05.
3 Results
3.1 Total cadmium in soils
The results shown in Table 3 indicate that increasing the amounts of stainless steel slag and ammonium humate led to significant decreases in the total soil cadmium concentration (p < 0.05). For each dose of ammonium humate, the increasing amounts of stainless steel slag continually led to significantly reduced total soil cadmium concentrations (p < 0.05). When the amount of ammonium humate was 0 and 100 g kg−1, increasing the amount of stainless steel slag from 100 to 200 and 300 g kg−1, significantly decreased the total soil cadmium concentration (p < 0.05). Similarly, when the amount of ammonium humate was 200 and 300 g kg−1, increasing the amount of stainless steel slag from 200 to 300 g kg−1, also led to significant decreases in the total soil cadmium concentration (p < 0.05).
With different amounts of stainless steel slag, as the amount of ammonium humate was increased from 100 to 200 g kg−1, the total soil cadmium concentration decreased significantly (p < 0.05).
Overall, the addition of stainless steel slag and ammonium humate resulted in removal rates for total cadmium from 0.93% to 16.30%. The maximum total cadmium removal rate was achieved when the amounts of both stainless steel slag and ammonium humate were 300 g kg−1.
3.2 Available cadmium in soils
Table 4 shows that, when the amounts of both stainless steel slag and ammonium humate increased, the available soil cadmium concentrations decreased significantly (p < 0.05). For each ammonium humate dose, increasing the amount of stainless steel slag resulted in significant decreases in the available cadmium concentrations (p < 0.05). When the amount of ammonium humate was 0 and 100 g kg−1, increasing the stainless steel slag dose significantly reduced the available soil cadmium concentration (p < 0.05). When the amount of ammonium humate was 200 and 300 g kg−1, increasing the stainless steel slag dose from 0 to 100 g kg−1 significantly decreased the available soil cadmium (p < 0.05); but increasing the stainless steel slag dose from 100 to 200 g kg−1 did not lead to a significant decrease in available soil cadmium concentration (p > 0.05).
For each amount of added stainless steel slag, as the ammonium humate dose increased, the decreases in the available soil cadmium concentrations were insignificant (p > 0.05). When the amount of stainless steel slag was 0 and 100 g kg−1, increasing the ammonium humate dose did not significantly reduce the available soil cadmium concentration (p > 0.05). Furthermore, when the amount of stainless steel slag was 200 and 300 g kg−1, increasing the ammonium humate dose from 100 to 200 g kg−1 significantly increased the available soil cadmium concentration (p < 0.05).
Overall, the treatments using different doses of stainless steel slag and ammonium humate removed 0.25% to 58.04% of the available soil cadmium. The maximum removal of available cadmium occurred with a stainless steel slag dose of 300 g kg−1 and ammonium humate dose of 100 g kg−1.
3.3 Soil physical and chemical properties
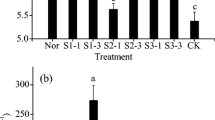
Figure 1 shows that, as both the stainless steel slag and ammonium humate doses increased, the soil pH also increased significantly (p < 0.05). For all ammonium humate doses, increasing the amount of stainless steel slag significantly increased the soil pH (p < 0.05). For all stainless steel slag doses, increasing the amount of ammonium humate from 0 to 200 g kg−1 increased the soil pH significantly (p < 0.05), but increasing the ammonium humate dose from 200 to 300 g kg−1 led to a slight decrease in soil pH. Overall, with different stainless steel slag and ammonium humate doses, the maximum increase in the soil pH was 34.94%, which occurred when the stainless steel slag dose was 300 g kg−1, and the ammonium humate dose was 200 g kg−1.
Figure 2 shows that the addition of stainless steel slag along did not significantly increase the soil organic matter content (p > 0.05). However, the combined application of stainless steel slag and ammonium humate significantly increased the soil organic matter content (p < 0.05). When the ammonium humate dose increased from 0 to 100 g kg−1, the increase in the soil organic matter was 129.12%.
Figure 3 shows that the addition of stainless steel slag alone did not increase the CEC in soils (p > 0.05), but the addition of ammonium humate at increasing doses significantly enhanced the CEC (p < 0.05). When the ammonium humate dose increased from 0 to 100 g kg−1, the soil CEC increased by 58.84%.
