Abstract
This study investigated the effects of AgNPs on pollutant removals in constructed wetlands (CWs) with different flow patterns and spatial distributions of silver. Before exposure to AgNPs, upward flow constructed wetland (UCW) had better nitrogen removal than down-flow CW (DCW). And 0.5 mg/L AgNPs evidently inhibited nitrogen and phosphorus removal, including ammonia, nitrate, and TP (total phosphorus), with average effluent concentrations increasing by 70.83% of NH4+-N in UCW, 18.75% of TP in UCW, and 28.33% and 25.06% of NO3--N in DCW and UCW, respectively, while COD (chemical oxygen demand) was not affected. Moreover, presence of 2 mg/L AgNPs slightly inhibited organic compounds and NH4+-N removal in two systems during stage 4 (dosing 2 mg/L AgNPs). However, the response of NO3--N and TN removal to 2 mg/L AgNPs in two systems were different, and nitrogen concentrations in effluent at the end of stage 4 significantly increased in DCW. Addition of 2 mg/L AgNPs significantly affected TP removal in two systems. Two wetlands showed high removal efficiencies of about 98% on AgNPs, indicating that CWs could provide a feasible approach for ecological restoration of nanoparticles pollution. This study also found that AgNPs mainly accumulated in the upper layer with the Ag content of 17.55–20.26 mg/kg dry weight in sand layer and 7.25–10.85 mg/kg dry weight in gravel layer. Plant roots absorbed AgNPs, with Ag content at 50.80–101.40 mg/kg and bioconcentration factors 2.80–5.00. The obtained results showed that up-flow CWs had better performance and higher resistance to the exposure of AgNPs pollution, compared with down-flow CWs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silver nanoparticles (AgNPs), as one of the mostly widely used engineered nanomaterials (ENMs), have been increasingly utilized in many industrial and commercial applications across many fields including cosmetics, energy, electronic devices, disinfectants, agriculture, and medicine (Deshmukhab et al. 2019; Nel et al. 2006; Tulve et al. 2015). However, as the result of the expanding usage of products containing AgNPs, corresponding concentrations of AgNPs in natural environment are inevitable to increase (Guo et al. 2019; Vogt et al. 2019), which has raised significant concerns for their environmental behaviors and potential threat. In general, via the sewer systems, municipal wastewater treatment plants (WWTPs) are known as the sink of AgNPs released from consumer products and therefore controlling the emissions of AgNPs from domestic and/or industrial sources to the natural water bodies (Benn and Westerhoff 2008; Kaegi et al. 2011). Although basing on the laboratory-scale bioreactors and investigations on the real WWTPs, AgNPs removal could reach about 90% by biological and mechanical treatment, mainly transforming to Ag2S and depositing to activated sludge or biosolids (Kaegi et al. 2011; Nowack 2010; Zhang et al. 2016), the result above does not support that we can underestimate the AgNPs discharged. For example, Li et al. (2013) found that mechanical and biological treatment of WWTPs in Germany could remove 95% of the AgNPs in the influent, with the AgNPs concentrations of < 12 ng/L in the effluent. In addition, previous papers documented that the AgNPs concentrations were 1.55 or 2.27–2.68 μg/g, in activated sludge and 0.98 ± 0.62 μg/g in the sediment near the discharged points of the WWTPs (Li et al. 2013; Vogt et al. 2019). Thereby, considering the results above, estimation of the impact of released AgNPs on the engineered treatment systems is necessitated owing to the increasing concerns over emergent nanoparticle contamination.
In order to purify the wastewater or secondary effluent, constructed wetlands (CWs) are one of the widely used engineered systems due to their easy operations and economic benefits, which mimic the ecological processes in natural wetlands (Tang et al. 2020; Vymazal 2007). During the purification process, contaminants like organic matters, nitrogen, phosphorus, and heavy metals are the main constituents of concern (Kropfelova et al. 2009; Sochacki et al. 2014; Vymazal and Březinová 2016). Recently, CWs have been used to treat emergent pollutions including nanoparticles (Huang et al. 2018; Liu et al. 2019; Xiao et al. 2021; Yang et al. 2020). These studies attempted to investigate the influences of nanoparticles on the treatment performance of CWs. For example, Huang et al. (2017a) reported that low levels of AgNPs caused the negative effects on the nitrogen and phosphorus removal, which further was confirmed by Liu et al. (2019). However, the impact of different designs and operation conditions such as water flow patterns and plants has received relatively less attention and requires further estimation. Basing on the previous studies, design and configuration of CWs could significantly affect the nutrient removal and microbial community structure (Sgroi et al. 2018; Vymazal 2007). Several studies reported that saturation conditions of substrate in CWs are the key factors controlling the treatment performance, for example, partially saturated CWs with better removal efficiency of total nitrogen (TN) (i.e., 35% vs 52%) (Sgroi et al. 2018; Zhang et al. 2018). Meanwhile, hydraulic flow direction (down- and up-flow or horizontal and vertical flow) is generally acknowledged as a remarkable impact factor to CWs (Dan et al. 2013; Huang et al. 2017b; Wang et al. 2021), which significantly affect the pollutant treatment performance of CWs (Zhang et al. 2018). In detail, CWs with different flow patterns potentially have different oxygen transfer and distribution, contact pattern between wastewater and substrate, and oxidation-reduction potential (ORP). Moreover, the changes of designs and operation conditions of CWs may affect the environmental transport and toxic effect of nanoparticles in these biological reactors. Previous studies confirmed that oxygen levels and redox conditions affected the presence of AgNPs in water phase, such as the presence of oxygen enhancing the release Ag+ from AgNPs (Sotiriou and Pratsinis 2010; Kittler et al. 2010) and lower dissolve oxygen inhibiting the Ag+ release and aggregation of AgNPs (Zhang et al. 2011). However, the impacts of hydraulic flow directions on pollutant performance and AgNPs distribution in CWs remains unknown. Therefore, investigating and revealing mechanisms of nutrient removal and AgNPs migration in response to hydraulic flow patterns in CWs are crucial for the proposal of optimal regulation strategy for controlling nanoparticle pollution in constructed wetlands.
