Abstract
Background
There is growing evidence supporting an association between obstructive sleep apnea syndrome (OSAS) and systemic vascular disorders. However, the data on choroidal microvasculature are limited. In recent years, choroidal thickness (CT) and choroidal vascularity index (CVI) have been of considerable interest as objective markers of choroidal vascularity. We hypothesized that the imbalance of vascular regulation in OSAS may adversely affect the CT and CVI and may help to assess the vascular risk in these patients.
Purpose
This study aimed to evaluate the choroidal morphology in patients with OSAS.
Materials and methods
Patients with moderate OSAS were included to this study. The subfoveal, nasal, and temporal CT were calculated. The choroidal area (CA) was binarized to the luminal area (LA) and stromal area (SA) using ImageJ software. The CVI was calculated as the proportion of the LA to the total CA.
Results
Of 40 eyes of 40 patients, the mean subfoveal CT was significantly decreased in the OSAS group in comparison to the controls (p = 0.032). The mean CA, LA, and SA were decreased in the OSAS group compared with the controls, but the differences did not reach a statistical significance (p = 0.132, p = 0.104, and p = 0.184, respectively). The CVI was not significantly changed in patients with OSAS (p = 1.000).
Conclusion
Unlike CT, there were no significant differences in choroidal structural parameters and CVI in patients with OSAS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is defined by repetitive episodes of upper airway obstruction, reduction of breath amplitude causing hypoxaemia and hypercapnia, and transient arousals from sleep. The diagnosis is a combination of clinical symptoms and polysomnographic findings. The prevalence of moderate to severe OSAS among adults is approximately 24% in men and 9% in women. OSAS prevalence in the Turkish population is around 14% according to the Turkish adult population epidemiology of sleep, a questionnaire-based study [1].
There is growing evidence supporting the association between OSAS and systemic vascular diseases [2,3,4,5]. Reduced oxygen supply can cause elevated blood pressure in OSAS, and reduced vascular response to the vasodilators can result in dysfunction of blood vessels and vascular endothelial disorders [2]. OSAS induces the release of vasoactive mediators like nitric oxide and endothelin [3]. Thus, OSAS can be associated with metabolic syndrome and cardiovascular disorders (hypertension, atrial fibrillation, myocardial infarction, and heart failure) [4].
The choroidal vascular structure is responsible for the majority of intraocular blood flow and supplies the retinal pigmented epithelium, retinal photoreceptors, and the prelaminar portion of the optic nerve. Choroidal flow can be altered by hemodynamic factors like blood flow and perfusion pressure. We hypothesized that, in OSAS, the imbalance of vascular regulation may adversely affect the ocular perfusion and the structure of choroidal vasculature. Most ocular vascular changes associated with OSAS have been studied with regard to choroidal thickness (CT), but the results are controversial [5, 6]. Although the CT is commonly used as a potential tool in research trials, it reflects only the total choroidal vasculature without any distinction about the inner morphology of the choroid.
Recently, the choroidal vascularity index (CVI) has drawn attention as a new objective marker of the choroidal vasculature. Sonoda et al. reported a method to assess the subfoveal vascular areas by binarized EDI-OCT images using the ImageJ software [7]. They reported on the ratio between the luminal area (LA) and total choroidal area (CA). Later, this ratio was renamed and suggested as a new parameter termed “CVI” to describe the vascular condition of the choroid. The choroidal vascularity was evaluated in healthy eyes, and the results showed that about 2/3 of the subfoveal choroid in a scan was comprised of vascular tissue [8]. According to the recent reports, the CVI is a more informative and reliable index of proportional change in the choroidal vasculature and improves our understanding of morphological changes in ocular and systemic diseases compared to the CT alone [9].
Therefore, in the current study, we evaluated the choroidal morphology using optical coherence tomography (OCT) in patients with OSAS and compared the results with healthy subjects.
Methods
This cross-sectional prospective study was conducted in treatment-naïve patients with newly diagnosed OSAS between May and August 2021. The study was approved by the Institutional Review Board of Ahi Evran University. Informed consent was obtained from each participant. As a control group, 40 healthy volunteers were selected among the people who attended the outpatient clinic for routine ophthalmological evaluation.
Full-night polysomnographic parameters were recorded, and patients who had apnea/hypopnea index (AHI) score ≥ 5 were diagnosed as having OSAS. The AHI was assessed using the American Academy of Sleep Medicine standards. The participants were classified according to the AHI as follows: without OSAS (< 5); mild OSAS (5<15); moderate OSAS (15<30); and severe OSAS (≥ 30) [10].
