Abstract
Aim
Obstructive sleep apnea syndrome (OSAS) is characterized by recurrent respiratory disorders in the upper airways during sleep. Although continuous positive airway pressure (CPAP) has been accepted to be the most effective treatment for OSAS, its role on inflammation remains debatable. In this study, our aim was to examine the influence of 3 months of CPAP treatment on tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), 8-isoprostane, and peroxynitrite levels in exhaled breathing condensates (EBC) and serum.
Methods
Thirty-five patients who were newly diagnosed as moderate or severe OSAS with full night polysomnography and used CPAP therapy regularly for 3 months were included in the study. Polysomnography, spirometric tests, fasting blood samples, and EBC were ascertained on entry into the study and after 3 months of treatment. All patients were assessed monthly for treatment adherence and side effects.
Results
We found that all polysomnographic parameters were normalized after CPAP therapy in the control polysomnogram. Also, all markers in EBC and nitrotyrosine and 8-isoprostane levels in serum were decreased significantly with CPAP treatment. Sedimentation rate, C-reactive protein, IL-6, and TNF-α remained unchanged in serum after treatment. We found that baseline nitrotyrosine levels were significantly correlated with apnea–hypopnea index, oxygen desaturation index, and percent time in SpO2 < 90 % (p < 0.01).
Conclusions
CPAP therapy has primarily a relevant impact on airways, and nitrotyrosine levels correlated well with severity of OSAS. This treatment decreases both inflammation and oxidative stress levels in airways in OSAS patients. Also, this treatment helps to decrease systemic oxidative stress levels in serum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS), which is characterized by repetitive complete or partial upper airway collapse occurring during sleep, is a common disorder affecting 4 % of men and 2 % of women in the general population [1]. The health impact of obstructive sleep apnea is enormous. Chronic intermittent hypoxia, increased sympathetic activation, enhanced state of inflammation, oxidative stress, and endothelial dysfunction are potential pathological mechanisms leading to OSAS-related mortality and morbidity [2, 3]. Recent evidence suggests that OSAS could be considered as a pro-atherosclerotic disease, independent of visceral fat amount [4]. Intermittent episodes of hypoxia as a result of transient cessation of breathing during sleep are major physiologic characteristic of OSAS and resemble ischemia–reperfusion injury. Intermittent nocturnal hypoxemia induces the production of oxygen-free radicals and therefore causes a state of low grade circulation and local inflammation [5]. Cell culture and animal studies have shown an increased inflammation in OSAS. Foresi et al. [6] showed the development of airway inflammation during sleep by measurement of airway inflammation and oxidative stress markers as pentane and exhaled nitric oxide in exhaled air of OSAS patients. Both Alberti et al. [7] and Ciftci et al. [8] pointed out increased levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in patients with sleep apnea compared to those in normal control subjects.
Current modalities of effective OSAS management include the maintenance of an adequate airflow during sleep using continuous positive airway pressure (CPAP), oral appliances, and curative surgery in the case of anatomical abnormalities [9, 10]. Although the most effective treatment for OSAS is CPAP, which acts as pneumatic splint, keeping the airway open during sleep, its effect on local and systemic inflammation is debatable. Skoczyński et al. [11] found that CPAP treatment caused a statistically significant rise of total inflammatory cell count in nasal lavage of OSAS patients, when compared with initial values. The compression applied by CPAP on the nasal wall can cause a mechanical stimulus that triggers inflammation. In another study, Kohler et al. [12] observed that 4 weeks of CPAP treatment has no beneficial effect on blood markers of inflammation and adiponectin in patients with moderate to severe obstructive sleep apnea. On the other hand, Fortuna et al. [13] showed decreased airway nitric oxide levels, reflecting the correction of upper airway inflammation after CPAP treatment. Similarly, Tamaki et al. [14] indicated that production of TNF-α by monocytes was significantly decreased after therapy. While improvements have been demonstrated, they are not always certain and the effect of CPAP on airway and systemic inflammation remains unclear. The aim of the study was to evaluate the influence of 3 months of CPAP treatment on both inflammation and oxidative stress markers in airway and serum.
