Abstract
This study examined whether subjective and objective sleep parameters (sleep stage, electroencephalography [EEG] power, heart rate variability) are related to the progression of sleep cycles using differences in the variables between two nights. We hypothesized that the association between night-to-night differences between subjective and objective sleep variables reflect the difference in objective sleep variables in the first sleep cycle. Seventy-seven healthy adults (23.8 ± 2.2 years; 41 females) participated in polysomnographic recordings on two consecutive nights. To extract the variables that represent the difference between the nights, the sleep parameters of Night 1 were subtracted from those of Night 2. Spearman’s rho was used to assess correlations between subjective sleep assessments and objective sleep parameters, with false discovery rate correction for multiple comparisons. Subjective sleep assessments were significantly correlated with whole-night sleep architecture and quantitative EEG activity, but not with heart rate variability during the night. Among sleep cycles, subjective sleep parameters were correlated with the objective sleep parameters in the first sleep cycle (“Ease of falling asleep” vs. waking after sleep onset [r = − 0.382], “Depth of sleep” vs. EEG theta power [r = 0.404], “Quality of sleep” vs. the percentage of stage N3 [r = 0.412] and EEG delta power [r = 0.458], all p < 0.05). These results suggest the importance of taking the difference among the nights into account when assessing subjective sleep quality. This study clarified that sleep in the first sleep cycle has a dominant influence on subjective sleep assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies used subjective and objective assessments to evaluate sleep. Subjective assessments of sleep using questionnaires are often compared with objective sleep measurements using polysomnography (PSG) [1, 2], actigraphy [3, 4], and heart rate variability (HRV) [4,5,6]. However, the correlation between subjective sleep quality and objective sleep measurements was only low to medium in strength and not consistent across studies [7, 8]. During the night, sleep stages change in relation to sleep cycles and from the initial to the late periods of sleep [9]. Currently, it remains to be elucidated how subjective assessments can represent cyclic changes and progression in sleep architecture.
A recent study reported that sleep progression and sleep architecture differed between adaptation and experimental nights in a sleep laboratory; moreover, the difference between the two nights (the first night effect) was most prominent in the first sleep cycle [10]. In addition, the correlation between subjective sleep quality and objective sleep assessment improved when the sleep data from multiple nights were taken into account. Moreover, confounders related to inter-individual variability, such as personality traits, preoccupations, and psychological stress, were minimized [11]. Therefore, the first night effect may be useful to assess the association between subjective and objective sleep evaluations in relation to sleep processes.
In this study, we hypothesized that the association between night-to-night differences between subjective and objective sleep variables reflect differences in the objective sleep variables in the first sleep cycle. The aim of the present study was to examine whether objective sleep measures are correlated with subjective evaluations of sleep based on the differences in subjective and the objective sleep parameters between two nights by electroencephalography (EEG) power and heart rate variability in sleep cycles.
Materials and methods
Participants
Participants (n = 103) aged between 20 and 35 years (49 women and 54 men, mean age 24.3 ± 2.9 years) were enrolled in the PSG study at Osaka University. The participants were recruited via flyers and word of mouth. To determine the correlation between subjective and objective sleep measurements, data from a previous study were used [10]. The patients were screened to exclude suspected sleep disorder. Data from 26 participants were excluded for the following reasons: sleep apnea syndrome (AHI ≥ 5 for both nights) (n = 19), and TST/TIB < 70% or sleep latency > 60 min (n = 7). As a result, 77 participants were included (41 women and 36 men, 20–33 years old, mean age 23.8 ± 2.2 years, BMI 20.5 ± 1.6 kg/m2). The sample size was warranted by a power analysis. To detect a weak correlation (p = ± 0.4, two-tailed) with an alpha of 0.05 and a power of 0.90, 61 participants were needed according to G*Power [12]. To ensure that participants in this study were healthy, all subjects completed the Pittsburgh Sleep Quality Index (PSQI) [13], Japanese version [14]; Self-rating Depression Scale (SDS) [15]; the Japanese version of the SDS [16]; and State Trait Anxiety Inventory (STAI) [17], Japanese version [18]. The PSQI is a subjective questionnaire to assess sleep quality and disturbances over a 1-month period and the SDS is a self-administered survey to quantify the depressed status of a patient. The patients completed a written informed consent form approved by the Research Ethics Committee of Osaka University Graduate School of Dentistry and Osaka University Dental Hospital. This study was approved by the Ethics Committee of Osaka University Dental Hospital and the Graduate School of Dentistry (H25-E9-5, H29-E48-3).