This study aims at assessing the response of constructed wetlands with different hydraulic flow patterns to exposure of AgNPs and silver spatial distribution in CWs. For this purpose, the major objectives of this study were to (1) investigate the influence of AgNPs on pollutant removal in down-flow and up-flow CWs, (2) measure the effluent concentrations of AgNPs to estimate the removal efficiency of CWs to AgNPs, and (3) determine the silver concentrations in wetland substrate and plant tissues and clarify silver spatial distribution in different components of two type wetlands.
Materials and methods
Silver nanoparticles
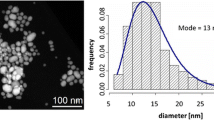
PVP-AgNPs stock suspension was purchased for Huzheng Nano Technology Co., Ltd., Shanghai, China. The stock suspension was stored in the brown bottle at 4 °C in the fridge. According to the manufacturer’s instruction, AgNPs stock suspension was 3000 ppm. The morphology and particle size were clarified with a transmission electron microscope (TEM, JEM-2100, JEOL, USA), which are in the range of 10–40 nm. The hydrodynamic diameter of AgNPs in DI water was measured at 58.1 nm, using dynamic light scattering (DLS) (Brookhaven BI-200SM, Brookhaven Instruments Corp., USA).
Mesocosm wetland setup and operation
Two laboratory-scale vertical flow constructed wetlands (VFCWs) with diameter and height of 0.3 m and 0.85 m, respectively, were set at the Jiulonghu Campus of Southeast University in Nanjing, China, as previously described (Cao et al. 2018). Briefly, The CWs consisted of three layers with depth of 65 cm, which were filled with different sizes of substrates from top to bottom as shown in Fig. 1: the surface layer contained a 40-cm-thick layer of 1–2-mm diameter sand, a 10-cm-thick layer of fine gravel of 3–5 mm, and a 15-cm-thick layer of zeolite (30–40-mm diameter) to facilitate the collection of effluent evenly. The wetland plants Iris pseudacorus was chosen in the present study due to its common plant species in eastern China. Each CW was planted with healthy and similar sizes at a density of 8 plants. After planting, all systems were flooded with tap water about 1 month to cultivate the plants. Two separate peristaltic pumps (BT100-1 L, Longer Pump, China) were employed to feed the influent (synthetic secondary wastewater) to experimental wetlands with downward and upward flow directions, referring as DCW and UCW, respectively. The water depth of all CWs was kept about 60 cm and the hydraulic loading rate was 0.1 m3/m2 d with a flow rate at 4.9 ml/min.
Chemicals
Synthetic secondary effluent was simulated in the laboratory and used as feed solution for the experimental wetland systems. A stock solution of synthetic secondary effluent was produced by dissolving the following reagents (purity > 99.7%) in distilled water: ammonium sulfate ((NH4)2SO4, 42 g/L), potassium nitrate (KNO3, 28 g/L), glucose (C6H12O6, 90 g/L), and monopotassium phosphate (KH2PO5, 4.5g/L). The synthetic secondary effluent had the chemical oxygen demand (COD) of 60 mg/L, total nitrogen (TN) of 23.3 mg/L, ammonium nitrogen (NH4+-N) of 14 mg/L, nitrate nitrogen (NO3--N) of 9.3 mg/L, and total phosphate (TP) of 1.5 mg/L. During experimental period, to ensure the growth of plants and microorganisms in the CWs, 5 ml of trace element solution was regularly added into the synthetic secondary effluent, containing magnesium sulfate (MgSO4, 75 g/L), ferrous sulfate (FeSO4, 5.25 g/L), zinc sulfate (ZnSO4, 0.195 g/L), sodium molybdate (Na2MoO4, 0.045 g/L), boric acid (H3BO3, 0.038 g/L), and copper sulfate (CuSO4, 0.045 g/L). In detail, the reagents were supplied by the Sinopharm Chemical Reagent, Co., Ltd., Shanghai, China, and Nanjing Chemical Reagent Co., Ltd., Nanjing, China. The synthetic secondary effluent was prepared freshly every 2 days in a feeding tank and then pumped into the VFCWs.
Analytical methods and sampling
The experiment began on March 9, 2018, and lasted a total of 249 days. The present experiment included a start-up stage ending on the June 16, 2018 (93 days), and an exposure stage extending from June 17, 2018, to November 17, 2018 (155 days). After 3 months operation, all systems continuously fed with synthetic secondary effluent had stable and high removal efficiencies of organic matter and nitrogen contaminants. On June 17, 2018, the synthetic wastewater containing 0.5 mg/L of AgNPs, which was obtained by adding certain volume of AgNPs stock suspensions into synthetic secondary effluent, were simultaneously pumped into two wetland systems (DCW and UCW, respectively) in the following 52-day operation (ending on August 6, 2018). On August 7, 2018, the synthetic secondary effluent containing 2 mg /L AgNPs was employed as influent to feed the wetland systems (DCW and UCW, respectively) in the following operation stage of 103 days (ending on November 17, 2018).
Water samples of influent and effluent in all systems were taken every 3 days and then stored in polyethylene bottles for measurements. The water quality parameters were measured as follows—NH4+-N, NO3--N, NO2--N, TN (total nitrogen), and TP (total phosphorus)—by a UV-1800 spectrophotometer (UV/Vis spectrophotometer, Jinghua Technology Instrument, Shanghai, China). COD (chemical oxygen demand) was measured using a HACH DR1010 (HACH, Loveland, Co). The variables above were analyzed in accordance with the Chinese NEPA standard methods. The pH and temperature were measured by a pH meter (PHSJ-4F, Thermo Fisher Scientific, Waltham, MA, USA).