Materials and methods
Subjects and methods
All consecutive 125 patients who were referred for overnight polysomnography because of symptoms of sleep apnea between January 2010 and June 2010 were included in the study. All of the patients’ polysomnography tests were performed in the same unit. All CPAP titrations were determined with full polysomnography. We included 35 patients who were diagnosed with moderate to severe OSAS with apnea hypopnea index ≥15 and used their CPAP treatment regularly with good adherence. CPAP adherence was defined as usage of this therapy more than 5 days in a week and more than 4 h nightly. Polysomnography, spirometry test, fasting blood samples, and exhaled breath condensates (EBC) were ascertained on entry into the study and after 3 months of treatment. All patients were assessed monthly for treatment adherence and side effects. Exclusion criteria were (1) patients who required nasal or other upper airway surgeries for the treatment or usage of CPAP; (2) patients who did not use their CPAP regularly; (3) patients with history of rheumotological or other systemic inflammatory diseases; (4) usage of inhaled, nasal or systemic corticosteroids, or other antiinflammatory drugs; and (5) history of asthma, COPD, rhinitis, or atopy. This was a prospective case control study. Informed consent was obtained from the patients and this study was approved by the ethics committee of our university.
Polysomnographic evaluation
Overnight polysomnography was recorded with a Grass polysomnograph (model PSG36-2, West Warwick, RI, USA), which recorded the following parameters: electrocardiogram, central, temporal and occipital electroencephalogram, bilateral electrooculogram, submental and anterior tibialis electomyogram, nasal airflow using a nasal cannula and pressure transducer, nasal–oral airflow using a thermistor, and respiratory effort using chest and abdominal piezoelectric belts. The electromyelogram, electrooculogram, and electroencephalogram leads were applied according to the International 10/20 electrode placement system. Oxyhemoglobin saturation was monitored using a pulse oximeter (Biox 3740; Ohmeda, Louisville, CO, USA). Sleep stages were scored according to the criteria of the American Academia of Sleep Medicine [15]. Apneas were defined as decrements in airflow ≥90 % from baseline for ≥10 s. Hypopneas were defined as a 30 % or greater decrease in flow lasting at least 10 s and associated with a 4 % or greater oxyhemoglobin desaturation. The number of apneas and hypopneas per hour of sleep was calculated to obtain the apnea–hypopnea index (AHI). Respiratory events were derived primarily from the nasal cannula pressure transducer. The oxygen desaturation index (ODI) was defined as the total numbers of episodes of oxyhemoglobin desaturation ≥4 % from the immediate baseline, ≥10 s but <3 min, divided by the total sleep time. OSAS severity was assessed as mild, moderate, and severe according to the AHI values of 5–14, 15–29, and more than 30, respectively.
Spirometry tests and measurements
All the participants’ height and weight were measured by the same person using the same equipment. Weight was measured by using a calibrated hospital scale with subjects dressed in normal indoor clothing without shoes. Height was measured against a wall using a fixed tape measure with subjects standing barefoot on a hard surface in centimeters. Body mass index (BMI) was calculated by dividing body weight to height square (kilogram per square meter). Patients were evaluated into two groups according to their BMI: BMI ≤ 29.9 (group 1, nonobese) and BMI ≥ 30 (group 2, obese).
Pulmonary function tests were performed by the standard method using a dry rolling seal spirometer to exclude patients with pulmonary diseases. Three technically adequate maneuvers were required and the best values for forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were accepted. Lung function spirometry and bronchodilator tests were performed before and after inhalation of salbutamol following international guidelines. A FEV1 > 80 % of predicted and/or a ratio of FEV1 to FVC > 70 % were considered to lie within normal limits in this clinical setting. Patients who presented spirometric values below these cutoffs were excluded.