Subjective sleep assessments
For this study, a sleep evaluation questionnaire was used to determine subjective sleep assessments (total sleep time, sleep latency, depth of sleep, quality of sleep) every morning using the following questions: “How long did you sleep last night? (hours and minutes)”, “How long did you need until you fell asleep compared with usual nights? (1: very long—5: very short)”, “How was your sleep depth compared with usual nights? (1: very light sleep—5: slept very soundly)”, and “How was your sleep quality compared with usual nights? (1: very bad—5: very good)”.
PSG and sleep stages
PSG recordings were performed on two consecutive nights in a sleep laboratory at Osaka University Graduate School of Dentistry. The measurement procedure was previously reported [10]. PSG recordings were performed using surface EEG (C3-A2, C4-A1, O1-A2, O2-A1, F3-A2, F4-A1, Fp1-A2, and Fp2-A1), bilateral electrooculography (EOG), lead II electrocardiography (ECG), and chin electromyography (EMG). Signals were amplified, filtered (EEG, EOG, and ECG: 0.3–70 Hz; EMG: > 10 Hz, with a 60-Hz hum filter), and recorded with a sampling frequency of 200 Hz using a software package (Embla N7000, REMbrandt™ PSG software, Natus Medical, Pleasanton, CA, USA). Oronasal thermal airflow, nasal pressure, chest and abdominal movements, arterial oxygen saturation, and body position were also recorded. Audio and video recordings were performed simultaneously. Sleep stages and respiratory events were scored according to the American Academy of Sleep Medicine criteria [19]. The apnea–hypopnea index (AHI) was calculated as the sum of all apneas and hypopneas with 3% O2 desaturation and/or EEG arousal divided by total sleep time. AHI exclusion criteria followed the AASM criteria [19].
Sleep cycles
A sleep cycle was defined as the time from the end of REM sleep to the end of the next REM sleep. The first sleep cycle was defined as the time from sleep onset to the end of the first REM sleep. To calculate the difference between two nights, the same number of sleep cycles was analyzed.
Quantitative analyses of cortical and cardiac activities
EEG signals (C4 referenced to the left ear) were quantified to estimate delta band (0.5–4.0 Hz), theta band (4.0–8.0 Hz), alpha band (8.0–12 Hz), sigma band (12.0–15.0 Hz), and beta band (15.0–23.0 Hz) amplitudes [20]. Quantitative EEG evaluation was performed using Fast Fourier transform (Bio Trend Professional Analysis, NoruPro Light Systems, Japan). Prior to analysis, epochs with artifacts were removed. The frequency resolution was 0.25 Hz and truncating errors were reduced by applying a Hanning window. The analysis window was 10.24 s with a 0.24-s overlap every 10 s, and the data for three units were averaged to obtain a value every 30 s. Because of non-normality, power densities were log-normalized before calculating the measures of interest.
Heart rate analysis was performed using the complex demodulation method [21]. The mean value of the instantaneous amplitude (in m) was calculated in 30-s windows using a computer program with a time scale of 0.1 s and a frequency resolution of 0.002 Hz (HRV LOG-Pro-DSA Analysis, NoruPro Light Systems). Following the removal of epochs with artifacts, density-spectrum array analysis was performed for low-frequency (LF: 0.04–0.15 Hz) and high-frequency (HF: 0.15–0.4 Hz) bands.
Statistical analysis
As not every variable in the data set was normally distributed, non-parametric tests were applied. Wilcoxon signed-rank tests were used to compare Night 1 and Night 2. The effect size was presented as Cohen's d, which is the mean preference index divided by the standard deviation. To compare subjective and objective sleep variables for two nights, the differences in variables between the nights were calculated by subtracting those of Night 1 from those of Night 2. Spearman’s rho (r) was used to assess correlations between the differences in subjective sleep assessments and objective sleep measurements. Multiple comparisons were accounted for by a false discovery (FDR) adjustment α = 0.05. Regression analysis was used to draw a scatter diagram. Statistical analyses were performed using commercially available software (IBM SPSS Statistics, version 24 for Windows, Chicago, IL). Results were significant when p values were less than 0.05.