Figure 4 shows that the addition of 100 g kg−1 of stainless steel slag significantly decreased the amount of soil aggregates (p < 0.05) compared with the control. Increasing the stainless steel slag dose from 100 to 300 g kg−1 did not to reduce the amount of soil aggregates further (p > 0.05). In contrast, the addition of ammonium humate at increasing doses increased the amount of soil aggregates (p < 0.05). The maximum increase in soil aggregates was 42.42%, when the stainless steel slag dose was 0 g kg−1 and the ammonium humate dose was 300 g kg−1.
4 Discussion
4.1 Effect on total Cd and available Cd in soils
Tables 3 and 4 indicate that treatment with stainless steel slag and ammonium humate led to maximum decreases in the total and available soil cadmium concentrations of 16.30% and 58.04%, respectively. These results are lower than the decreases reported by Deng et al. (2011), which may be related to the shorter incubation time used in their study, as a longer time may be necessary to remove cadmium to low concentrations.
Some researchers indicated soil pH and CEC were the dominant factors for decreasing availability of heavy metal (Shi et al. 2009a, b). Some studies showed the available soil cadmium concentration was significantly affected by the soil pH (Li et al. 2008) and found some chemical amendments could decrease soil Cd availability through rising soil pH (Liang et al. 2005). Others suggested remediation of soils was also affected by the CEC (Castaldi et al. 2005).
The removal of both total and available cadmium by stainless steel slag may be attributed to the increased high pH (13.96), porosity (14.50%), and large surface area (2.31 m2 g−1) that are necessary for satisfactory adsorption (Gupta 1998; Ali and Gupta 2006; Gupta and Rastogi 2009). In this study, Figs. 1 and 3 indicated stainless steel slag addition significantly increased soil pH, then decrease total and available cadmium.
Some scientists found organic amendment could decrease the bioavailability of heavy metals in soil (Tordoff and Baker 2000; Shi et al. 2009a; Gupta et al. 2009), thus permitting the re-establishment of vegetation at contaminated sites. This relies on the ability of the humic substance to re-distribute heavy metals from available form to non-available ones (McGrath and Cegarra 1992; Gupta et al. 1998, 2004, 2007c). In this study, ammonium humate effectively decreased total cadmium in soil in this study. Addition of ammonium humate significantly increase soil CEC due to its effective components humic acid (52.22%) and total N (5.60%), which contains numerous active functional groups, including carbonyl, carboxyl, hydroxyl, and phenolic hydroxyl groups, many of which can form complex reactions with cadmium (Wang et al. 2005; Chen and Wang 2006). The reduction in total soil Cd most resulted from the formation of Cd complexes with ammonium humate. Ammonium humate is alkaline; the addition can increase soil alkaline (Fig. 1) and so decrease total cadmium in soil.
But ammonium humate could not effect on the soil available cadmium almost when the stainless steel slag dose was low (<100 g kg−1). And when the amount of stainless steel slag was 200 or 300 g kg−1, increasing dose of the ammonium humate led to significant increase of available cadmium in soil. That because: (1) when the stainless steel dose was low, the preferential adsorption of Cd on the stainless steel slag, as the stainless steel slag has a greater capacity for Cd adsorption than ammonium humate (Gupta et al. 1997); (2) when the stainless steel dose was high, ammonium humate as a competitive adsorbent lead to the desorption of some Cd ions from the surface of the stainless steel slag, with some of the Cd ions remaining in the soil, elevating the available soil cadmium (Srivastava et al. 1997); (3) The addition of ammonium humate could increase water-soluble fraction of heavy metal in soil due to bioavailability of organic matter (Shi et al. 2009a).
4.2 Effect on soil physical and chemical properties
Figure 1 shows the increasing soil pH with the addition of stainless steel slag, which is similar to the results of study in which silicate induced pH rise in the soils (Liang et al. 2005). The soil pH increased at lower ammonium humate doses, but decreased at higher doses (200 and 300 g kg−1). The maximum soil pH was found when the ammonium humate dose was 200 g kg−1. The ammonium humate used in this study had a pH of 9.72 and increased soil pH on its own. Humic substances are a heterogeneous mixture of polyacidic compounds with a considerable buffering capacity over a wide pH range, due to the dissociation of the acidic functional groups (Ceppi et al. 1999). The buffering capacity of soil is of considerable practical importance in most processes that control the optimum supply of nutrients to crops, most of which act within a narrowly defined pH range (García-Gil et al. 2004). In this study, increasing the ammonium humate dose enhanced the soil buffering capacity, which explains slight decrease in soil pH at high ammonium humate doses.
