Abstract
Purpose
The incidence of obstructive sleep apnea (OSA) in interstitial lung disease (ILD) has been reported at different frequencies in several studies. The aims of our study were to evaluate the frequency of OSA in ILD and to analyze the relationship between polysomnography (PSG) findings and pulmonary function, disease severity, parenchymal involvement, and Epworth Sleepiness Scale (ESS) scores.
Methods
ILD patients with parenchymal involvement were evaluated. The disease severity was assessed using an index consisting of body mass index (BMI), carbon monoxide diffusion capacity, the Modified Medical Research Council dyspnea scale, and the 6-min walking distance. All of the patients had lung function, chest X-ray, PSG, ESS scoring, and an upper airway examination. Patients with a BMI ≥ 30 or significant upper airway pathologies were excluded.
Results
Of 62 patients, 50 patients comprised the study group (14 male, 36 female; mean age 54 ± 12.35 years, mean BMI 25.9 ± 3.44 kg/m2) with diagnoses of idiopathic pulmonary fibrosis (IPF; n = 17), stage II–III sarcoidosis (n = 15), or scleroderma (n = 18). The frequency of OSA was 68 %. The mean apnea–hypopnea index (AHI) was 11.4 ± 12.5. OSA was more common in IPF patients (p = 0.009). The frequency of rapid eye movement-related sleep apnea was 52.9 %. The frequency of OSA was higher in patients with a disease severity index ≥3 (p = 0.04). The oxygen desaturation index and the AHI were higher in patients with diffuse radiological involvement (p = 0.007 and p = 0.043, respectively).
Conclusions
OSA is common in ILD. PSG or at minimum nocturnal oximetry should be performed, particularly in patients with functionally and radiologically severe disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is estimated to occur in aproximately 2–4 % of healthy adults [1, 2]. The morbidity and the mortality of OSA are high especially when it occurs concomitantly with other respiratory diseases [3]. Interstitial lung disease (ILD) is a chronic and restrictive lung disease with high morbidity and mortality. It is associated with hypoxemia that progresses to respiratory failure, pulmonary hypertension, and death [4]. There have been few studies describing OSA in patients with ILD. The incidence of OSA in these studies has been reported to be between 17 and 88 % [5–9]. However, some of these studies included small numbers of patients. Additionally, important risk factors for OSA such as obesity and upper airway pathologies were not excluded in these studies. In the study design of these investigations, there was no upper airway examination, and patients with Mallampati classification of 3 and 4 were included in some of these studies. Additionally, most of these previous studies included only patients with idiopathic pulmonary fibrosis (IPF).
In ILD, decreased lung volumes, increased respiratory work, decreased in the activity of intercostal muscles, increased upper airway resistance, and collapse may occur during sleep, especially during the rapid eye movement (REM) period [6, 7, 10]. Cough-related arousals, nocturnal desaturations, and increased respiratory drive disrupt sleep architecture and decrease REM sleep and sleep efficiency in patients with ILD [5, 6]. Earlier studies that were performed with small groups showed that the desaturation during sleep was mild and less severe in patients with ILD than in patients with chronic obstructive lung disease [11, 12]. Oxygen desaturations are unlikely to be of clinical importance in patients with ILD [11–13]. Therefore, the frequency and significance of OSA in ILD are still unclear. The aims of our study were to describe the incidence of OSA in patients with ILD and to analyze the relationship between OSA and ILD severity as determined by a scoring method. The secondary aim was to analyze the relationships between polysomnography (PSG) findings with forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), carbon monoxide diffusion capacity (DLCO), and daytime sleepiness confirmed by the Epworth Sleepiness Scale (ESS).
Materials and methods
This prospective study was conducted at Istanbul University, Istanbul Medical Faculty, from November 2009 to February 2010. The study group included consecutive patients with ILD who have been followed up at the Department of Pulmonology. All of the patients were informed about the study and voluntarily signed their informed consent. The study was carried out according to the principles of the Helsinki Declaration. The study was approved by the Istanbul University Istanbul Medical Faculty Institutional Board (2009/894).