Exclusion criteria were as follows: patients with systemic vascular diseases that might have ocular involvement; intraocular pressure more than 21 mmHg; choroidal, retinal, and optic nerve pathology; myopia greater than − 6.0 diopters, or an axial length > 26.5 mm; prior ocular intervention; use of corticosteroid and phosphodiesterase-5 inhibitors; and smoking tobacco and caffeine and/or alcohol consumption within 24 h. Control subjects with symptoms of OSAS were also excluded from the study.
All participants underwent a detailed ophthalmologic examination, including best-corrected visual acuity, slit-lamp examination, Goldmann applanation tonometry, and OCT imaging. For OCT, the eye with the highest scan score index was selected. All OCT examinations were done within the same time interval (between 1:00 pm and 3:00 pm). An experienced staff technician captured the enhanced depth imaging (EDI) OCT images. Only one eye per participant was included.
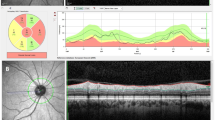
EDI-OCT (SPECTRALIS®, SD-OCT; Heidelberg Engineering, Germany) was conducted by the technique described by Spaide et al. [11]. Any participant in which the OCT scan had a maximum image quality score of less than 15 was excluded. The CA was binarized to LA and stromal area (SA) using ImageJ (version 1.50a; National Institutes of Health). The CA was measured manually at 3000 mm wide, with margins of 1500 mm nasal and 1500 mm temporal from the foveal center horizontally. The binarization was performed using the Niblack auto local threshold. White pixels were accepted as SA and dark pixels as LA [7]. The CVI was calculated as the proportion of the LA to the total CA (Fig. 1).
Statistical evaluation was done using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). A Shapiro–Wilk test was done for all variables to detect deviations from a normal distribution. The differences among groups were evaluated by Student t and Mann–Whitney U tests. For the analyses comparing the study groups, Bonferroni corrections for multiple comparisons were performed, and a p-value < 0.05 was considered significant.
Results
The current study included 40 eyes of 40 patients (30 men) with OSAS and 40 eyes of 40 healthy subjects (30 men). The mean age was 55.5 ± 8.1 years (range, 42–71) in the OSAS group and 56.3 ± 6.9 years (range, 41–72) in the control group (p = 0.468). The mean intraocular pressure, body mass index, and axial length values were similar in the OSAS and control groups (p = 0.524, p = 0.468, and p = 0.442, respectively). The demographic data and clinical parameters are shown in Table 1.
On structural OCT, the mean subfoveal CT was 288. 5 ± 68.1 μm in the OSAS group and 313.34 ± 89.3 μm in the control group (p = 0.032).
The mean CA was 0.847 ± 0.216 mm2 and 0.967 ± 0.290 mm2 (p = 0.132); the mean LA was 0.619 ± 0.159 mm2 and 0.708 ± 0.221 mm2 (p = 0.104); and the mean SA was 0.228 ± 0.062 mm2 and 0.259 ± 0.073 mm2 (p = 0.184) for the OSAS group and the controls, respectively. The mean CVI was 73.15% ± 2.64 in the OSAS group and 73.39% ± 3.09 in the control group. The difference in the mean CVI between the OSAS group and controls did not reach a statistical significance (p = 1.000).
Structural OCT measurements and statistical comparisons are given in Table 2.
Discussion
Obstructive sleep apnea syndrome is characterized by intermittent upper airway obstruction during sleep with synchronous hypoxia and hypercapnia and is a substantial risk factor for systemic vascular diseases. Recent experimental and clinical data have pointed to the clinical significance of this sleep-related breathing disorder. High prevalence in the population and association with cardiovascular complications, such as systemic and pulmonary hypertension and atherosclerosis, have contributed to the recent increase of scientific reports about OSAS. Nonetheless, there are still various unsolved issues in the pathogenesis of this common clinical disorder [4].