To quantify concentrations of AgNPs retained in the wetland systems, the soil samples and plant tissues were collected at the end of the experiment. The sand samples in 0–20-cm and 20–40-cm depth and the gravel samples in 40–50-cm depth were taken from two wetlands and then were measured the total silver concentrations using the ICP-AES with a method of the United States Environmental Protection Agency (EPA) 3050b, as previously reported (Huang et al. 2018). Briefly, after natural drying, filtering with 20 mesh, the soil samples were digested by HNO3 at 200 °C in a digestion system. The plant tissues, such as roots, shoots, and leaves, collected from two wetlands were washed with tap water to remove the adhered soil and dried at 60 °C for 24 h and finely powdered using mortar and pestle. The silver concentration was determined for each sample by ICP-AES after digestion and analysis.
To investigate the removal efficiency of AgNPs in two wetlands, the effluent wastewater was sampled 5 times during the whole experimental period and then analyzed the silver concentration after HNO3 and HCl digestion according to procedure of EPA 200.8 using ICP-MS with a detection concentration of 0.0001 μg L-1.
Calculation of removal efficiencies and statistical analysis
The removal efficiency was calculated as percentage removal for each parameter, calculated by R = (1-Ce/Ci) × 100, where Ci and Ce are the influent and effluent concentrations in mg/L, respectively.
The factors of bioconcentration and translocation of silver in the wetland substrate and test plants were calculated as follows:
-
Bioconcentration factors (BCFs):
$$ {C}_{root}/{C}_{substrate} $$
where Csbustrate and Croot are the silver concentrations in substrate and roots (mg/kg dry weight), respectively. The BCFs value shows the ability of a plant to uptake and accumulate an element from surroundings in its roots (Soda et al. 2012).
-
Translocation Factors (TFs):
$$ {C}_{sand2}/{C}_{sand1} $$$$ {C}_{gravel}/{C}_{sand1} $$$$ {C}_{gravel}/{C}_{sand2} $$$$ {C}_{leaf}/{C}_{root} $$
where Csand1, Csand2, Cgravel, Croot, and Cleaf are the silver concentrations in sand 0–20-cm depth, sand 20–40-cm depth, gravel 40–50-cm depth, roots, and leaves (mg/kg dry weight), respectively. The TFs value shows the silver mobility within the soil layer and plant, and a larger TFs value indicates higher translocation capability (Deng et al. 2004).
The concentrations of COD, NH4+-N, TN, NO3--N, and TP in influent and effluent were used to measure the removal rates (%). Some results were expressed as mean ± standard deviation. An analysis of variance (ANOVA) was used to test the significance of results, and p < 0.05 was statistically significant.
Results and discussions
The effect of AgNPs on the organic matter removal
As shown in Fig. 2, before exposing AgNPs, COD concentrations of effluent in two wetlands kept stable at about 10.1 ± 3.85 and 8.2 ± 2.69 mg/L, with removal rates of 82.75% and 83.19% for down- and up-flow CWs, which had no statistical differences. The results suggested that the flow directions of CWs had no impact on the removal of organic matters. After exposing to 0.5 mg/L AgNPs, the effluent concentrations of COD were in the range of 10.56–11.69 mg/L, and the removal rates were in the range of 84.66–86.2% (Table 1). Compared with stage 2, the average removal efficiency of organic matters showed no significant difference in two systems (as shown in boxplot in Fig. 2). These observations indicated that 0.5 mg/L of AgNPs did not affect COD, which is in consistent with the studies by Cao et al. (2019) and Huang et al. (2017). However, when increasing AgNPs concentration to 2 mg/L in the influent, COD effluent concentration of DCW significantly increased from approximately 11.4 to 28.83 mg/L for 16-day exposure, whereas COD concentrations in UCW effluent kept stable in the range of 4.5–13.1 mg/L (mean value of 10.56 mg/L) during the operational stage 4. The different responses of DCW and UCW to AgNPs exposure at 2 mg/L might result from different flow directions, which affect the oxygen transfers, contacts efficiency between wastewater and substrate (Wang et al. 2021), thereby leading to the different inhibition degree in two flow types. Moreover, with extending experiment time, the effluent COD decreased to about 8.79 mg/L in DCW. Furthermore, the relative efficiency of organic matters of stage 4 in UCW significantly higher than that of stage 2 (p < 0.05). The above results indicated that high AgNPs concentrations slightly inhibited the removal of organic matters. A study by Zhang et al. (2016) found that 1 and 10 mg/L AgNPs increased COD effluent concentrations and lower the purification of organic compounds, consistent with this study. In CWs, the microorganisms mainly including heterotrophic bacteria play an essential role for the purification of organic matters, which were reported good resistance to the toxic substrates (Emma et al. 2014), resulting in two experimental wetlands with good treatment performance of organic matters.
Variations of COD concentrations in influent and effluent and removal efficiency of organic matters in two systems throughout the experimental period. Relative removal efficiencies of organic matters were calculated by comparing with average removal efficiency of before exposing AgNPs (stage 2) with standard deviations. Asterisks (*) above the error bars represented significant differences compared to before exposing AgNPs (ANOVA, p < 0.05)
The effect of AgNPs on the nitrogen removal
Figure 3a–d shows the changes of the removal of NH4+-N, NO3--N, NO2--N, and TN in downward and upward flow CWs. Before adding AgNPs into synthetic wastewater, especially during the operational stage 2, the ammonia concentrations of effluent in two systems kept stable, with the mean concentrations of 1.13 ± 0.55 and 1.20 ± 0.81 mg/L. And there is no statistical difference of NH4+-N removal between two wetlands (Table 1). In contrast, the effluent NO3--N concentrations stabilized at approximately 7.96 and 1.63 mg/L, with the average NO3--N concentrations of 8.26 ± 1.89 and 3.95 ± 2.14 mg/L in downward and upward flow CWs, respectively. The constructed wetlands with upward flow direction had higher NO3--N removal efficiency (p < 0.05), compared to DCW. Figure 3d also showed that NO2--N and TN concentrations of effluent in DCW were higher, with the poor TN treatment performances, compared with UCW (Table 1). The above results illustrated that the hydraulic flow directions of CWs significantly influenced the ultimate nitrogen removal, specially affecting the NO3--N and TN removal. And constructed wetlands with wastewater flowing bottom to top significantly promoted the nitrogen removal from wastewater. The explanations might be that the different flow directions would affect the oxygen transfer, contacts efficiency between wastewater and substrate (Wang et al. 2021). In general, the downward flow CWs have good oxygen conditions, contributing to the organic compounds and NH4+-N removal, whereas low oxygen levels or anaerobic conditions are often found in the upward flow CWs, which promotes denitrification process. On the other hand, the top layer of substrate plays an important role in decontamination of organic matters in influent, leading to the low levels of carbon source for the denitrification process in the bottom layer of downward flow CWs. In upward flow CWs, denitrifying bacteria could utilize the organic matters in influent as carbon sources for its growth and then enhancing the nitrogen removal.