The inclusion criteria were ILD with parenchymal involvement, clinical stability, ≥18 years of age, and a PaO2 > 60 mmHg at rest on room air. The diagnosis of IPF was confirmed using the American Thoracic Society/European Respiratory Society Consensus Statement criteria [14]. The diagnosis of scleroderma was confirmed using the American Rheumotology Society Consensus Statement criteria [15]. Pulmonary involvement in scleroderma patients was confirmed using HRCT and lung function tests (spirometry and DLCO testing). All sarcoidosis patients had histopathologic diagnosis, and disease staging was performed by a radiologist via chest X-ray.
The exclusion criteria were treatment changes in the last 3 months, a diagnosis of neurological disease or severe psychiatric disease, lower respiratory tract airway infection in the last month, total sleep time less than 4 h on PSG, a chronic pulmonary disease except ILD, drug usage affecting sleep architecture (benzodiazepine, narcotic drugs), body mass index (BMI) ≥ 30, and failure to perform spirometry. An upper airway examination was performed in all of the patients. Patients with high Mallampati classification scores (3 or 4) or anatomic upper airway obstructions such as significant nasal septal deviation, nasal polyps, concha hypertrophy, or tonsillar hypertrophy were excluded.
Assessment of clinical parameters and pulmonary function
Characteristic OSA symptoms (snoring, witnessed apnea, excessive daytime sleepiness) were recorded. The ESS was used to measure daytime sleepiness. An ESS score of ≥10 was considered to be excessive daytime sleepiness. The BMI was calculated using Khosla and Lowe’s formula (weight [kg] / height2 [m2]) [16]. Spirometry and DLCO testing (ZAN 74N) were performed according to approved standards [17]. Arterial blood gas analysis was performed on room air after 15 min of rest using Radiometer ABL 5. The 6-min walking test was performed in accordance with the American Thoracic Society guidelines [18].
Assessment of disease severity
The severity of ILD was determined using a scoring method that included the BMI, DLCO results, MMRC dyspnea score, and 6MWD. The scores ranged between 0 and 10 (Table 1). Patients with scores ≥3 were compared with patients whose scores were <3.
Assessment of chest X-ray findings
Posteroanterior chest X-rays were divided into three zones for each lung (upper, middle, and lower zones) by two crossed lines from the second and fourth costae. Each of the three zones contained one third of the craniocaudal distance of the lung on a frontal radiograph and was evaluated separately. One point was given for each zone of involved lung parenchyma with interstitial images. If the number of involved zones was higher than three, it was considered to be diffuse radiological pulmonary involvement. Patients with diffuse radiological pulmonary involvement were compared with the patients with nondiffuse involvement.
Assessment of polysomnographic findings
All-night PSG was performed using a 44-channel Compumedics E instrument. The following recordings were made using a computerized workstation: multiple channels of the electroencephalogram (central and occipital), two channels of the electrooculogram, two channels of the electromyogram, the oronasal flow (using a thermistor and a nasal pressure transducer), the respiratory effort (using abdominal and thoracic strain gauges), the oxygen saturation (using pulse oximetry), snoring sounds (using a microphone), submental and anterior tibialis monitoring, and body position [19]. The PSG findings were scored in 30-s windows following the recommended criteria of the American Academy of Sleep Medicine 2007 guideline [20]. Recordings with more than 4 h of sleep time were evaluated. The sleep efficiency, percentage of time in different sleep stages, apnea index (AI), hypopnea index (HI), apnea–hypopnea index (AHI), arousal index, oxygen desaturation index (ODI), REM AHI, REM ODI, percentage of total sleep time with O2 saturation <90 %, mean oxygen saturation, and lowest oxygen saturation levels were evaluated. Obstructive apnea was defined as a cessation of airflow ≥90 % compared with baseline for ≥10 s, while there was evidence of a persistent respiratory effort. Hypopnea was defined as an amplitude reduction of ≥30 % in airflow for ≥10 s that was associated with an oxygen desaturation of ≥4 % [20]. PSG records were scored by a trained technician and controlled by a sleep specialist. OSA was diagnosed if the AHI was ≥5/h. The OSA severity was graded as mild (AHI 5–15/h), moderate (AHI 15–30/h), or severe (AHI >30/h) [1, 20]. REM-related OSA was considered present when the AHI was ≥5, the NREM AHI was less than 15, and the REM/NREM AHI was ≥2 [1, 20]. An ODI higher than 10 was considered as pathologic [21].
