Abstract
Purpose
Adaptive servo-ventilation (ASV) is a positive pressure ventilator support system to normalize ventilation in patients with Cheyne-Stokes respiration (CSR). The latest generation enhanced ASV device (PaceWave™; ResMed) has a new feature—auto-adjustment of EPAP. This study tested the hypothesis that enhanced ASV with auto-adjustment of EPAP (PaceWave™) is non-inferior to conventional ASV (AutoSet™CS).
Methods
This prospective, randomized, crossover, single-center study enrolled adult patients with stable heart failure (HF) and moderate-to-severe sleep-disordered breathing (SDB) who had been receiving conventional ASV therapy for at least 4 weeks. Patients received conventional ASV for one night and enhanced ASV on another night. Support settings for the two ASV devices were similar, with fixed expiratory positive airway pressure (EPAP) set to between 4 and 10 cm H2O and variable EPAP set to between 4 and 15 cm H2O. Full polysomnography was performed during ASV therapy on both nights. Endpoints were the number of nocturnal respiratory events and oxygen desaturations, and changes in blood pressure (BP).
Results
Levels of EPAP were comparable during the use of enhanced and conventional ASV, but minimum and maximum inspiratory pressure support values were significantly higher with the PaceWave™ device. All measures of apnea and hypopnea, and oxygen saturation, were significantly improved during ASV therapy with either device. There were no significant changes in BP or heart rate.
Conclusions
Enhanced ASV is non-inferior to ASV with fixed EPAP in patients with chronic HF and CSR, with a trend towards better control of respiratory events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
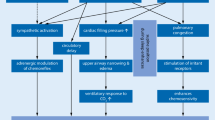
Sleep-disordered breathing (SDB) is increasingly recognized as an important comorbidity in patients with heart failure (HF). The prevalence of SDB in patients with systolic HF is likely to be as high as 50–60 %, substantially greater than in the general population [1–4]. In the majority of HF patients, the SDB pattern is predominant central sleep apnea (CSA) with Cheyne-Stokes respiration (CSR) [4–6].
Despite the more widespread availability of data on the incidence and impact of SDB in patients with HF, it remains an under-diagnosed and under-treated comorbidity [7] because SDB has negative effects on outcomes in patients with HF [8–11]. Effective treatment is therefore an important goal.
Observational data suggest that positive airway pressure therapy improves cardiac parameters [12–14] and is a significant independent predictor of survival in patients with chronic HF [11]. Adaptive servo-ventilation (ASV) is a positive pressure ventilator support system to normalize ventilation in patients with CSA-CSR [15, 16]. Since its first use more than a decade ago, evidence regarding the beneficial effects of ASV in HF patients with CSA-CSR is accumulating. These include improvements in quality of life, exercise tolerance, cardiac and cardiovascular function, and rehospitalization rate [15, 17–24].
One of the most widely-used ASV devices is the ResMed AutoSet™CS/VPAP Adapt™, which operates using a fixed expiratory positive airway pressure (EPAP) to which a variable amount of inspiratory pressure support (inspiratory positive airway pressure; IPAP) is added. This has been extensively utilized in clinical settings for over 10 years. A new feature has been added to the latest generation enhanced ASV device (PaceWave™; ResMed)—auto-adjustment of EPAP.
This study was designed to test the hypothesis that enhanced ASV with automatic EPAP adjustments (ASVAuto, PaceWave™) is non-inferior to conventional ASV with respect to reducing nocturnal respiratory events and preventing oxygen desaturations. A secondary aim of the study was to assess changes in blood pressure (BP) to determine the safety of auto-titrating EPAP.
Materials and methods
Study design
This study was a prospective, randomized, crossover, observational, single-center study comparing the efficacy of conventional ASV therapy (AutoSet™CS, VPAP Adapt SV; ResMed) and enhanced ASV with automatic adjusting EPAP (ASVAuto, PaceWave™; ResMed) for the suppression of nocturnal respiratory events and prevention of oxygen desaturations.
Patients
Adult patients (age ≥21 years) with chronic stable HF (NYHA functional class ≥ II) and moderate-to-severe nocturnal SDB (apnea-hypopnea-index ≥15/h, with the majority of events being central) were included. All patients have been receiving conventional ASV therapy (AutoSet™CS) for at least 4 weeks before the study and were able to fully understand the study information and participation requirements. Titration of conventional ASV therapy was conducted under full polysomnography (PSG) surveillance, and EPAP was titrated by experienced technicians to resolve any upper airway obstruction. In addition, all patients received at least one follow-up PSG investigation, providing the opportunity to readjust any pressure settings, including EPAP. Thus, EPAP used in this study was manually adjusted to the optimal level for each patient.