Variations of NH4+-N (a), NO3--N (b), NO2--N (c), and TN (d) concentrations in influent and effluent and removal efficiency of two systems throughout experimental period. Relative removal efficiencies of NH4+-N, NO3--N, NO2--N, and TN were calculated by comparing with average removal efficiency or average concentration of before exposing AgNPs (stage 2) with standard deviations. Asterisks (*) above the error bars represented significant differences compared to before exposing AgNPs (ANOVA, p < 0.05)
After obtaining stable treatment performances, two systems were exposed to 0.5 mg/L of AgNPs for about 50 d. And effluent NH4+-N concentrations in DCW were stable at about 1.00 mg/L, with the removal rate of 93.2%, whereas effluent NH4+-N in UCW increased to about 2.87 mg/L (Fig. 3a). And the average NH4+-N concentrations were calculated as 1.00 ± 0.32 and 2.05 ± 0.65 mg/L, corresponding removal efficiencies of 93.2 ± 2.04% and 86.0 ± 4.21% in two systems, respectively (Table 1). On the other hand, as shown in Fig. 3, the NO3--N and TN concentrations in effluent increased to about 1.99- and 2.02-fold (max values) in DCW and 4.89- and 4.84-fold (max values) in UCW of these in stage 2 (average values of last 10 days), respectively. And the relative efficiency of TN and NO3--N in stage 3 was lower than that in stage 2 (before exposure to 0.5 mg/L AgNPs). The above results showed that addition of AgNPs at 0.5 mg/L evidently inhibited the nitrogen removal, including the ammonia and nitrate, in accordance with the studies by Liu et al. (2019) and Yang et al. (2020) that 1 mg/L AgNPs and TiO2 NPs significantly suppressed the NH4+-N and TN removal. Compared between two wetlands, NH4+-N removal in downward flow CW showed better resistance to the AgNPs than upward flow CW, possibly due to the high oxygen level in DCW, which easily reduced the toxicity of AgNPs (Sotiriou and Pratsinis 2010; Kittler et al. 2010), while the lower dissolve oxygen in UCW resulted in the inhibition of Ag+ release from AgNPs (Zhang et al. 2011) and kept high toxicity of nanoparticles. For NO3--N and TN, average effluent concentrations increased by 28.33% and 22.06% in DCW and 25.06% and 14.64% in UCW, respectively (Table 1). Inhibition of AgNPs on the TN and NO3--N removal, which replied on the denitrification process (Fu et al. 2016), mainly resulting from the suppressions of the microbial activities (especially denitrifying bacteria), and the toxicity of AgNPs to microbes is generally associated with the specific properties of AgNPs and their release of Ag+ (Sheng and Liu 2017). Figure 3c and Table 1 also confirmed that the NO2--N concentrations in effluent of two systems showed the upward trend during stage 3. Yazdanbakhsh et al. (2019) reported that the exposure to Ag-Fe NPs suppressed the TN removal by affecting the integrity of cell membrane (LDH) and key enzymes activities. And Liu et al. (2019) found that only about 5% AgNPs released the Ag+ in the effluent. In view of the above, effects of AgNPs on the nitrogen treatment possibly depended on the combined effects of particle-specific characteristics and toxicological effects of released Ag+. At the end of stage 3, the N removal in two systems recovered to the level of stage 2 after about 50 days exposure (Fig. 3).
And during stage 4, the influent with 2 mg/L AgNPs was fed into the two test systems; the results are shown in Fig. 3. The effluent NH4+-N concentrations slightly increased to 3.27–3.24 mg/L after about 30 days exposure in the initial days of stage 4 and then gradually declined to about 0.5–1.0 mg/L in the end of stage 4. The observations further confirmed that the negative impacts of AgNPs on the NH4+-N removal, and higher levels of AgNPs caused higher inhibition (Liu et al. 2019). On the other hand, the TN and NO3- concentrations in effluent showed different variations. TN and NO3--N concentrations significantly raised in the presence of 2 mg/L AgNPs for about 74 d in DCW. Conversely, the TN and NO3--N in effluent of UCW kept stable at about 2.50 and 3.00 mg/L during the stage 4. And the NO2--N concentrations in effluent of DCW were greater than that of UCW (Fig. 3c). The obtained results indicated that exposure of AgNPs hindered the NO3--N transformations (accumulations of NO2--N in DCW), and subsequently reduced TN removal. The different responses of two systems to the exposure of AgNPs at 2 mg/L might be owing to the hydraulic flow directions of wastewater, which would significantly change the oxygen levels and redox conditions in wetland substrates. In DCW, the good oxygen levels contributed to the Ag+ released from AgNPs, which accounted for essential roles on the toxicity of AgNPs (Sintubin et al. 2011), restraining the NO3--N transformations. On the contrary, the low oxygen conditions in upward flow CW possibly restrained the release of Ag+ and aggregation of AgNPs (Zhang et al. 2011; Xiu et al. 2012), keeping a stable state (e.g., as nanoparticles). And with the increasing operation of CWs, massive organic matters would aggregate on the wetland substrates, such as the growth of biofilms and humic substrates, leading to the aggregation or immobilization of AgNPs (Baalousha et al. 2013). On the other hand, the presence of S2- in anaerobic environment would interact with the Ag+ and form sulfide precipitations, and then lowered the toxicity of AgNPs (Reinsch et al. 2012). To sum up, up-flow CWs had better ability of resistance to AgNPs toxicity, compared to downward flow CWs.