Statistical analysis
Means and standard deviations were calculated for the normally distributed continuous variables, while medians and intraquartile ranges were calculated for the variables without normal distributions. All of the data are presented as mean ± SD. Categorial variables are reported as percentages. Comparison analyses were assessed using the chi-squared test, the Wilcoxon test, Fisher’s exact test, and the Mann–Whitney U test. The Pearson correlation coefficient was used to examine the relationship between the PSG data and the pulmonary function test results. All p values were two tailed, and values less than or equal to 0.05 were considered statistically significant.
Results
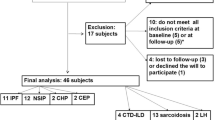
A total of 62 patients were enrolled in the study. Four patients with upper airway pathologies [nasal septal deviation (one), nasal polyps (one), concha hypertrophy (one), high Mallampati classification score (one)] and eight obese patients (BMI ≥ 30) were excluded. The study group included 50 patients with diagnoses of IPF (n = 17), sarcoidosis (n = 15), or scleroderma (n = 18). Thirty-six (72 %) of the patients were female. The mean age was 53.92 ± 12.19 years, and the mean BMI was 25.94 ± 2.32 kg/m2. At the time of study, 48 % (n = 24) of the patients were receiving prednisolone, 22 % (n = 11) were receiving azathioprine, and 14 % (n = 7) were receiving methotrexate therapy. The following comorbidities were reported in 30 % (n = 15) of the patients: hypertension, ischemic heart disease, and diabetes mellitus.
Sleep-related symptoms, ESS scores, and pulmonary function
The mean percentages of FVC, FEV1, and DLCO were normal for the whole group (Table 2). Ten patients (one sarcoidosis, two scleroderma, and seven IPF) were using supplemental oxygen therapy only during exercise due to exercise-induced desaturation. The patients had complaints of sleep-related symptoms such as snoring (40 %), witnessed apneas (6 %), and excessive daytime sleepiness (10 %). The mean ESS score was 5.42 ± 3.26. ESS scores > 10 were found in only five patients. In comparing the IPF patients to the other study participants, they were mostly male, were older, had lower PaO2 measurements, had lower predicted FVC, and DLCO percentages (p < 0.001, p < 0.002, p = 0.001, p = 0.03, and p = 0.001, respectively) (Table 2).
Disease severity and chest X-ray findings
The mean disease severity index score was 3.24 ± 2.25. Disease severity scores were higher in the patients with IPF than either scleroderma or sarcoidosis patients (p = 0.043, and p = 0.000, respectively). Diffuse parenchymal involvement on chest X-ray was found in 18 patients (IPH = 11, sarcoidosis = 4, and scleroderma = 3). The demographic characteristics, pulmonary function test results, ESS scores, and severity index scores of the study groups are presented in Table 2.