Exclusion criteria were decompensated HF, myocardial infarction, or resuscitation within the last 3 months, any stroke with swallowing disorders or persistent hemiparesis, untreated restless legs syndrome, alcohol or drug abuse, known cancer, pregnancy, or any conditions that could interfere with patients participating in and completing the protocol and/or unsuitability for enrolment as determined by the investigator.
Use of ASV devices
Patients were admitted to our sleep laboratory for two consecutive nights. They were alternately allocated to receive either conventional or enhanced ASV on the first night and the other form of ASV on the second night; the first patient was allocated to conventional ASV treatment on the first night. Support settings for the two ASV devices were similar, with fixed EPAP set between 4 and 10 cm H2O and variable EPAP set between 4 and 15 cm H2O.
Assessments
Full PSG was performed during therapy on both nights according to the 2007 American Academy of Sleep Medicine (AASM) guidelines [25]. Blinded analysis of PSG recordings was conducted independently of the study investigators.
Standard oscillometric upper arm BP measurements were performed during the study. In the first instance, all patients had to pass a “BP test” with ASV at default settings and then with EPAP set to 10 cm H2O for at least 15 min each, with BP recorded every minute. A patient was deemed to failed this blood pressure test if they had: any sign of systemic hypotension (e.g., neurological symptoms such as dizziness, headache, blurred vision, etc.) and/or a >40 mmHg fall in systolic BP (or a fall of ≥20 % if baseline systolic BP was ≤100 mmHg), and/or a sustained decrease in mean arterial pressure to <60 mmHg. BP recordings during PAP therapy testing were taken before “lights out” at night and immediately after “lights on” in the morning.
Ethics
The study protocol was approved by the local ethical committee (Reg.-No. 19/2011) and announced to German authorities (Bundesamt für Arzneimittel und Medizinprodukte). In addition, the trial was registered at clinicaltrials.gov (NCT01405313). All patients gave written informed consent to participate in the trial, which was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The study was monitored by the local study organization (IKFE-HDZ, Bad Oeynhausen, Germany) and sponsored by ResMed Inc., Sydney, Australia.
Statistics
A power calculation using data from an unpublished study conducted by the study sponsor showed that a minimum sample size of 16 was required to demonstrate a difference of 0.75 events/h with 80 % power. To allow for drop-outs and erroneous data, 21 subjects were recruited. Data from all 21 patients were included in the analysis.
For analyses of repeated measurements, ANOVA for repeated measures and Bonferroni’s or Tukey tests for pairwise comparisons were used. When normality testing (Shapiro-Wilk) failed, either Mann–Whitney rank sum test or Kruskal–Wallis one-way analysis of variance on ranks testing was used to evaluate differences between groups. A p value <0.05 was considered to be statistically significant. All values are expressed as mean ± standard deviation, for some parameters median values and ranges are also presented. All statistical analyses were performed using SigmaPlot® 12.0 software (Systat Software Inc., Erkrath, Germany).
Results
A total of 21 patients with HF, nocturnal CSR, and pre-established conventional ASV therapy (AutoSet™CS) were randomized and included in the study. Mean duration of prior ASV treatment before randomization was 23.6 ± 20.0 months (median 17.7 months). During this period, ASV efficacy was evaluated periodically with adjustment of pressure settings or mask fittings, ensuring optimal device settings for conventional ASV treatment prior to randomization. Demographics, clinical characteristics, and concomitant medication for all enrolled patients are reported in Table 1.
Pressure support
Levels of EPAP were comparable during the use of enhanced ASV compared with the conventional ASV (Table 2). In contrast, minimum and maximum IPAP values were significantly higher with the new PaceWave™ device.
Respiratory and sleep parameters
All measures of apnea and hypopnea significantly improved during ASV therapy, both with the conventional and enhanced devices. Comparable, statistically significant improvements were also documented for oxygen saturation (Table 3).