The effect of AgNPs on the phosphorus removal
As shown in Fig. 4, two experimental wetlands had good and stable purification ability for phosphorus pollution. At the end of stage 2, the effluent TP concentrations were stable at approximately 0.13 and 0.11 mg/L in DCW and UCW, with the removal rate of 90.44% and 90.53%, respectively. And the average effluent concentrations of TP in stage 1 and stage 2 (no AgNPs) were 0.18 ± 0.09 and 0.16 ± 0.04 mg/L in DCW and 0.17 ± 0.10 and 0.14 ± 0.05 mg/L in UCW, with the mean removal efficiencies of 89.11 ± 6.52% and 88.71 ± 3.24% in DCW and 89.71 ± 7.65% and 89.88 ± 3.3% in UCW, respectively. The observed phenomena suggested that the constructed wetlands with downward flow and upward flow showed similar phosphorus removal treatment. And there was no statistical difference in two experimental systems (p < 0.05, Table 1). During stage 3, the synthetic wastewater containing 0.5 mg/L of AgNPs was pumped into DCW and UCW; the effluent TP concentrations gradually increased from approximately 0.14–0.16 to 0.25–0.375 mg/L, with the removal rates reducing by 10.16% and 23.71%, respectively. And Table 1 also showed that the average effluent concentrations of TP were calculated as 0.16 ± 0.05 mg/L and 0.19 ± 0.08 mg/L. With extending exposure time and increasing AgNPs concentrations to 2 mg/L during the operational stage 4, TP concentrations in effluent of two wetlands continuously raised to 1.06–1.35 mg/L (max values), which were near the influent TP concentrations (about 1.5 mg/L) after exposing to 2 mg/L AgNPs for about 30 days. And the relative efficiencies of TP of DCW and UCW in stage 3 and stage 4 were significantly lower than that in stage 2 (before AgNPs addition). The above results proved that AgNPs negatively affected the phosphorus removal in constructed wetlands (p < 0.05), confirming the study by Huang et al. (2020) that TP removal efficiency decreased under 0.20 mg/L AgNPs for about 90 days. The adsorption and immobilization of phosphorus are known as the essential pathways of phosphorus removal in constructed wetlands. In detail, the binds of phosphate anion and metal cations like calcium or aluminum to poor soluble phosphate, or the reactions between phosphorus and minerals such as iron and aluminum oxides promote the phosphorus purifications from wastewater (Drizo et al. 1997). Hence, the AgNPs negatively charged could easily interact with these components, including sorption, aggregation/agglomeration, and precipitation. As a result, the competition of phosphate anion and AgNPs for binding sites in wetland substrate leads to the decline of TP removal. Figure 4 also showed that at the end of stage 4, the TP removal gradually recovered after chronic exposure of AgNPs. And the average effluent concentrations of TP in two systems were 0.57 ± 0.33 and 0.50 ± 0.61, respectively, with the removal at 60.33% and 65.13%. The observed results suggested that addition of AgNPs significantly decreased the phosphorus removal and upward flow constructed wetlands had higher treatment performance and better resistant ability to AgNPs pollution (p < 0.05), compared to downward flow constructed wetlands.
Variations of TP concentrations in influent and effluent and removal efficiency of two systems throughout the experimental period. Relative removal efficiencies of TP compared with before exposing AgNPs with standard deviations in each term. Asterisks (*) above the error bars represented significant differences compared to before exposing AgNPs (ANOVA, p < 0.05).
Fate and migration of AgNPs in the vertical flow constructed wetlands
The removal behaviors of AgNPs in two VFCWs with different flow directions
Table 2 shows the effluent AgNPs concentrations from different sampling ports and removal efficiency of AgNPs in two wetlands. During stage 4, water samples collected effluent from sampling ports 1 and 2 and system outlet were analyzed several times by ICP-MS. The effluent concentrations from system outlets were 23.26 ± 11.38 and 16.86 ± 7.38 μg/L, with the removal efficiencies of 98.83% and 99.16% in DCW and UCW, respectively. The results suggested that CWs could efficaciously remove the nanoparticles from wastewater, implying the potential engineering systems to control the nanoparticle pollution (Hannele et al. 2017), confirming the findings by Huang et al. (2018). In downward flow CW, effluent Ag concentrations from sampling port s1 and 2 were 580.2 ± 20.43 and 56.9 ± 10.12 μg/L, respectively. In contrast, the Ag concentrations in effluent of sampling ports 1 and 2 were measured as 32.5 ± 15.54 and 370.1 ± 34.87 μg/L, respectively. These phenomena above indicated that the sand layer in constructed wetlands played an essential role for removal of AgNPs, because of the richness of organic matters, such as biofilms and organic pollutants and high ionic strength, promoting the aggregation and deposition of AgNPs (He et al. 2019). And the gravel layer in upward flow CW also was observed a certain capacity to retain the AgNPs (Table 2), which might be owing to the cellular polymeric substrates (EPS) on the surface of gravel, immobilizing and entrapping the nanoparticles (Gonzalez et al. 2015).