Polysomnographic data
The percentages of sleep efficiency, slow-wave sleep, and REM sleep were found to be decreased in all 50 patients with ILD. The arousal index was higher than 10 in 64 % of the patients. A diagnosis of OSA was made in 68 % (n = 34) of the patients (19 mild, 11 moderate, and 4 severe). The AHI was 11.41 ± 12.52. Most of the respiratory events were hypopneas rather than apneas. REM-related sleep apnea was determined to be present in 52.9 % (n = 18/34) of the patients. Oxygen desaturation was significant during REM. The ODI was higher than 10 in 52 % (n = 26) of the patients. The OSA diagnosis rate was higher in male patients than in female patients (92.8 and 58.3 %, respectively, p = 0.02). There were no significant differences for smoking history, comorbidities, immunosuppressive therapy usage, characteristic symptoms of OSA, ESS score, lung function between patients with and without an OSA diagnosis. Half of the patients with OSA were receiving corticosteroid therapy. The PSG data did not differ between the patients who were using corticosteroids and the patients who were not. There was no correlation between age and the AHI. There was a weak correlation between the BMI and the AHI (r = 0.404, p = 0.004). The AHI and the ODI showed no correlation with lung function. There were no significant differences for the AHI between the patients who were using oxygen during exercise and the patients who were not. However, 70 % of the patients (n = 7/10) who were using oxygen during exercise had an OSA diagnosis.
The ODI was statistically different between patients with and without OSA (17.4 ± 3.4 and 3.3 ± 0.7, respectively, p = 0.001). There was a strong correlation between the ODI and the AHI (r = 0.883, p = 0.000). There was a weak positive correlation between the ODI and the BMI (r = 0.351, p = 0.012). The PSG data and nocturnal oximetry parameters are given in Table 3.
The OSA diagnosis rate was higher in the patients who had severity index ≥ 3 (p = 0.04). Additionally, the severity index score was correlated with the sleep efficiency, percentage of REM sleep, mean oxygen saturation, and time spent with saturation <90 % (r = −0.308, p = 0.03; r = 0.310, p = 0.028; r = −0.502, p = 0.000; and r = 0.370, p = 0.008, respectively).
Diffuse parenchymal pulmonary involvement as determined by chest X-ray was related to a higher AHI, increased stage 2 sleep, decreased stage 3 sleep, and higher REM AHI when compared to the nondiffuse involvement (p = 0.043, p = 0.026, p = 0.012, and p = 0.013, respectively). In addition, the mean nocturnal saturation was significantly lower, and the total sleep time with O2 saturation <90 % and the ODI were significantly higher in subjects with diffuse radiological involvement (p = 0.002, p = 0.024, and p = 0.007, respectively).
Comparison of PSG data in ILD subgroups
An OSA diagnosis was observed at a rate of 82.3 % (n = 14) in the IPF patients, 55.5 % (n = 10) in the scleroderma patients, and 66.6 % (n = 10) in the sarcoidosis patients. The frequency of OSA was higher in the IPF patients (p = 0.009). When we compared PSG data of the three groups, only the mean saturation was significantly decreased in the IPF patients (p = 0.03) (Table 3). Snoring was mostly reported in the IPF group (p = 0.04), but it was not related to an OSA diagnosis. Other sleep-related symptoms were not different between the ILD subgroups.
There was no correlation between the disease severity scores and the PSG data in the patients with IPF. However, the disease severity index score was correlated with sleep efficiency, mean oxygen saturation, and time spent with saturation <90 % in the scleroderma patients (r = −0.586, p = 0.01; r = −0.705, p = 0.001; and r = 0.665, p = 0.003, respectively). In the sarcoidosis patients, disease severity was only correlated with time spent with saturation <90 %.
Discussion
Our study revealed a high prevalence of OSA (68 %) in patients with ILD. The prevalence of OSA was 82.3 % in the IPF patients, 55.5 % in the scleroderma patients, and 66.6 % in the sarcoidosis patients. The OSA severity was mostly mild. The respiratory events were predominantly hypopneas rather than apneas. The percentages of sleep efficiency and REM sleep were decreased.