Exhaled breath condensates and fasting serums
EBC was collected over 10–15 min of quiet breathing using a condenser EcoScreen (Jaeger, Germany), according to standard protocol using a nasal clip. After rinsing their mouths, the recruited subjects breathed tidally through a mouthpiece that was connected through a unique one-way valve into a cooled collection tube where vapors, aerosols, and moisture in the breath condensed along the walls of the tube. The design of the system prevented salivary contamination of EBC. Each subject was asked to breathe through the device, while wearing a nose clip, for 10 min, and more than 1 ml of EBC could be collected from each subject. EBC was transported to the analytical laboratory in tightly closed and cooled containers and stored at −70 °C until analysis/further examination. EBC samples and fasting sera were collected in the morning of the day from 9 to 10 a.m. Concentrations of IL-6, TNF-α, 8-isoprostane, and nitrotyrosine were determined by two-site sandwich quantitative enzyme-linked immunosorbent assay using commercially available kits (Chemikline). The marker concentration was expressed in picogram per milliliter. The concentration of all markers in the samples was calculated by comparison to the curve obtained with different concentrations of standards included in each kit. Tests were done twice on each sample for validation.
Statistical analysis
Parametric data are expressed as mean ± SEM. Parametric data were compared using Student’s t test. Nonparametric data comparisons were made using Mann–Whitney U test between the two groups. Comparisons between baseline and end of treatment data were made using Wilcoxon’s signed rank test (two-tailed). Correlations between different parameters were tested with Spearman’s rank correlation test. A p value of <0.05 was considered significant for all analyses, which were performed with SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total 125 patients had polysomnography test during the study period and 78 of them were diagnosed as moderate to severe OSAS. Eight of them had chronic obstructive pulmonary diseases, 2 of them had allergic rhinitis, and 17 of them were offered nasal surgery, and therefore, 51 patients were included in the study. CPAP treatment was started, and at the end of final control, we determined that 11 patients on auto-CPAP and 24 patients on CPAP therapy were using their machine at least five nights in a week and more than 4 h nightly at the end of final control. So the results presented were an analysis of 35 subjects (21 men), with a mean age 52.5 ± 10.3 years. According to the records that were gathered from the PAP device, mean usage was 5.8 (range varies between 4.2 and 7.7) h/nightly and mean usage was 5.7 (range varies between 5.1 and 7.0) days/week. Mean CPAP pressures were 10.7 ± 2.2 (range varies between 7.6 and 14.1) cmH2O.
As expected, we found that all polysomnographic parameters were abolished after CPAP therapy in control polysomnogram (Table 1). The changes in percent sleep stages (stages 1, 2, and 3 and REM) before and after CPAP therapy in polysomnogram were 12.7 ± 2.3 vs 6.5 ± 2.1, 58.9 ± 5.9 vs 50.7 ± 3.5, 14.4 ± 4.2 vs 22.1 ± 3.2, and 12.3 ± 3.8 vs 19.8 ± 2.9, respectively.
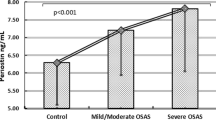
After the treatment period, we found that all of the marker levels changed significantly in EBC (Table 2). The mean nitrotyrosine and 8-isoprostane levels were also changed significantly in serum, but we could not find any significant differences for the other markers in serum. Sedimentation rates before and after treatment were 21.6 ± 20.55 and 17.9 ± 16.1 mm/h and C-reactive protein values before and after treatment were 8.3 ± 8.5 and 6.17 ± 4.3, respectively (p > 0.05) (Table 3). We found that baseline nitrotyrosine levels in EBC were significantly correlated with mean ODI (r = 0.47; p = 0.004) and total sleep time below saturation 90 % (minutes) (r = 0.5; p = 0.002) (Fig. 1).