The fate of AgNPs in two VFCWs with different flow directions
As shown in Table 3, the silver content in different substrate layers of two wetlands were measured at the end of the experiment (stage 4). Ag concentrations in sand layer of downward flow constructed wetland (DCW) were measured as 17.55 ± 0.83 mg/kg (0–20-cm depth) and 20.26 ± 0.50 mg/kg (20–40-cm depth), which were higher than that in gravel layer of 7.25 ± 0.40 mg/kg. The above results showed that the majority of AgNPs were retained in the upper layer of DCW, probably owing to the wastewater flow direction (from top to bottom) and richness of organic matters and ionic strength, which entrap the AgNPs from water phase. The translocation factors (TFs) in DCW were calculated as 0.41–0.36 between gravel layer and sand layer, implying the weak transport ability of AgNPs in wetland substrate. A study by Cao et al. (2019) reported that the TFs of AgNPs in soil layer were approximately 0.16, which was lower than this study, and the reason behind is that the soil had a lot of organic matters, fine soil particles and high ionic strength, showing strong interception ability, compared with sand layer. In contrast, the Ag contents in UCW substrate were measured as 10.85 ± 0.31 mg/kg, 18.51 ± 0.07, and 4.71 ± 0.38 mg/kg in gravel, 20–40-cm depth and 0–20-cm-depth sand layer along with the flow direction from bottom to top. And the translocation factors (TFs) between gravel and 20–40-cm-depth sand of 1.71, which was higher than that in DCW of 0.36. The reason is that the particle size of gravel is in the range of 5–30 mm, and wastewater flowed from the bottom into sand layer, resulting in high transport ability of AgNPs through gravel layer, whereas the sand particle size of 1–2 mm and biofilms on the sand surfaces lower the migration of AgNPs. The observed facts suggested that most AgNPs mainly accumulated in the sand layer, which explained high removal efficiency of sand layer (Table 2). The present results confirmed the study by Auvinen et al. (2016), who found that sand had better removal (85%), compared with gravel layer (67%).
Table 4 summarizes the average values obtained for the silver contents in the roots, leaves, and bioconcentration factors (BCFs) or translocation factors (TFs) of the test plant. The observed results showed that test plants could uptake the Ag from surroundings, and the silver content reached 101.40 ± 2.86 and 51.80 ± 1.73 mg/kg dry weight in roots, which was approximately 3.34- and 7.11-fold of the one in leaves for DCW and UCW, respectively. These observations indicated that vegetations in CWs could uptake AgNPs from surroundings, and the assimilated AgNPs mainly accumulated in roots, confirming the study by Li et al. (2018) that Ag concentration in wheat roots was about 2-fold of that in shoot. And the silver concentrations in leaves of test plants were as high as 30.33 ± 0.17 and 7.29 ± 0.33 mg/kg dry weight for DCW and UCW, respectively, indicating that the absorbed Ag by plant roots was transported to the leaves (Ebbs et al. 2016). And the translocation factor (Cleaf/Croot) was 0.30 and 0.14 in DCW and UCW, in consistent with the study by Cao et al. (2018) that the TFs value in wetland plant Iris pseudacorus was about 0.18 in presence of 100 μg/L AgNPs. The bioconcentration factors (BCFs, Cleaf/Croot) were calculated as 5.00 and 2.80 for DCW and UCW, respectively, indicating that the hydraulic flow directions could affect the enrichment capacity of plants. The reason might be that the wastewater containing AgNPs flow from surface layer and lead to the accumulation of Ag in upper layer (Table 3), and plant roots mainly located in the upper layer, resulting in the easy contact and uptake of roots and AgNPs and then higher content observed in plant roots of DCW, compared with upward flow CW.
Conclusions
This study investigated the effects of AgNPs on the pollutant removals in constructed wetlands (CWs) with different hydraulic flow directions and the spatial distribution of silver. Before exposing to AgNPs, upward flow constructed wetland (UCW) had the better nitrogen removal, compared with down-flow CW (DCW). During stage 3 (dosing 0.5 mg/L AgNPs), addition of AgNPs evidently inhibited the nitrogen and phosphorus removal, with the average concentrations increasing by 70.83% of NH4+-N in UCW, 18.75% of TP in UCW, and 28.33% and 25.06% of NO3--N in DCW and UCW, respectively, while there was no inhibition on the removal of organic matters. Furthermore, the presence of 2 mg/L AgNPs slightly inhibited the organic compounds and NH4+-N removal in two systems during stage 4 (dosing 2 mg/L AgNPs). However, the response of NO3--N and TN removal to 2 mg/L AgNPs in two systems were different, and nitrogen concentrations in effluent at the end of stage 4 significantly increased in downward flow CW. And addition of 2 mg/L AgNPs significantly affected the TP removal in two systems. Compared with up-flow CWs, down-flow CWs showed better resistance to the nanoparticle inhibition. The present study also found high removal of AgNPs over 98%, indicating that CWs could provide a feasible approach for ecological restoration of nanoparticles pollution. This study also found that AgNPs mainly accumulated in the upper layer with the Ag content of 17.55–20.26 mg/kg dry weight in sand layer and 7.25–10.85 mg/kg dry weight in gravel layer. And the roots of wetland plants absorbed the AgNPs with content at 50.80–101.40 mg/L and the bioconcentration factors 2.80–5.00 in two systems. And the translocation factors of roots and leaves in two systems were in the range of 0.14–0.30. The obtained results showed that the up-flow CWs had better performance and higher resistance to the exposure to AgNPs pollution, compared with down-flow CWs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Auvinen H, Sepulveda VV, Rousseau DPL, Liang GD (2016) Substrate- and plant-mediated removal of citrate-coated silver nanoparticles in constructed wetlands. Environ Sci Pollut Res 23:21920–21926. https://doi.org/10.1007/s11356-016-7459-6