In the literature, there are few studies about sleep-related breathing disorders in ILD [5–9, 22]. The incidence of OSA in these studies was between 17 and 88 % [5–7, 9]. Most of these studies investigated sleep-related breathing disorders in IPF. However, these studies had some methodological problems such as including only patients with IPF and not excluding patients with obesity (BMI ≥ 30) or upper airway pathologies that could cause OSA. Anatomical defects of the upper respiratory tract and obesity are important risk factors for OSA. OSA has been considered to be related to obesity and a high Mallampati classification score in IPF patients [6, 7, 23]. In contrast, Aydogdu et al. studied 37 patients with ILD, and they did not find a relationship between OSA diagnosis and BMI, even though they did not exclude obese patients [5]. Lancaster et al. reported a weak correlation between OSA and BMI in IPF patients. They noted that only 5 of 44 OSA patients had a normal BMI [7]. In addition, Mermigkis et al. found a positive correlation between AHI and BMI in IPF patients [6]. To exclude the possible effects of these factors, we excluded patients with BMI ≥ 30 and upper airway pathologies that could cause OSA. In our study, there was a weak correlation between the AHI and the BMI. In light of these data, the effect of obesity on OSA in ILD patients is still unknown. There may be different mechanisms for OSA in this population other than obesity. The role of inflammation as a factor in OSA has been speculated upon in previous studies [24–27]. Corticosteroid may increase the risk for OSA by causing obesity. Nearly half (n = 24) of our study population was on corticosteroid treatment, but there was no significant difference for OSA diagnosis between patients receiving corticosteroids and patients not receiving corticosteroids. In addition, the AHI and the ODI were similar between these groups. Therefore, it seems that the high incidence of OSA in our study could not be related to corticosteroid usage and obesity. Restrictive pulmonary diseases are characterized by decreased lung volumes that can reduce upper airway stability and increase resistance due to decreased traction on the upper airway. These changes can facilitate upper airway collapse, especially during REM sleep when functional residual capacity is further reduced due to the inactivity of the intercostal muscles [28–30]. A study in 20 rabbits demonstrated that increases in lung volume resulted in caudal tracheal traction that mediated a decrease in upper airway collapsibility due to a decrease in upper airway extraluminal tissue pressure [31]. Based on this theory, pulmonary function impairment has been concluded to be a predictor of OSA in ILD [9]. In the literature, there have been conflicting reports on this subject [6, 7]. In a previous study, a negative correlation between the FVC and the AHI was found [6]. Mermigkis et al. found that the REM AHI was significantly correlated with total lung capacity (p = 0.03, r = −0.38) [32]. In addition, DLCO was correlated with mean oxygen saturation during sleep (p = 0.02, r = 0.39) [32]. The authors concluded that obese IPF patients with significantly decreased pulmonary function parameters may have an increased risk for OSA, particularly during REM sleep [32]. In contrast to these findings, Lancaster et al. did not find any correlation between FVC, lung volumes, and DLCO [7]. Moreover, we did not find any relationship between the AHI and pulmonary function parameters in our nonobese IPF, sarcoidosis, and scleroderma patients, which supports the data of Lancester et al. A technical problem may explain these results. The respiratory function tests were applied in a sitting position when patients were awake, but the PSG data are recorded in a lying position during sleep. Thus, these tests were not applied under the same conditions.
The mean predicted FVC percentages and DLCOs of our scleroderma and sarcoidosis patients were normal. The pulmonary function tests in our IPF patients were slightly affected (FVC = 71 ± 21.73 %, DLCO = 55.53 ± 19.33 %). There was no relationship between pulmonary function and the AHI. However, the number of subjects in our study was not sufficient to make a broad inference. This relationship should be evaluated in a larger series. The inclusion of patients with mild disease might be the reason for not finding any relationship between pulmonary function and the AHI. However, the FVC of our IPF patients was similar to previous studies, and the DLCO was slightly higher than in previous studies [6, 7, 32].