Figures 2, 3, and 4 indicate that the addition of stainless steel slag had no effect on soil organic matter and CEC, and decreased the amount of soil aggregates. The stainless steel slag could not organically fuse with soil and influence on all aspects of soil physical and chemical properties like organic or mineral addition, but that can play an independent assistant, which is not fused with soil in essence, for remediating Cd-polluted soil utilizing high pH, porosity, and large surface area (Wang and Cai 2006; Shi et al. 2009a; Gupta et al. 2009).
In contrast, the addition of ammonium humate increased the soil organic matter, CEC, and amount of soil aggregates. It is well known that humic substances (HA) contribute up to 85–90% of soil organic matter and can improve soil properties such as aggregation, water holding capacity, microbial growth, organic matter mineralization, and the solubility and availability of micronutrients (e.g., Fe, Zn, and Mn) (Chen and Aviad 1990; Ayuso et al. 1996; Sharif et al. 2002). Consequently, HA is utilized in agriculture as a fertilizer, plant growth promoter, nutrient carrier, and soil conditioner (Nisar and Mir 1989). Humic substances can integrate nitrogen into their structure either directly, through chemical reactions, or indirectly, through microbial activities and the subsequent decomposition of microbial biomass (Clinton et al. 1995). Several reports indicate that NH +4 –N could be fixed to soil organic matter and that increasing the amount of nitrogen in soil facilitates the conversion of organic matter.
5 Conclusions
Both ammonium humate and stainless steel slag could be applied individually for the remediation of heavy-metal-contaminated soils; due to their physical and chemical properties, each material only contributes to some aspects of the soil remediation process. Individually, neither material meets all the requirements for soil remediation.
In this study, we found that the co-application of ammonium humate and stainless steel slag not only significantly decreased the total and available soil cadmium concentrations but also improved the soil physical and chemical properties. In particular, the co-application of these materials increased soil pH, organic matter, and CEC and promoted the formation of soil aggregates. This study has proven the feasibility of these materials for the remediation of Cd-polluted soil.
References
Ali I, Gupta VK (2006) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ayuso M, Hernandez T, Garcia C, Pascual JA (1996) Stimulation of barley growth and nutrient absorption by humic substances originating from various organic materials. Bioresource Technol 57:251–257
Castaldi P, Santona L, Melis P (2005) Heavy metal immobilization by chemical amendments in a polluted soil and influence on white lupin growth. Chemosphere 60:365–371
Ceppi SB, Velasco MI, De Pauli CP (1999) Differential scanning potentiometry: surface charge development and apparent dissociation constant of natural humic acids. Talanta 50:157–1063
Chen Y, Aviad T (1990) Effect of humic substances on plant growth. In: MacCarthy P (ed) Humic substances in soil and crop sciences: selected readings. ASA-SSSA, Madison, pp 161–186
Chen CL, Wang XK (2006) Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind Eng Chem Res 45:9144–9149
Clinton PW, Newman RH, Allen RB (1995) Immobilization of 15N in forest litter studied by 15N CPMAS NMR spectroscopy. Eur J Soil Sci 46:551–556
Deng THB, Gu HH, Qiu RL (2011) Effects of steel slag application on multi-metal contaminated soil and heavy metal uptake of rice. J Agro-Environ Sci 30:455–460
García-Gil JC, Ceppi SB, Velasco MI, Polo A, Senesi N (2004) Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 121:135–142
Ghabbour EA, Shaker M, El-Toukhy A, Abid IM, Davies G (2006) Thermodynamics of metal cation binding by a solid soil-derived humic acid: binding of Fe(III), Pb(II), and Cu(II). Chemosphere 63:477–483
Giannis A, Gidarakos E, Skouta A (2007) Application of sodium dodecyl sulfate and humic acid as surfactants on electrokinetic remediation of cadmium-contaminated soil. Desalination 211:249–260
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interf Sci 271:321–328
Gupta VK, Rastogi A (2008a) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloid Surf B 64:170–178
Gupta VK, Rastogi A (2008b) Sorption and desorption studies of chromium(VI) from nonviable Cyanobacterium nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Rastogi A (2008c) Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash—a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Mohan D, Sharma S (1998) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environment 19:129–136