Discussion
The inflammation in airways is believed to be the consequence of the repeated mechanical trauma related to the obstruction and vibration of the airway and forceful inspiratory effort against a closed airway passage during apneas. Furthermore, reoxygenation after a brief period of hypoxia may predispose to cell stress by increased oxidative stress and inflammation. Activation of proinflammatory cytokines in the upper airway can promote abnormalities in upper airway reflexes, upper airway collapsibility, and pharyngeal muscle dysfunction and amplify upper airway narrowing thereby worsening the frequency and duration of apneas during sleep [16, 17]. These unfavorable processes may increase the severity of OSAS setting up a vicious cycle. So understanding the influence of treatment in the basis of both intermittent hypoxia and inflammation within airways and serum could provide insight into pathophysiological pathways for OSAS consequences. Guasti et al. [18] pointed out that in patients showing similar cardiovascular risk factors, cytokine production from peripheral blood mononuclear cells and polymorphonuclear leukocytes was not modified during CPAP therapy. Devouassoux et al. [19] demonstrated that inflammatory pattern in induced sputum remained unchanged, and an airway hyperresponsiveness was increased after 1 month of CPAP therapy. Further, Vgontzas et al. [20] showed that CPAP decreased daytime sleepiness and blood pressure (p < 0.05), but did not affect fasting glucose, insulin, adiponectin, IL-6, TNF-α, or TNF-r1 levels. However, Mota et al. [21] observed a significant decrease in blood pressure and serum triglyceride levels in patients with less severe OSAS and with a better therapeutic compliance to automatic CPAP. Also, Hegglin et al. [22] found that long-term CPAP positively affects TNF-α levels even in men with poor adherence to CPAP. Yokoe et al. [23] showed a significant reduction in serum levels of C-reactive protein (CRP) and IL-6 of 30 OSAS patients when compared with 14 obese controls after 1 month of treatment with CPAP. Carpagnano et al. [24] reported higher levels of IL-6 and 8-isoprostane in the EBC of 18 patients with OSAS when compared with 10 obese subjects and 15 healthy subjects. After treatment with CPAP for two nights within 1 week, they found a significant reduction of 8-isoprostane in EBC. Similarly, we also found that CPAP decreased all inflammatory and oxidative stress markers in EBC but just nitrotyrosine and 8-isoprostane levels in serum. The reason for these conflicting results could be related to CPAP adherence and sampling methods. Weaver et al. [25] proved that effectiveness of CPAP treatment rises with increased hours of use. Four hours of CPAP use per night was proven effective for impaired OSAS patients in order to achieve normal scores in the Epworth Sleepiness Scale. In our study, we only included patients with good CPAP adherence. We preferred to measure markers in EBC as it is a noninvasive method for studying the composition of airway lining fluid and also useful for monitoring and assessing the efficacy of therapy with various inflammatory and oxidative stress biomarkers in the respiratory tract [26, 27].
Reactive oxygen species damages cell membranes mainly through a process called lipid peroxidation. These compounds are also implicated in ischemia–reperfusion injury that occurs following repletion of oxygen or restoration of blood flow to tissue [28]. Previous reports have shown a positive relationship between severity of OSAS and inflammatory markers. Petrosyan et al. [29] found that 8-isoprostane and LTB4 in EBC are positively correlated with the apnea–hypopnea index. Devouassoux et al. [19] demonstrated IL-8 in sputum supernatant was correlated to AHI. In our study, we found that nitrotyrosine levels in EBC were significantly correlated with mean ODI and desaturation values. All our patients had AHI greater than 15 and we only included moderate to severe OSAS patients. So we thought that with more heterogeneous group, the correlations could be more obvious.
A possible limitation of our study is that it was not a randomized controlled trial. However, such a study is difficult to perform, since it would be unethical to leave patients with confirmed OSAS untreated. We prefer to compare values before and after treatment. Our patient number is relatively small but this is because of our strict inclusion criteria. We tried to exclude all comorbid and cofounding factors. The strength of our study is that we compare multiple inflammatory and oxidative stress markers in both airways and serum.