Results
Subject characteristics
Sleep quality assessed by the PSQI in all participants was 4.5 ± 2.0 (range: 0–21, cut-off score: ≥ 5.5). The average score on the SDS and trait anxiety were within the standard range (SDS; range: 20–80, cutoff score: > 40, average score for all participants: 39.2 ± 5.4; STAI, range: 20–80, cutoff score: > 44, average score for all participants: 41.5 ± 9.5).
Descriptive statistics of the sleep variables
Descriptive statistics of the sleep variables are listed in Table 1. Many sleep variables for Night 1 significantly differed from those for Night 2 (Table 1 and Supplementary materials 1 and 2). Night 1 was characterized by delayed sleep onset, longer wake time, lower slow wave sleep, and higher arousal. As previous studies have reported [20, 22], EEG power and HRV differed among sleep stages (Supplementary materials 1 and 2: EEG delta power increased and LF and HF amplitude of HRV decreased with increasing sleep depth during NREM sleep period). Subjectively, the sleep of Night 1 was characterized by lower ratings for “Total sleep time”, “Ease of falling asleep”, “Depth of sleep”, and “Quality of sleep”. Correlations were found between “Depth of sleep” and “Quality of sleep” (r = 0.648, p = 0.000), between “Ease of falling asleep” and “Quality of sleep” (r = 0.472, p = 0.000). Subjective “Total sleep time” was not correlated with other questions.
Correlations for an entire night
A total of 108 correlations were performed. After FDR correction, 10 significant associations remained (p < 0.05) (bold in Table 2). Significant correlations were found when using the difference between the nights: subjective “Total sleep time” was related to objective “Total sleep time” (r = 0.597, p = 0.000). Moreover, subjective “Ease of falling asleep” was related to sleep onset latency (r = − 0.468, p = 0.002), stage N2 latency (r = − 0.515, p = 0.000), and stage N3 latency (r = − 0.590, p = 0.000). Subjective “Depth of sleep” was related to sleep efficiency (total sleep time/sleep period time) (r = 0.407, p = 0.024), stage N3 time (r = 0.434, p = 0.008), wakefulness after sleep onset (r = − 0.405, p = 0.026), and EEG delta power in the NREM sleep period (r = 0.427, p = 0.011). Subjective “Quality of sleep” was related to stage N3 time (r = 0.407, p = 0.024) and EEG delta power in the NREM sleep period (r = 0.438, p = 0.007). There were no correlations between subjective sleep assessments and heart rate variability.
Correlations for each sleep cycle
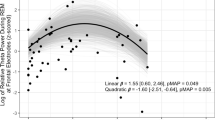
Correlations between objective sleep parameters and subjective sleep evaluation for each sleep cycle are presented in Table 3 (for the first cycle) and Supplementary materials 3, 4, and 5 (for the second, third, and fourth cycles, respectively). The results of linear regression analysis in the first sleep cycle are shown in Fig. 1a–e. The numbers of subjects were 77 for the first and second sleep cycles, 65 for the third sleep cycle, and 36 for the fourth sleep cycle. A total of 84 correlations were found for each sleep cycle. After FDR correction, four (first sleep cycle) significant correlations remained (p < 0.05) (bold in Table 3). The highest correlations were found in the first sleep cycle: subjective “Ease of falling asleep” was related to wakefulness after sleep onset (r = − 0.382, p = 0.049) (Table 3, Fig. 1b), whereas subjective “Depth of sleep” was related to EEG theta power (r = 0.404, p = 0.022) (Table 3, Fig. 1a). Moreover, subjective total “Quality of sleep” was related to stage N3 (%), EEG delta power, and EEG theta power, with an r ranging from 0.412 (p = 0.016) to 0.458 (p = 0.002) (Table 3, Fig. 1c–e). In the third sleep cycle, there was only one significant correlation observed between subjective and objective sleep parameters: subjective “Quality of sleep” was related to EEG delta power in the NREM sleep period (r = 0.419, p = 0.043) (Supplementary material 4). There were no correlations between subjective sleep assessments and heart rate variability in each sleep cycle (Table 3 and Supplemental materials 3, 4, and 5).