Although several reports have suggested an underlying vascular etiology, the pathophysiology of the ocular manifestations of OSAS is not totally understood [4, 12]. As a vascular tissue, the choroidal layer of the eye is essential for proper retinal function [13]. Being highly vascularized makes it susceptible to the changing microvascular dynamics as occurs in OSAS. Rich choroidal innervation of the sympathetic nervous system is responsible for the adaptation of choroidal vascular resistance. In OSAS, each apnea or hypopnea event results in hypoxemia, and the balance between the vasodilators and the vasoconstrictors is disturbed [14, 15]. Hence, expecting choroidal structural alterations and CVI in patients with OSAS patients is reasonable. As far as we know, this is the first research demonstrating the choroidal morphology and CVI in untreated patients with OSAS.
In this study, results showed that the subfoveal CT was significantly decreased in the OSAS group when compared with the healthy subjects. The relationship between OSAS and CT has been previously analyzed; however, different studies have shown inconsistent results [5, 6]. Xin et al. evaluated the CT changes in patients with OSAS who had no pathological changes in their fundus, using SD-OCT [5]. According to their results, patients with OSAS had decreased subfoveal and nasal CT. He et al. performed a meta-analysis to evaluate the CT changes in OSAS [16]. The meta-analysis suggested that the thickness of choroid was decreased in patients with OSAS. Ozge et al. assessed the impact of OSAS on CT in comparison to the control subjects [6]. They did not find any significant differences in the subfoveal and temporal CT between the groups. Contrary to the expectation, OSAS group had significantly thicker choroid than the control group, at 500 and 1500 μm nasal to the fovea in both eyes. Çolak et al. compared retinal capillary plexus vessel densities, CT, foveal avascular zone, and optic disc vessel densities measurements between the healthy subjects and patients with OSAS using OCT angiography [17]. Their findings showed that, in patients with severe OSAS, deep parafoveal and perifoveal vascular densities were decreased and choroidal layer got thinner. Numerous physiologic variables such as age, refraction, timing of the measurement, and ethnicity can affect the CT measurements. All these factors may have caused possible discrepancies in the outcomes of the studies.
In OSAS, episodes of airway occlusion during sleep with consequent hypoxemia and hypercapnia induce various autonomic, hemodynamic, and neurohumoral responses. These responses promote acute systemic vascular changes and sustained effects that continue in the daytime even though breathing becomes normal. Peripheral sympathetic vasoconstriction is an important autonomic response to hypoxemia and hypercapnia in the blood vessels. This reflex response increases the blood pressure as well as the level of circulating catecholamines. Intermittent hypoxemia, together with fluctuations in the blood flow and blood pressure, can lead to oxidative stress, endothelial dysfunction, and atherosclerosis. Endothelial dysfunction and atherosclerosis can also induce blood vessel diameter changes and modify vasoreactivity in patients with OSAS, which may result in decreased structural parameters and CT even during wakefulness [18, 19].
The choroid is an important vascular bed often involved in ocular and systemic diseases. The choroid may show a microvascular alteration that might be the local manifestation of a vascular disorder. Binarization of OCT images can give further information on the relationship between OSAS and the vascular system. The early alterations may lead to the use of the choroidal parameters, like CVI, as a noninvasive screening or prognostic tool for OSAS-related vascular disorders, and therefore, repeated OCT imaging may be useful for guidance of the vascular alterations in order to avoid further damage.
In this study, choroidal structural parameters (CA, LA, and SA) and CVI were decreased in the OSAS group though the difference did not reach statistical significance. The change in CT may depend on changes in the choroidal vessels, stromal tissue, or both. Therefore, the choroidal thinning was most probably related to the decrease of both the LA and SA in the patient group. The CVI is defined as the proportion of LA to the total CA, expressed as a percentage. In patients with OSAS, in which both the LA and SA were decreased simultaneously, the CVI did not reveal a significant difference with respect to the controls. Accordingly, the CVI does not show the choroidal vascular changes in patients with OSAS. Further analysis of CVI variations may help to clarify this issue.
There are some limitations of the current study. The sample size was relatively small, which may affect the strength of the statistical comparisons. The cross-sectional feature of the study did not allow us to assess changes over time. Only patients with moderate OSAS were included. The results may therefore change in other subgroups of OSAS. The CVI is not a reliable measure of blood flow velocity, so it does not provide any knowledge about dynamic flow. As the patients were newly diagnosed with OSAS and as OSAS is a chronic disease, studies with a long-term follow-up may provide more insight into the clinical relevance of the current findings.