In conclusion, a minimum 4-h usage of CPAP treatment nightly decreases all inflammatory and oxidative parameters significantly in the airways and just oxidative stress markers in serum in moderate to severe OSAS patients. CPAP therapy has a relevant impact upon inflammation especially in airways and has a marginal effect on some parameters of systemic inflammation in moderate to severe OSAS patients. Exhaled breath techniques in OSAS provide an easy, noninvasive way to detect or monitor local upper airway inflammation, and nitrotyrosine levels in EBC provide further evidence for severity of OSAS.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Kohler M, Stradling JR (2010) Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7:677–685
Ryan S, Taylor CT, McNicholas WT (2009) Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med J 85:693–698
Quercioli A, Mach F, Montecucco F (2010) Inflammation accelerates atherosclerotic processes in obstructive sleep apnea syndrome (OSAS). Sleep Breath 14:261–269
Takama N, Kurabayasi M (2009) Influence of untreated sleep-disordered breathing on the long-term prognosis of patients with cardiovascular disease. Am J Cardiol 103:730–734
Foresi A, Leone C, Olivieri D, Cremona G (2007) Alveolar-derived exhaled nitric oxide is reduced in obstructive sleep apnea syndrome. Chest 132:860–867
Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, Gallai V (2003) Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res 12:305–311
Ciftci TU, Kokturk O, Bukan N, Bilgihan A (2004) Relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 21:87–91
Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J Jr, Friedman L, Kapen S, Kapur VK, Kramer M, Lee-Chiong T, Owens J, Pancer JP, Swick TJ, Wise MS (2006) American Academy of Sleep Medicine Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 29:375–380
Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Owens J, Pancer JP (2006) American Academy of Sleep Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. Sleep 29:240–243
Skoczyński S, Ograbek-Król M, Tazbirek M, Semik-Orzech A, Pierzchała W (2008) Short-term CPAP treatment induces a mild increase in inflammatory cells in patients with sleep apnoea syndrome. Rhinology 46:144–150
Kohler M, Ayers L, Pepperell JC, Packwood KL, Ferry B, Crosthwaite N, Craig S, Siccoli MM, Davies RJ, Stradling JR (2009) Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax 64:67–73
Fortuna AM, Miralda R, Calaf N, González M, Casan P, Mayos M (2011) Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med 105:630–636
Tamaki S, Yamauchi M, Fukuoka A, Makinodan K, Koyama N, Tomoda K, Yoshikawa M, Kimura H (2007) Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern Med 48:1255–1262
Iber C, Ancoli-Israel S, Chesson A, Quan SF (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. American Academy of Sleep Medicine, Westchester
McNicholas WT (2009) Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis 51:392–399
Salerno FG, Carpagnano E, Guido P, Bonsignore MR, Roberti A, Aliani M, Vignola AM, Spanevello A (2004) Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med 98:25
Guasti L, Marino F, Cosentino M, Maroni L, Maresca AM, Colombo F, Maio RC, Castiglioni L, Saporiti F, Loraschi A, Gaudio G, Bernasconi A, Laurita E, Grandi AM, Venco A (2011) Cytokine production from peripheral blood mononuclear cells and polymorphonuclear leukocytes in patients studied for suspected obstructive sleep apnea. Sleep Breath 15:3–11
Devouassoux G, Lévy P, Rossini E, Pin I, Fior-Gozlan M, Henry M, Seigneurin D, Pépin JL (2007) Sleep apnoea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol 119:597–603
Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, Pejovic S, Chrousos GP (2008) Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest 38:585–595
Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA (2011) APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 15:665–672
Hegglin A, Schoch OD, Korte W, Hahn K, Hürny C, Münzer T (2012) Eight months of continuous positive airway pressure (CPAP) decrease tumor necrosis factor alpha (TNFA) in men with obstructive sleep apnea syndrome. Sleep Breath 16:405–412
Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M (2003) Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107:1129–1134
Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V, Foschino MP (2008) Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res 151:45–50
Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI (2007) Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30:711–719
Hunt J (2007) Exhaled breath condensate: an overview. Immunol Allergy Clin North Am 27:587–596
Rosias PP, Robroeks CM, Kester A, den Hartog GJ, Wodzig WK, Rijkers GT, Zimmermann LJ, van Schayck CP, Jöbsis Q, Dompeling E (2008) Biomarker reproducibility in exhaled breath condensate collected with different condensers. Eur Respir J 31:934–942
Yamauchi M, Kimura H (2008) Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complication. Antioxid Redox Signal 10:735–768
Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E, Roussos C, Gratziou C (2008) Exhaled breath markers in patients with obstructive sleep apnea. Sleep Breath 12:207–215
Acknowledgments
This study is supported by Fatih University Scientific Research Fund under project no. P53011005_2.
Conflict of interest
All authors have indicated no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karamanlı, H., Özol, D., Ugur, K.S. et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath 18, 251–256 (2014). https://doi.org/10.1007/s11325-012-0761-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-012-0761-8