Scatter diagram of the subjective sleep evaluation and the objective sleep parameters that were significantly correlated in the first sleep cycle. Objective sleep parameters on the vertical axis show values obtained by subtracting Night 1 from Night 2. The subjective evaluation on the horizontal axis shows the value obtained by subtracting the Night 1 score (from 1 point to max 5 points) from the Night 2 score (from 1 point to max 5 points)
Discussion
This study investigated the relationship between subjective sleep assessments and objective sleep measurements in sleep cycles using the differences in the variables between two nights. Subjective sleep assessments were significantly correlated with objective sleep measurements for sleep architecture, but not with heart rate variability during the night. When analyzing sleep cycles, subjective sleep parameters were most correlated with objective sleep parameters in the first sleep cycle. Therefore, subjective sleep assessments of the entire night of sleep may be influenced by the sleep architecture in the first sleep cycle.
Previous studies examined the relationship between subjective sleep assessment and objective sleep variables for a single night and found that subjective sleep quality was correlated with many sleep variables such as total sleep time, sleep efficiency, wake time after sleep onset, sleep onset latency, and the amount of sleep stages of light and deep sleep [2, 23, 24]. However, the correlation between subjective sleep assessment and objective sleep variables has been inconsistent between studies, as a significant correlation was not always found with specific sleep variables [7, 8]. The correlation between subjective and objective sleep assessments may be obscured in cross-sectional studies because inter-individual differences in daily sleep fluctuations cannot be controlled. In addition, there are many confounding factors affecting physiological sleep, such as physiological stress [25], and subjective evaluations such as personality traits and anxiety [26, 27]. In the present study, inter-individual differences in daily sleep fluctuations were minimized and differences in the variables between two nights were analyzed to improve the correlation [11]. In this study population, subjective sleep assessment and objective sleep variables significantly differed between the two nights, which support the findings of previous studies for the first night effect (sleep quality in subjective assessment and objective sleep architecture was lower in Night 1 than in Night 2) [10].
In this study, subjective sleep quality was assessed by four questions (Total sleep time, Ease of falling asleep, Quality of sleep, and Depth of sleep). The correlations of these questions with objective sleep parameters remained even after FDR adjustment. Unlike insomnia patients, young healthy subjects were able to accurately recognize their own sleep [28]. Total sleep time was correlated with objective total sleep time, suggesting that the time domain of subjective sleep assessment reflects that of objective sleep assessment in healthy subjects. In addition, “Ease of falling asleep” was correlated with the latency of sleep stages, especially sleep onset latency. This finding is supported by a previous study, which demonstrated that falling asleep cannot be recognized until sleep spindles appear after stage N1 [29]. The delay of sleep onset consists of a prolonged period with the concomitant appearance of EEG signs for wakefulness and sleep, and replacement of arousal and sleep [30, 31].
As previously reported, the subjective assessment of sleep quality was correlated with many sleep variables including total sleep time, sleep efficiency, wake time after sleep onset, sleep onset latency, and the amount of sleep stages of light and deep sleep [1, 2, 23, 24]. The present study partially supports these previous findings. In addition, the two questions about the quality domain of subjective sleep assessment, ‘Quality of sleep’ and ‘Depth of sleep’, similarly represent objective sleep variables regarding the amount and quality of slow-wave sleep such as the time in Stage N3 and EEG delta power. Thus, the two subjective assessments were significantly correlated. However, ‘Depth of sleep’ may further represent additional aspects of sleep related to sleep fragmentation or stability, as it was further correlated with wake time after onset and sleep efficiency. Furthermore, unlike patients with chronic fatigue syndrome [5] or depression symptoms [6], no correlation was found between subjective sleep assessments and heart rate variability, although heart rate variability during sleep significantly differed between the two nights. Therefore, subjective sleep assessments were correlated with objective assessments quantified by analysis of EEG activity in healthy individuals.
In this study, we further investigated whether objective sleep measurements in a specific period of sleep rather than overall sleep, were reflected in the subjective sleep assessment. This analysis was encouraged by correlations between “Depth of sleep” and “Quality of sleep”, and between “Ease of falling asleep” and “Quality of sleep” because these subjective assessments reflected the objective measurements characterizing the initial period of sleep such as sleep latency, the amount of stage N3, and delta EEG power. The hypothesis was supported by sleep cycle-by-cycle analysis, as significant correlations were found between subjective assessment and objective sleep measurements of the first sleep cycle.
These results may be associated with the unique physiological processes of transition from wakefulness to sleep and further into slow-wave sleep in the first sleep cycle. The wake time after sleep onset in the first sleep cycle was significantly correlated with the subjective evaluation of sleep onset, “Ease of falling asleep” (r = − 0.382, p < 0.001) (Table 3, Fig. 1b). This suggests that the more frequent appearance of waking periods before and after sleep onset, a factor for delayed sleep onset and unstable sleep onset processes (as discussed above), was reflected in the subjective evaluation. This may be because increased arousal in sleep onset is related to the recognition of environmental or endogenous conditions [32].