During treatment with either ASV device, there was a significant decrease from baseline in total sleep time and time spent in N2 sleep (Table 3). In addition, there was a significant increase versus baseline in the arousal index during conventional ASV (Table 3). There were no differences in sleeping position during the nights with conventional or enhanced ASV therapy.
Blood pressure
All enrolled patients passed the initial BP testing protocol. BP measurements taken at the beginning and the end of every ASV treatment night are presented in Table 4. There were no significant differences in BP and heart rate between the conventional and enhanced ASV devices.
Discussion
This study confirms that the new enhanced ASV device with auto-adjustment of EPAP (PaceWave™) is non-inferior to conventional ASV with fixed EPAP (AutoSet™CS2) in patients with HF and CSA-CSR, but may provide improved control of respiratory events.
ASV has been described as the most effective treatment option for CSR-CSA in patients with HF [26]. In addition to ameliorating SDB, ASV has been shown to have a variety of beneficial effects in HF patients.
The use of ASV for one night decreased cardiac overload, improved renal function, and reduced N-terminal pro-brain natriuretic peptide (NT-pro BNP) levels in 50 patients with SDB and HF with left ventricular dysfunction [13]. Looking at longer term therapy, more than 6 months of ASV treatment at a mean EPAP of 6 mmHg decreased NYHA class from 2.5 to 1.6 and significantly decreased plasma BNP, accompanied by significant improvements in left ventricular ejection fraction and left ventricular pressures. Furthermore, a significantly greater proportion of ASV versus non-ASV recipients was event-free during follow-up (p < 0.01) [27]. Other studies have reported similar positive outcomes [14, 20]. The positive effects of ASV in patients with HF are not limited to those with left ventricular dysfunction. Patients with HF and preserved left ventricular function have also experienced improvements in HF symptoms and cardiac function as a result of using ASV [28]. A comprehensive overview of all these trials can be found in Oldenburg et al. 2012 [29]. In another study, noninvasive ventilation which included pressure support as well as conventional continuous positive airway pressure (CPAP) increased cardiac index to a significantly greater extent than CPAP alone [30]. Overall, it is thought that the beneficial effects of ASV in patients with HF are secondary to the reductions in the elevated sympathetic tone in this group [26].
To date, assessments of the hemodynamic and cardiac effects of positive airway pressure therapy in patients with HF have used devices with low and fixed levels of end-expiratory pressure (EEP), such as the AutoSet™CS product included in the current study. The newer PaceWave™ ASV device includes an algorithm that automatically adjust EEP over a wide range. The results of this trial indicate that PaceWave™ is noninferior to fixed EEP devices and has the potential to expand the range of benefits associated with ASV therapy in HF patients. By enrolling only patients with central sleep apnea, we were able to test the new algorithm for any inappropriate increase in EPAP as a consequence of central apnea, which did not occur. However, the inclusion of only central apnea patients mean that we can draw only limited conclusions regarding the suppression of coexisting obstructive events.
Careful monitoring of symptoms and hemodynamic data is recommended during initiation of any positive airway pressure therapy in patients with HF [29]. High EPAP in severe HF patients with low filling pressures and/or low resting BP may result in unexpected drops in BP [18]. However, the EPAP automatically set by the intelligent algorithm in the PaceWave™ device was comparable to that manually titrated when the AutoSet™CS was used. In contrast, mean IPAP chosen by the PaceWave™ algorithm was statistically higher compared with the AutoSet™CS2 device. This had no obvious effect on hemodynamics or sleep parameters, as measured in this study. As a result, we did not evaluate pCO2 and respiratory parameters such as tidal volume or respiratory rate, but no effects on sleep quality were seen. However, whether numerically higher IPAP levels contribute to changes in adherence to ASV therapy needs to be determined.
Therefore, it is likely that no additional precautions need to be taken when using the new product. It does, however, highlight the need to individualize ASV therapy, including the recommended assessment of initial hemodynamic responses. It is not yet clear whether decreases in BP during the initiation of ASV are transient or sustained, or whether these hypotensive effects will influence compliance with therapy [29].