Baalousha M, Nur Y, Romer I, Tejamaya Lead JR (2013) Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci Total Environ 454:119–131. https://doi.org/10.1016/j.scitotenv.2013.02.093
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42(11):4133–4139. https://doi.org/10.1021/es7032718
Cao C, Huang J, Yan CN, Liu JL, Hu Q, Guan WZ (2018) Shifts of system performance and microbial community structure in a constructed wetland after exposing silver nanoparticles. Chemosphere 199:661–669. https://doi.org/10.1016/j.chemosphere.2018.02.031
Cao C, Huang J, Guo Y, Yan CN, Xiao J, Ma YX, Liu JL, Guan WZ (2019) Long-term effects of environmentally relevant concentration of Ag nanoparticles on the pollutant removal and spatial distribution of silver in constructed wetlands with Cyperus alternifolius and Arundo donax. Environ Pollut 252:931–940. https://doi.org/10.1016/j.envpol.2019.05.144
Dan A, Yang Y, Dai YN, Chen CX, Wang SY, Tao R (2013) Removal and factors influencing removal of sulfonamides and trimethoprim from domestic sewage in constructed wetlands. Bioresour Technol 146:363–370. https://doi.org/10.1016/j.biortech.2013.07.050
Deng H, Yea ZH, Wong MH (2004) Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ Pollut 132:29–40. https://doi.org/10.1016/j.envpol.2004.03.030
Deshmukhab SP, Patilac SM, Mullania SB, Delekar SD (2019) Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C 97:954–965. https://doi.org/10.1016/j.msec.2018.12.102
Drizo A, Frost CA, Smith KA (1997) Phosphate and ammonium removal by constructed wetlands with horizontal subsurface flow, using shale as a substrate. Water Sci Technol 35(5):95–102. https://doi.org/10.1016/S0273-1223(97)00057-7
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ Sci Nano 3(1):114–126. https://doi.org/10.1039/c5en00161g
Emma J, Wan-Tack I, Dong-Hoon K, Mi-Sun K, Seoktae K, Hang-Sik S, SoRyong C (2014) Different susceptibilities of bacterial community to silver nanoparticles in wastewater treatment systems. J Environ Sci Health A 49:685–693. https://doi.org/10.1080/10934529.2014.865454
Fu GP, Yu TY, Ning KL, Guo ZP, Wong MH (2016) Effects of nitrogen removal microbes and partial nitrification-denitrification in the integrated vertical-flow constructed wetland. Ecol Eng 95:83–89. https://doi.org/10.1016/j.ecoleng.2016.06.054
Gonzalez AG, Mombo S, Leflaive J, Lamy A, Pokrovsky OS, Rols JL (2015) Silver nanoparticles impact phototrophic biofilm communities to a considerably higher degree than ionic silver. Environ Sci Pollut Res 22:8412–8424. https://doi.org/10.1007/s11356-014-3978-1
Guo Z, Zeng G, Cui K, Chen A (2019) Toxicity of environmental nanosilver: mechanism and assessment. Environ Chem Lett 17:319–333. https://doi.org/10.1007/s10311-018-0800-1
Hannele A, Ralf K, Diederik PLR, Gijs DL (2017) Fate of silver nanoparticles in constructed wetlands-a microcosm study. Water Air Soil Pollut 228:97. https://doi.org/10.1007/s11270-017-3285-9
He JZ, Wang DJ, Zhou DM (2019) Transport and retention of silver nanoparticles in soil: effects of input concentration, particle size and surface coating. Sci Total Environ 648:102–108. https://doi.org/10.1016/j.scitotenv.2018.08.136
Huang J, Cao C, Yan CN, Hu Q, Guan WZ (2017a) Impacts of silver nanoparticles on the nutrient removal and functional bacterial community in vertical subsurface flow constructed wetlands. Bioresour Technol 243:1216–1226. https://doi.org/10.1016/j.biortech.2017.07.178
Huang X, Zheng J, Liu C, Liu L, Liu Y, Fan H (2017b) Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: effect of hydraulic flow direction and substrate type. Chem Eng J 308:692–699. https://doi.org/10.1016/j.cej.2016.09.110
Huang J, Cao C, Yan CN, Guan WZ, Liu JL (2018) Comparison of Iris pseudacorus wetland systems with unplanted systems on pollutant removal and microbial community under nanosilver exposure. Sci Total Environ 624:1336–1347. https://doi.org/10.1016/j.scitotenv.2017.12.222
Huang J, Xiao J, Guo Y, Guan WZ, Cao C, Yan CN, Wang MY (2020) Long-term effects of silver nanoparticles on performance of phosphorus removal in a laboratory-scale vertical flow constructed wetland. J Environ Sci 87:319–330. https://doi.org/10.1016/j.jes.2019.07.012
Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45(9):3902–3908. https://doi.org/10.1021/es1041892
Kittler S, Greulich C, Diendorf J, Koller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22(16):4548–4554. https://doi.org/10.1021/cm100023p
Kropfelova L, Vymazal J, Svehla J, Stichova J (2009) Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech Republic. Environ Pollut 157(4):1186–1194. https://doi.org/10.1016/j.envpol.2008.12.003
Li L, Hartmann G, Doblinger M, Schuster M (2013) Quantification of nanoscale silver particles removal and release from municipal wastewater treatment plants in Germany. Environ Sci Technol 47(13):7317–7323. https://doi.org/10.1021/es3041658
Li Y, Chen HY, Wang F, Zhao FR, Han XM, Geng HH, Gao L, Chen HL, Yuan RF, Yao J (2018) Environmental behavior and associated plant accumulation of silver nanoparticles in the presence of dissolved humic and fulvic acid. Environ Pollut 243:1334–1342. https://doi.org/10.1016/j.envpol.2018.09.077
Liu XB, Yang XY, Hu XB, He Q, Zhai J, Chen Y, Xiong Q, Vymazal J (2019) Comprehensive metagenomic analysis reveals the effects of silver nanoparticles on nitrogen transformation in constructed wetlands. Chem Eng J 358:1552–1560. https://doi.org/10.1016/j.cej.2018.10.151
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627. https://doi.org/10.1126/science.1114397
Nowack B (2010) Nanosilver revisited downstream. Science 330(6007):1054–1055. https://doi.org/10.1126/science.1198074
Reinsch BC, Levard C, Li Z, Ma R, Wise A, Gregory KB, Brown GE Jr, Lowry GV (2012) Sulfidation of silver nanoparticles decreases Escherichia coli growth inhibition. Environ Sci Technol 46:6992–7000. https://doi.org/10.1021/es203732x