The amounts of stage N3 and EEG delta and theta power in the first sleep cycle were significantly positively correlated with the subjective evaluation of “Depth of sleep” and “Quality of sleep” (Table 3 Fig. 1a, c, d, and e). On the other hand, no significant correlation was found in the other sleep cycles, except for between ‘Depth of sleep’ and delta EEG power in the third sleep cycle. This can be explained by Stage N3 being predominant in the first sleep cycle [9], which is characterized by higher delta and theta power than other sleep stages [10, 33]. Moreover, Stage N3 is associated with a higher arousal threshold [34], the level of which becomes higher as delta EEG power increases [35, 36]. As previously reported, in the first night, delayed sleep onset impedes sleep progression and leads to a decreased amount of stage N3 in the first sleep cycle, although the total amount of stage N3 did not differ between the two nights [10]. Thus, cortical EEG power reflects the difference in physiological quality of sleep between the two nights and this difference was subjectively recognized in healthy subjects.
This study had several limitations. First, the two nights of PSG were executed in the sleep laboratory. Ideally, testing should be performed at home to have more typical “normal” nights. In addition, data from more nights were necessary to observe differences. Second, a standard definition of subjective sleep quality has not been identified. In this study, in addition to “Quality of sleep”, we also used the factor “Depth of sleep”. There was no specific boundary between the two questions, but the correlation between these questions and sleep parameters differed. This suggests that differences in the terminology and definition used in the questionnaire may affect subjective sleep assessments. Many studies noted that if the definition of sleep quality was generalized, it would benefit future studies because comparisons between similar definitions can be employed [8, 11, 37, 38].
The present study found night-to-night variations in subjective and objective sleep measurements. Few studies have investigated the correlation between subjective sleep evaluation and objective sleep measurements based on the differences in objective sleep measurements between two nights for each sleep cycle using PSG, EEG power, and heart rate variability. In most of the subjective and sleep parameters, moderate or higher correlations were found when considering the difference between nights, which is consistent with previous reports [11]. On the other hand, there were no correlations between subjective sleep evaluations and heart rate variability. Furthermore, based on sleep cycle-by-cycle analysis, subjective sleep assessments were correlated with EEG-related sleep measurements in the first sleep cycle. These results suggest the importance of taking differences among the nights into account when assessing subjective sleep quality. This study clarified that sleep in the first sleep cycle has a dominant influence on subjective sleep assessments in healthy young subjects.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Westerlund A, Lagerros YT, Kecklund G, Axelsson J, Åkerstedt T. Relationships between questionnaire ratings of sleep quality and polysomnography in healthy adults. Behav Sleep Med. 2016;14:185–99.
Rosipal R, Lewandowski A, Dorffner G. In search of objective components for sleep quality indexing in normal sleep. Biol Psychol. 2013;94:210–20.
Goelema MS, Regis M, Haakma R, van den Heuvel ER, Markopoulos P, Overeem S. Determinants of perceived sleep quality in normal sleepers. Behav Sleep Med. 2019;17:388–97.
Faerman A, Kaplan KA, Zeitzer JM. Subjective sleep quality is poorly associated with actigraphy and heart rate measures in community-dwelling older men. Sleep Med. 2020;73:154–61.
Burton AR, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res. 2010;204:71–8.
Guo X, Su T, Xiao H, Xiao R, Xiao Z. Using 24-h heart rate variability to investigate the sleep quality and depression symptoms of medical students. Front Psychiatry. 2022;12:1–10.
McCarter SJ, Hagen PT, St. Louis EK, Rieck TM, Haider CR, Holmes DR, et al. Physiological markers of sleep quality: a scoping review. Sleep Med Rev. 2022;64:101657.
Cudney LE, Frey BN, McCabe RE, Green SM. Investigating the relationship between objective measures of sleep and self-report sleep quality in healthy adults: a review. J Clin Sleep Med. 2022;18:927–36.
Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25:131–43.
Shirota A, Kamimura M, Kishi A, Adachi H, Taniike M, Kato T. Discrepancies in the time course of sleep stage dynamics, electroencephalographic activity and heart rate variability over sleep cycles in the adaptation night in healthy young adults. Front Physiol. 2021;12: 623401.