There are a number of factors to discuss in relation to the limitations of the current study. First is the short duration, but the trial was designed as a preliminary investigation to determine the non-inferiority of a device using a new algorithm (PaceWave™) compared with existing ASV therapy. All patients had previously been treated with ASV, meaning that they had adapted to therapy. A study of one night of each treatment would not have been possible in treatment-naïve patients due to the time taken to adapt to using ASV therapy and to sleep with the device throughout the night, which can be several days or even weeks. Secondly, the open-label nature of the study including a non-perfect randomization in whom the investigator knew the sequence of devices which were used. Both could potentially introduce bias into the results. However, the primary outcome was recording of respiratory events, which is outside the control of either the patient or the clinical and trial staff, and is therefore considered to be objective. In addition, assessment of PSGs was undertaken by the investigators unaware of the device used. Another factor to note is that total sleep time was reduced compared with baseline in both treatment periods of this study. This may have been a result of patients being outside their usual surroundings in a less familiar sleep laboratory setting. Also, the slight increase in N3 sleep documented represents an improvement in sleep quality meaning that although sleep quantity reduced slightly, the quality of sleep increased, with the patients better able to reach and maintain deep sleep; this is considered to be a positive effect of the therapy devices. Finally, although HF was comparatively mild in the patients enrolled in this study, all met the inclusion criteria of having moderate-to-severe SDB (AHI ≥15/h) with the majority of events being of a central nature. This was the most important factor in determining whether the new algorithm would be able to control CSA-CSR, and the degree of HF was not relevant to the ability of the algorithm to control SDB.
Additional studies are needed to evaluate the exact hemodynamic response during PaceWave™ therapy, including blood pressure, heart rate, stroke volume, cardiac output, and pulmonary and systemic vascular resistance. The ongoing multinational, multicentre, randomized, parallel, SERVE-HF trial has been designed to assess the effects of the addition of ASV to optimal medical management compared with medical management alone in patients with symptomatic chronic HF, left ventricular ejection fraction ≤45 %, and predominant CSA. The primary combined endpoint is the time to first event of all-cause death, unplanned hospitalization (or unplanned prolongation of a planned hospitalization) for worsening of chronic HF, cardiac transplantation, resuscitation of sudden cardiac arrest, or appropriate life-saving shock for ventricular fibrillation or fast ventricular tachycardia in implantable cardioverter defibrillator patients. This study will provide important data on the effects of ASV treatment on morbidity and mortality, as well as the cost-effectiveness of this therapy, in patients with chronic HF and predominantly CSA/CSR. The first patient was randomized in February 2008, and the study is expected to be completed in mid 2015 [31]. In addition, the trend towards better sleep quality with PaceWave™ needs to be investigated in future large-scale studies.
Enhanced ASV is non-inferior to ASV with fixed EPAP in patients with chronic HF and CSA. There is a trend towards better control of respiratory events with enhanced versus standard ASV. Further randomized trials should focus on comparing the two devices with respect to the number of respiratory events, sleep architecture, and hemodynamic events.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Javaheri S, Parker TJ (1998) Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 97:2154–2159
Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT (2009) In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: Report of prevalence and patient characteristics. J Card Fail 15:739–746
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V (2007) Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 9:251–257
Schulz R, Blau A, Börgel J, Duchna HW, Fietze I, Koper I, Prenzel R, Schädlich S, Schmitt J, Tasci S, Andreas S, working group Kreislauf und Schlaf of the German Sleep Society (DGSM) (2007) Sleep apnoea in heart failure. Eur Respir J 29:1201–1205
Vazir A, Hastings PC, Dayer M, McIntyre HF, Henein MY, Poole-Wilson PA, Cowie MR, Morrell MJ, Simonds AK (2007) A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail 9:243–250
Kahwash R, Kikta D, Khayat R (2011) Recognition and management of sleep-disordered breathing in chronic heart failure. Curr Heart Fail Rep 8:72–79
Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT (2010) Sleep apnea testing and outcomes in a large cohort of medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med 183:539–546
Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, Richards AM, Tan HC, Yeo TC (2011) Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med 7:616–621
Khayat R, Abraham W, Patt B, Brinkman V, Wannemacher J, Porter K, Jarjoura D (2012) Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 18:534–540
Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, Schroll S, Montalvan S, Riegger GA, Pfeifer M, Arzt M (2011) Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail 13:68–75
Karavidas A, Kapsimalis F, Lazaros G, Markozanes E, Arapi S, Cholidou K, Matzaraki V, Kyrkou K, Tsiachris D, Matsakas E, Pyrgakis V, Alchanatis M (2011) The impact of positive airway pressure on cardiac status and clinical outcomes in patients with advanced heart failure and sleep-disordered breathing: a preliminary report. Sleep Breath 15:701–709
Yoshihisa A, Suzuki S, Miyata M, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y (2012) A single night’ beneficial effects of adaptive servo-ventilation on cardiac overload, sympathetic nervous activity, and myocardial damage in patients with chronic heart failure and sleep-disordered breathing. Circ J 76:2153–2158
Koyama T, Watanabe H, Igarashi G, Terada S, Makabe S, Ito H (2011) Short-term prognosis of adaptive servo-ventilation therapy in patients with heart failure. Circ J 75:710–712
Oldenburg O, Schmidt A, Lamp B, Bitter T, Muntean BG, Langer C, Horstkotte D (2008) Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail 10:581–586
Teschler H, Döhring J, Wang YM, Berthon-Jones M (2001) Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 164:614–619
Oldenburg O, Bitter T, Lehmann R, Korte S, Dimitriadis Z, Faber L, Schmidt A, Westerheide N, Horstkotte D (2011) Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol 100:107–115
Oldenburg O, Bartsch S, Bitter T, Schmalgemeier H, Fischbach T, Westerheide N, Horstkotte D (2012) Hypotensive effects of positive airway pressure ventilation in heart failure patients with sleep-disordered breathing. Sleep Breath 16:753–757
Hastings PC, Vazir A, Meadows GE, Dayer M, Poole-Wilson PA, McIntyre HF, Morrell MJ, Cowie MR, Simonds AK (2010) Adaptive servo-ventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol 139:17–24
Koyama T, Watanabe H, Kobukai Y, Makabe S, Munehisa Y, Iino K, Kosaka T, Ito H (2010) Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J 74:2118–2124
Randerath WJ, Nothofer G, Priegnitz C, Anduleit N, Treml M, Kehl V, Galetke W (2012) Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest 142:440–447
Pepperell JC, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, Davies RJ (2003) A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med 168:1109–1114
Philippe C, Stoïca-Herman M, Drouot X, Raffestin B, Escourrou P, Hittinger L, Michel PL, Rouault S, d’Ortho MP (2006) Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a 6 month period. Heart 92:337–342
Haruki N, Takeuchi M, Kaku K, Yoshitani H, Kuwaki H, Tamura M, Abe H, Okazaki M, Tsutsumi A, Otsuji Y (2011) Comparison of acute and chronic impact of adaptive servo-ventilation on left chamber geometry and function in patients with chronic heart failure. Eur J Heart Fail 13:1140–1146
Iber C, Ancoli-Israel S, Chesson A, Quan SF, eds (2007) American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification, 1st ed. American Academy of Sleep Medicine, Westchester, Illinois
Monomura S-I (2013) Treatment of Cheyne-Stokes respiration-central sleep apnea in patients with heart failure. J Cardiol 59:110–116
Yoshihisa A, Shimizu T, Owada T, Nakamura Y, Iwaya S, Yamauchi H, Miyata M, Hoshino Y, Sato T, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, S-i S, Takeishi Y (2011) Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J 52:218–223
Bitter T, Westerheide N, Hossain MS, Lehmann R, Prinz C, Kleemeyer A, Horstkotte D, Oldenburg O (2011) Complex sleep apnoea in congestive heart failure. Thorax 66:402–407
Oldenburg O (2012) Cheyne-Stokes respiration in chronic heart failure. Circ J 76:2305–2317
Yoshida M, Kadokami T, Momii H, Hayashi A, Urashi T, Narita S, Kawamura N, Ando S-I (2012) Enhancement of cardiac performance by bilevel positive airway pressure ventilation in heart failure. J Card Fail 18:912–918
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho M-P, Erdmann E, Levy P, Simonds A, Somers VK, Zannad F, Teschler H (2013) Rationale and design of the SERVE-HF study: adaptive servo-ventilation treatment of sleep-disordered breathing with predominant central sleep apnoea in patients with chronic heart failure. Eur J Heart Fail 15:937–943
Acknowledgments
Medical writing assistance was provided by Nicola Ryan, independent medical writer, on behalf of ResMed. Furthermore, we thank the ResMed Science Center, in particular Dr. Holger Woehrle and Dr. Adam Benjafield, for their support in preparing, conducting and interpretation of the study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oldenburg, O., Spießhöfer, J., Fox, H. et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath 19, 795–800 (2015). https://doi.org/10.1007/s11325-014-1083-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1083-9