The strength of our study is the first evaluation of choroidal structural parameters and CVI in a group of patients with OSAS. We compared a patient group, comprised of newly diagnosed and untreated OSAS, with age- and sex-matched control subjects. Although a detailed history regarding associated comorbidities, concomitant medications, physical activities, and dietary habits was recorded from all participants, only patients free of any other vascular risk factors were included to avoid an influence on the choroidal reactivity.
In conclusion, CT was the only parameter to be significantly decreased in patients with newly diagnosed OSAS compared with healthy control subjects. Unlike CT, the changes in choroidal structural parameters and CVI appear to be less able to identify the vascular risk in patients with OSAS. Therefore, CVI alone may not be a good index to show the choroidal vascular changes in OSAS. Further studies with a larger sample size and in different subgroups of OSAS may be useful to clarify the effect of this common disease on choroidal vascular structure.
References
Demir A, Ardic S, Firat H et al (2015) for the TAPES Investigation Committee. Prevalence of sleep disorders in the Turkish adult population epidemiology of sleep study. Sleep Biol Rhythms 13:298–308
Priou P, Gagnadoux F, Tesse A et al (2010) Endothelial dysfunction and circulating microparticles from patients with obstructive sleep apnea. Am J Pathol 177:974–983. https://doi.org/10.2353/ajpath.2010.091252
Fuchsjäger-Mayrl G, Luksch A, Malec M et al (2003) Role of endothelin-1 in choroidal blood flow regulation during isometric exercise in healthy humans. Invest Ophthalmol Vis Sci 44:728–733. https://doi.org/10.1167/iovs.02-0372
Li M, Li X, Lu Y (2018) Obstructive sleep apnea syndrome and metabolic diseases. Endocrinology 159(7):2670–2675. https://doi.org/10.1210/en.2018-00248
Xin C, Wang J, Zhang W et al (2014) Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye (Lond) 2:415–421. https://doi.org/10.1038/eye.2013.307
Ozge G, Dogan D, Koylu MT et al (2016) Retina nerve fiber layer and choroidal thickness changes in obstructive sleep apnea syndrome. Postgrad Med 12:317–322. https://doi.org/10.1080/00325481.2016.1159118
Sonoda S, Sakamoto T, Yamashita T et al (2014) Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci 55:3893–3899. https://doi.org/10.1167/iovs.14-14447
Agrawal R, Gupta P, Tan KA et al (2016) Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep 6:21090
Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, Vagge A, Di Zazzo A, Forlini M, Finocchio L, Carnevali A et al (2020) Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med 9:595. https://doi.org/10.3390/jcm9020595
Iber C, Ancoli-Israel S, Chesson Jr A, et al (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Am Acad Sleep Med. Westcheste(IL)
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500. https://doi.org/10.1016/j.ajo.2008.05.032
Zamarrón C, Valdés Cuadrado L, Alvarez-Sala R (2013) Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med 2013:521087. https://doi.org/10.1155/2013/521087
Delaey C, Van De Voorde J (2000) Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res 32:249–256. https://doi.org/10.1159/000055622
Papst N, Demant E, Niemeyer G (1982) Changes in pO2 induce retinal autoregulation in vitro. Graefes Arch Clin Exp Ophthalmol 219:6–10. https://doi.org/10.1007/BF02159971
Budhiraja R, Parthasarathy S, Quan SF (2007) Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 3(4):409–15
He M, Han X, Wu H, Huang W (2016) Choroidal thickness changes in obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath 20:369–378
Çolak M, Özek D, Özcan KM, Eravcı FC, Karakurt SE, Karakuş MF, Evren KÖ (2021) Evaluation of retinal vessel density and foveal avascular zone measurements in patients with obstructive sleep apnea syndrome. Int Ophthalmol 41(4):1317–1325. https://doi.org/10.1007/s10792-020-01690-0
Yun CH, Jung KH, Chu K et al (2010) Increased circulating endothelial microparticles and carotid atherosclerosis in obstructive sleep apnea. J Clin Neurol 6:89–98. https://doi.org/10.3988/jcn.2010.6.2.89
Lanfranchi P, Somers VK (2001) Obstructive sleep apnea and vascular disease. Respir Res 2:315–319. https://doi.org/10.1186/rr79
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Medical Ethical Committee of the Ahi Evran University Faculty of Medicine (2021–11/127).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özcan, G., Temel, E., Örnek, K. et al. Choroidal vascularity index in obstructive sleep apnea syndrome. Sleep Breath 26, 1655–1659 (2022). https://doi.org/10.1007/s11325-021-02538-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02538-2