The severity of OSA was predominantly found to be mild in ILD patients in previous studies [5, 32]. Aydogdu et al. studied a mixed group with different diagnoses of ILD, and they reported an OSA diagnosis rate of 64.8 % in ILD patients [5]. The severity of OSA was also mostly mild in their study. In a recent study, 34 IPF patients were evaluated and the frequency of OSA was 59 %, and mild OSA was common (44 %) [32]. However, there are two studies in which OSA severity was reported as predominantly severe [6, 7]. Mermigkis et al. reported a 61 % OSA rate in IPF patients, and 45 % of their OSA patients had severe OSA [6]. However, this study was retrospective and included only patients referred to the sleep laboratory due to a high clinical suspicion for OSA. These circumstances could be the reason for the high rate of severe OSA. In addition, Lancaster et al. reported an OSA diagnosis rate of 88 % in their IPF subjects, and 68 % of these patients had severe OSA [7]. In our study, OSA severity was predominantly mild (55.8 %), and the prevalence of mild OSA was 50 % (n = 7) in the IPF patients, 55.5 % (n = 5) in the scleroderma patients, and 70 % (n = 7) in the sarcoidosis patients.
Disturbance of sleep architecture and the increase in OSA in ILD especially occur during REM sleep [6, 7, 10, 32, 33]. REM-related sleep apnea was found in 52.9 % of the patients with OSA. This high rate is consistent with previous reports [6, 7].
Previous studies have mostly investigated OSA only in IPF patients [6, 7, 32]. The remaining studies included a mixed population with different diagnoses of ILD in small numbers [5, 8]. Therefore, in these studies, there was no chance to compare the data from different types of ILD. Our study was designed to investigate OSA not only in patients with IPF, a disease affecting only the lungs, but also scleroderma and sarcoidosis, which affect multiple organ systems. Therefore, we had the chance to compare different types ILD. Similar to our study, Aydogdu et al. included different diagnoses of ILD such as IPF (n = 18), sarcoidosis (n = 7), and other interstitial lung diseases (n = 12) in small numbers, and they reported an OSA diagnosis rate of 64.8 % in ILD patients [5]. Aydogdu et al. compared the PSG findings from patients with IPF and a mixed group of different ILDs. There were only seven patients with sarcoidosis in their population, but there were no patients with scleroderma. They did not find any differences in the PSG data between IPF and the other diagnoses [5]. When we compared patients with IPF and the other study populations (patients with scleroderma and sarcoidosis with pulmonary involvement), the frequency of OSA was higher in our IPF patients. The mean saturation was found to be decreased in IPF patients. Nocturnal hypoxemia in IPF could be explained by the fibrosis in the alveolar–arterial gas transfer unit. The IPF patients were predominantly male, while scleroderma and sarcoidosis patients were predominantly female in our study. The mean age of the IPF patients was higher than that of the scleroderma and sarcoidosis patients. Being male and older could explain the higher frequency of OSA in our IPF patients. However, the frequency of OSA was also high in our scleroderma and sarcoidosis patients with parenchymal involvement which consisted mostly of nonobese, middle-aged women with low ESS scores. Previously, sleep apnea was reported to be frequent in sarcoidosis patients, possibly due to factors such as steroid usage, neurosarcoid, or upper airway obstruction [10, 23, 34]. In a study by Turner et al., OSA was reported to occur in 17 % of sarcoidosis patients, which was significantly higher than the prevalence in controls [10]. In our scleroderma group, a 55 % diagnosis rate of OSA was found. In a previous study, decreased sleep efficiency and REM sleep and increased AI and slow-wave sleep were reported in scleroderma patients, but the frequency of OSA was not found to be increased [35]. There are no other studies in the literature against which to compare our data in scleroderma patients.