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Premvir S, Nafisur R (2004) Adsorption behavior of Hg(II), Pb(II), andCd(II) from aqueous solution on Duolite C-433: asynthetic resin. J Colloid Interf Sci 275:398–402
Gupta VK, Mittal A, Gajbe V, Mittal J (2006) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Ali I, Saini VK (2007a) Adsorption studies on the removal of vertigo blue 49 and orange DNA 13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interf Sci 315:87–93
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007b) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interf Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007c) Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas MML (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Env Sci Tec 39:783–842
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interf Sci 342:135–141
Hrudey SE, Chen W, Rousseau CG (1995) Bioavailability in environmental risk assessment. Lewis Pub, Boca Raton, FL
Karaca A, Naseby DC, Lynch JM (2002) Effect of cadmium contamination with sewage sludge and phosphate fertiliser amendments on soil enzyme activities, microbial structure and available cadmium. Biol Ferti Soils 35:428–434
Kim DH, Shin MC, Choia HD, Seo CI, Baek K (2008) Removal mechanisms of copper using steel-making slag: adsorption and precipitation. Desalination 223:283–289
Li P, Wang XX, Zhang TL, Zhou DM, He YQ (2008) Effects of several amendments on rice growth and uptake of copper and cadmium from a contaminated soil. J Environ Sci 20:449–155
Li H, Shi WY, Shao HB, Shao MA (2009) The remediation of the lead-polluted garden soil by natural zeolite. J Hazard Mater 169:1106–1111
Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liu SY, Gao J, Yang YJ, Yang YC, Ye ZX (2010) Adsorption intrinsic kinetics and isotherms of lead ions on steel slag. J Hazard Mater 173:558–562
McGrath SP, Cegarra J (1992) Chemical extractability of heavy metals during and after long-term applications of sewage sludge to soil. J Soil Sci 43:313–321
Moreno JL, Hernández T, Pérez A, García C (2002) Toxicity of cadmium to soil microbial activity: effect of sewage sludge addition to soil on the ecological dose. Appl Soil Ecol 21:149–158
Moreno JL, García C, Hernández MT (2003) Toxic effect of cadmium and nickel on soil enzymes and the influence of adding sewage sludge. Eur J Soil Sci 54:377–386
Moreno JL, Sánchez-Marín A, Hernández MT, Garcia C (2006) Effect of cadmium on microbial activity and a ryegrass crop in two semiarid soil. Environ Manag 37:626–633
Nisar A, Mir S (1989) Lignitic coal utilization in the form of HA as fertilizer and soil conditioner. Sci Tech Develop 8:23–26
Pehlivan E, Arslan G (2006) Comparison of adsorption capacity of young brown coals and humic acids prepared from different coal mines in Anatolia. J Hazard Mater B 138:401–408
Sharif M, Khattak RA, Sarir MS (2002) Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun Soil Sci Plant Anal 33:3567–3580
Shi WY, Shao HB, Li H, Shao MA, Du S (2009a) Co-remediation of the lead-polluted garden soil by exogenous natural zeolite and humic acids. J Hazard Mater 167:136–140
Shi WY, Shao HB, Li H, Shao MA, Du S (2009b) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J Hazard Mater 170:1–6
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Enviorn Eng-ASCE 123:461–468
Tang XY, Zhu YG, Bei YS, Duan J, Tang L (2006) The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ Int 32:682–689
Thakur SK, Tomar NK, Pandeya SB (2006) Influence of phosphate on cadmium sorption by calcium carbonate. Geoderma 130:240–249
Tordoff GM, Baker AJM (2000) Current approaches to the revetation and reclamation of metalliferous mine wastes. Chemosphere 41:219–228
Wang X, Cai QS (2006) Steel slag as an iron fertilizer for corn growth and soil improvement in a pot experiment. Pedosphere 16:519–524
Wang XK, Chen CL, Du JZ, Tan XL, Xu D, Yu SM (2005) Effect of pH and aging time on the kinetic dissociation of 243Am(III) from humic acid-coated γ-Al2O3: a chelating resin exchange study. Environ Sci Technol 39:7084–7088
Acknowledgments
This research was supported by the Grand Science and Technology Special Project of Shanxi Province (20111101016); Science & Technology Key Program of Shanxi Province (20090311072); the Key Research Program of State Key Laboratory of Loess and Quaternary Geology; Initial Funding from the Institute of Earth Environment, Chinese Academy of Sciences; the West Light Foundation of the Chinese Academy of Sciences; and the Technology Center of the Shanxi Taigang Stainless Steel Co. Ltd.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Zhuo, L., Li, H., Cheng, F. et al. Co-remediation of cadmium-polluted soil using stainless steel slag and ammonium humate. Environ Sci Pollut Res 19, 2842–2848 (2012). https://doi.org/10.1007/s11356-012-0790-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0790-7