Goelema M, Leufkens T, Haakma R, Markopoulos P. Determinants of self-reported sleep quality in healthy sleepers and patients. Cogent Psychol. 2018;5:1–14.
Lakens D. Sample size justification. Collabra Psychol. 2022;8:1–28.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh sleep quality index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97:165–72.
Zung WWK. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70.
Fukuda K, Kobayashi S. Jiko-hyoka-shiki yokuutsu-sei shakudo no kenkyuu (a study on a self-rating depression scale). Psychiatr Neurol Jpn. 1973;75:673–9.
Spielberger CD, Gorsuch RL, Rushene RE. Stai manual for the state-trait anxiety inventory: (self-evaluation questionnaire). Palo Alto: Consulting Psychologists Press; 1970.
Spielberger CD. State-trait anxiety inventory. Japanese translation rights arranged with Mind Garden, Inc. through Japan UNI Agency, Inc., Tokyo; 2000.
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL. American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events : rules, terminology, and technical specifications, version 2.2. Am Acad Sleep. 2016.
Šušmáková K. Human sleep and sleep EEG Institute of Measurement Science, Slovak Academy of Sciences. Meas Sci Rev. 2004;4:59–74.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;17:354–81.
Mateos-Salgado EL, Ayala-Guerrero F. Comparison of autonomic activity between N2 and N3 stages of NREM sleep: evaluation through heart rate variability metrics. Sleep Biol Rhythms. 2021;19:181–6.
Keklund G, Åkerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–20.
O’Donnell D, Silva EJ, Münch M, Ronda JM, Wang W, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J Sleep Res. 2009;18:254–63.
Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–9.
Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:365–76.
Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93.
Lecci S, Cataldi J, Betta M, Bernardi G, Heinzer R, Siclari F. Electroencephalographic changes associated with subjective under- and overestimation of sleep duration. Sleep. 2020;43:1–12.
Hori T, Hayashi M, Morikawa T (1994) Topographical EEG changes and the hypnagogic experience. In: Ogilvie RD, Harsh JR (eds). American Psychological Association, Washington, p 237–53
Nittono H, Momose D, Hori T. The vanishing point of the mismatch negativity at sleep onset. Clin Neurophysiol. 2001;112:732–9.
Ogilvie RD, Wilkinson RT. The detection of sleep onset: behavioral and physiological convergence. Psychophysiology. 1984;21:510–20.
Tamaki M, Nittono H, Hayashi M, Hori T. Examination of the first-night effect during the sleep-onset period. Sleep. 2005;28:195–202.
Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clin Neurophysiol. 2001;112:992–6.
Kato T. Sleep bruxism and its relation to obstructive sleep apnea-hypopnea syndrome. Sleep Biol Rhythms. 2004;2:1–15.
Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93.
Campbell IG, Higgins LM, Darchia N, Feinberg I. Homeostatic behavior of fast Fourier transform power in very low frequency non-rapid eye movement human electroencephalogram. Neuroscience. 2006;140:1395–9.
Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–93.
Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9:10–7.
Acknowledgements
We would like to thank technicians (Kataoka N., Takahashi C., Nakamura M., Teshima Y., Maekawa T., Yamamoto A., Koda S., Hirai N., Iwaki A., Nishida M., Nakamura U., and Nonoue S.) for their technical assistance and sleep stage scoring.
Funding
This study was funded by Grants-in-Aid for Scientific Research (B) (#25293393) from the Japan Society for the Promotion of Science (JSPS), by funds from the Intractable Oral Disease at Osaka University Graduate School of Dentistry and the Center of Innovation Science and Technology Based Radical Innovation and Entrepreneurship Program, and partially by a Grant-in-Aid for Challenging Research (exploratory, #17K19753) from the JSPS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this manuscript.
Ethics statement
Studies involving human participants were reviewed and approved by the Research Ethics Committee of Osaka University Graduate School of Dentistry and Osaka University Dental Hospital (H25-E9-5, H29-E48-3).
Informed consent
The patients/participants provided written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirota, A., Kamimura, M., Katagiri, A. et al. Subjective sleep assessments are correlated with EEG-related sleep measurements of the first sleep cycle in healthy young adults. Sleep Biol. Rhythms 21, 211–219 (2023). https://doi.org/10.1007/s41105-022-00437-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-022-00437-x