Another factor that differentiates our study from previous studies is the evaluation of the relationship between the AHI and the disease severity of ILD. There is only one study that evaluated the relationship between the AHI and the disease severity of ILD [5]. In that study, the authors combined several clinical, radiological, and physiological parameters and created a scoring system (the CRP scoring system). They did not find a relationship between their CRP scoring system and the AHI. In our study, we created a disease severity index and evaluated the relationship between this severity index and the AHI. Similar to that previous study, we did not find a relationship between the AHI and disease severity. In contrast, our findings showed that the frequency of OSA increased when the disease severity score was higher than 3. Additionally, the severity index score was inversely correlated with sleep efficiency, the percentage of REM sleep, and mean oxygen saturation levels and positively correlated with the time spent with saturation <90 %. There was no correlation between the disease severity score and the PSG data in patients with IPF. However, the disease severity index score was correlated with sleep efficiency, the mean oxygen saturation level, and the time spent with saturation <90 % in scleroderma patients. In sarcoidosis patients, the disease severity was only correlated with the time spent with saturation <90 %.
Additionally, the AHI and ODI were significantly higher in subjects with diffuse radiological involvement in our study. In the English language literature, we could not find any study that had evaluated the relationship between PSG parameters and radiological involvement in ILD.
Krishnan et al. reported excessive daytime sleepiness and worse sleep efficiency in IPF patients [36]. However, they did not confirm this finding by PSG. In our study, sleep efficiency was decreased according to the PSG data, especially in IPF patients. The ESS scores did not differ between patients with or without OSA, and they were also not correlated with the AHI in our study, which is in agreement with the results of Lancester et al. [7].
One of the important limitations of our study is that we could not repeat PSG to minimize the first-night effect of PSG. Another limitation is in the comparison of sarcoidosis patients and IPF patients because there is too much disparity between the two populations with respect to their sex ratios and mean ages that may affect the frequency of OSA. However, in our study population, only the sex ratio and mean age were statistically different between patients with IPF and patients with sarcoidosis.
In conclusion, although we excluded obese patients and patients with upper airway pathologies causing OSA, we found a high frequency of OSA in patients with ILD. Respiratory disturbance was worse during REM sleep. A high disease severity index score was related to an OSA diagnosis. A higher AHI was related to diffuse radiological involvement. The ODI was high in our study group, especially in IPF and sarcoidosis patients. In light of these data, we suggest that PSG studies should be performed in patients with radiologically or clinically advanced ILD. Such data may facilitate early recognition of clinically significant OSA, while treatment may reduce morbidity and improve quality of life.
References
American Academy of Sleep Medicine (2005) International classification of sleep disorders, 2nd ed: diagnostic and coding manual. American Academy of Sleep Medicine, Westchester
Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5:263–276
Rasche K, Orth M (2009) Sleep and breathing in idiopathic pulmonary fibrosis. J Physiol Pharmacol 5:13–14
Pitsiou G, Bagalas V, Boutou A, Stanopoulos I, Argyropoulou-Pataka P (2013) Should we routinely screen patients with idiopathic pulmonary fibrosis for nocturnal hypoxemia? Sleep Breath. pmid:22562264/doi:10.1007/s11325-012-0716-0.