Sgroi M, Pelissari C, Roccaro P, Sezerino PH, García J, Vagliasindi FGA, Ávila C (2018) Removal of organic carbon, nitrogen, emerging contaminants and fluorescing organic matter in different constructed wetland configurations. Chem Eng J 332:619–627. https://doi.org/10.1016/j.cej.2017.09.122
Sheng ZY, Liu Y (2017) Potential impacts of silver nanoparticles on bacteria in the aquatic environment. J Environ Manag 191:290–296. https://doi.org/10.1016/j.jenvman.2017.01.028
Sintubin LS, Gusseme BD, Meeren PV, Pycke BFG, Verstraete W, Boon N (2011) The antibacterial activity of biogenic silver and its mode of action. Appl Microbiol Biotechnol 91:153–162. https://doi.org/10.1007/s00253-011-3225-3
Sochacki A, Surmacz-Górska J, Faure O, Guy B (2014) Polishing of synthetic electroplating wastewater in microcosm upflow constructed wetlands: effect of operating conditions. Chem Eng J 237:250–258. https://doi.org/10.1016/j.cej.2013.10.015
Soda S, Hamada T, Yamaoka Y, Ike M, Nakazato H, Saeki Y, Kasamatsu T, Sakurai Y (2012) Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol Eng 39:63–70. https://doi.org/10.1016/j.ecoleng.2011.11.014
Sotiriou GA, Pratsinis SE (2010) Antibacterial activity of nanosilver ions and particles. Environ Sci Technol 44(14):5649–5654. https://doi.org/10.1021/es101072s
Tang SY, Liao YH, Xu YC, Dang ZZ, Zhu XF, Ji GD (2020) Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour Technol 314:123759. https://doi.org/10.1016/j.biortech.2020.123759
Tulve NS, Stefaniak AB, Vance ME, Rogers K, Mwilu S, LeBouf RF, Schwegler-Berry D, Willis R, Thomas TA, Marr LC (2015) Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children's potential exposures. Int J Hyg Environ Health 218(3):345–357. https://doi.org/10.1016/j.ijheh.2015.02.002
Vogt R, Mozhayeva D, Steinhoff B, Schardt A, Spelz BTF, Philippe A, Kurtz S, Schaumann GE, Engelhard C, Schönherr H, Lamatsch DK, Wanzenböck J (2019) Spatiotemporal distribution of silver and silver-containing nanoparticles in a prealpine lake in relation to the discharge from a wastewater treatment plant. Sci Total Environ 696:134034. https://doi.org/10.1016/j.scitotenv.2019.134034
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380(1-3):48–65. https://doi.org/10.1016/j.scitotenv.2006.09.014
Vymazal J, Březinová T (2016) Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: a review. Chem Eng J 290:232–242. https://doi.org/10.1016/j.cej.2015.12.108
Wang YM, Zhou J, Shi SH, Zhou J, He XJ, He L (2021) Hydraulic flow direction alters nutrients removal performance and microbial mechanisms in electrolysis-assisted constructed wetlands. Bioresour Technol 325:124692. https://doi.org/10.1016/j.biortech.2021.124692
Xiao J, Huang J, Wang MY, Huang MJ, Wang Y (2021) The fate and long-term toxic effects of NiO nanoparticles at environmental concentration in constructed wetland: enzyme activity, microbial property, metabolic pathway and functional genes. J Hazard Mater 413:125295. https://doi.org/10.1016/j.jhazmat.2021.125295
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275. https://doi.org/10.1021/nl301934w
Yang XY, Chen Y, Guo FH, Liu XB, Su XX, He Q (2020) Metagenomic analysis of the biotoxicity of titanium dioxide nanoparticles to microbial nitrogen transformation in constructed wetlands. J Hazard Mater 384:121376. https://doi.org/10.1016/j.jhazmat.2019.121376
Yazdanbakhsh AR, Rafiee M, Daraei H, Amoozegar MA (2019) Responses of flocculated activated sludge to bimetallic Ag-Fe nanoparticles toxicity: performance, activity enzymatic, and bacterial community shift. J Hazard Mater 366:114–123. https://doi.org/10.1016/j.jhazmat.2018.11.098
Zhang WZ, Yao Y, Li KG, Huang Y, Chen YS (2011) Influence of dissolved oxygen on aggregation kinetics of citrate-coated silver nanoparticles. Environ Pollut 159(12):3757–3762. https://doi.org/10.1016/j.envpol.2011.07.013
Zhang ZH, Gao P, Li MQ, Cheng JQ, Liu W, Feng YJ (2016) Influence of silver nanoparticles on nutrient removal and microbial communities in SBR process after long-term exposure. Sci Total Environ 569:234–243. https://doi.org/10.1016/j.scitotenv.2016.06.115
Zhang L, Lyu T, Zhang Y, Button M, Arias CA, Weber KP, Brix H, Carvalho PN (2018) Impacts of design configuration and plants on the functionality of the microbial community of mesocosm-scale constructed wetlands treating ibuprofen. Water Res 131:228–238. https://doi.org/10.1016/j.watres.2017.12.050
Funding
The research was funded by the National Natural Science Foundation of China (Grant No. 51479034) and Fundamental Research Funds for the Central Universities (Grant No. 2242016R30008). The work was also supported by “The scientific research foundation of graduate school of Southeast University” (Grant No. YBPY1862).
Author information
Authors and Affiliations
Contributions
Chong Cao designed the study and analyzed the samples and wrote the manuscript; Juan Huang participated in writing through reviewing and editing; Chun-ni Yan contributed to the writing of the final version of the manuscript; Xin-xin Zhang collected the samples and analyzed the samples. All authors have read and approved the final version of the paper.
Corresponding author
Ethics declarations
Ethics approval
All scholarly contributions by other authors, tables, graphs, data sources, etc. are cited properly. No any unethical content is added.
Consent to participate
All authors agree to participate in the revision stage of this paper and will appreciate the comments of editor and reviewers.
Consent for publication
All authors give consent to publish this paper after due process by the editorial board.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Alexandros Stefanakis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, C., Huang, J., Yan, Cn. et al. Hydraulic flow direction alters impacts of AgNPs on pollutant removal and silver spatial distribution in vertical flow constructed wetlands. Environ Sci Pollut Res 28, 67736–67747 (2021). https://doi.org/10.1007/s11356-021-15350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15350-y