Aydogdu M, Ciftci B, Guven S, Ulukavak CT, Erdogan Y (2006) Assessment of sleep with polysomnography in patients with interstitial lung disease. Tuberk Toraks 54:213–221
Mermigkis C, Chapman J, Golish J, Mermigkis D, Budur K, Kopanakis A, Polychronopoulos V, Burgess R, Foldvary-Schaefer N (2007) Sleep-related breathing disorders in patients with ıdiopathic pulmonary fibrosis. Lung 185:173–178
Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA (2009) Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest 136:772–778
Bye PT, Issa F, Berthon-Jones M, Sullivan CE (1984) Studies of oxygenation during sleep in patients with interstitial lung diseases. Am Rev Respir Dis 129:27–32
Turner GA, Lower EE, Corser BC, Gunther KL, Baughman RP (1997) Sleep apnea in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 14:61–64
Aronson RM, Carley DW, Onal E, Wilborn J, Lopata M (1991) Upper airway muscle activity and thoracic volume dependence of upper airway resistance. J Appl Physiol 70:430–438
Midgren B, Hansson L, Eriksson L, Airikkala P, Elmqvist D (1987) Oxygen desaturation during sleep and exercise in patients with interstitial lung disease. Thorax 42:353–356
McNicholas WT, Coffey M, Fitzgerald MX (1986) Ventilation and gas exchange during sleep in patients with interstitial lung disease. Thorax 41:777–782
Midgren B (1990) Oxygen desaturation during sleep as a function of the underlying respiratory disease. Am Rev Respir Dis 141:43–46
American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Intertitial Pneumonias (2002) Am J Respir Crit Care Med 165:277–304
Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23:581–590
Khosla T, Lowe FR (1967) Indices of obesity derived from body weight and height. Br Soc Med 21:122–128
American Thoracic Society (1995) Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 152:1107–1136
ATS statement (2002) Guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–117
Polysomnography Taske Force, American Sleep Disorders Association Standards of Practice Committee (1997) Practice parameters for the indications for polysomnography and related procedures. Sleep 20:406–422
Iber C, Ancoli-Israel S, Stuart Quan M (2007) The American Academy of Sleep Medicine manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. American Academy of Sleep Medicine, Westchester
Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y (2012) Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 114:993–1000
Drent M, Verbraecken J, van der Grinten C, Wouters E (2000) Fatigue associated with obstructive sleep apnea in a patient with sarcoidosis. Respiration 67:337–340
Poulain M, Doucet M, Major GC, Drapeau V, Sériès F, Boulet LP, Tremblay A, Maltais F (2006) The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ 174:1293–1299
Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH (2008) Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117:2270–2278
Depalo A, Carpagnano GE, Spanevello A, Sabato R, Cagnazzo MG, Gramiccioni C, Foschino-Barbaro MP (2008) Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med 263:70–78
Carpagnano GE, Spanevello A, Sabato R, Depalo A, Palladino GP, Bergantino L, Foschino Barbaro MP (2010) Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res 155:35–43
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2002) Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 122:1162–1167
Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, Hoffstein V (1986) Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med 315:1327–1331
Sériès F, Cormier Y, Lampron N, La Forge J (1988) Increasing the functional residual capacity may reverse obstructive sleep apnea. Sleep 11:349–353
Heinzer RC, StanchinaML MA, Fogel RB, Patel SR, Jordan AS, Schory K, White DP (2005) Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 172:114–117
Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC (2007) Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep 30:179–186
Mermigkis C, Stagaki E, Tryfon S, Schiza S, Amfilochiou A, Polychronopoulos V, Panagou P, Galanis N, Kallianos A, Mermigkis D, Kopanakis A, Varouchakis G, Kapsimalis F, Bouros D (2010) How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath 14:387–390
Brian BK, Jesse D, Octavian I (2008) The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath 12:259–264
Verbraecken J, Hoitsma E, van der Grinten CP, Cobben NA, Wouters EF, Drent M (2004) Sleep disturbances associated with periodic leg movements in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 21:137–146
Prado GF, Allen RP, Trevisani VM, Toscano VG, Earley CJ (2002) Sleep disruption in systemic sclerosis (scleroderma) patients: clinical and polysomnographic findings. Sleep Med 3:341–345
Krishnan V, McCormack MC, Mathai SC, Agarwal S, Richardson B, Horton MR, Polito AJ, Collop NA, Danoff SK (2008) Sleep quality and health-related quality of life in idiopathic pulmonary fibrosis. Chest 134:693–698
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pihtili, A., Bingol, Z., Kiyan, E. et al. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath 17, 1281–1288 (2013). https://doi.org/10.1007/s11325-013-0834-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-013-0834-3