Abstract
Purpose
Continuous positive airway pressure (CPAP) is the therapy of choice for the treatment of obstructive sleep apnea (OSA). Not all patients can use CPAP therapy with adequate compliance. There is a need to develop more comfortable modes. Auto bi-level Pressure Relief-Positive Airway Pressure (ABPR-PAP) can be an alternative. We conducted a prospective double-blind, randomised trial to evaluate the efficacy and compliance of ABPR-PAP compared with CPAP in OSA patients.
Methods
We included 35 CPAP naive patients (age 53.3 ± 10.3 years, BMI 31.0 ± 5.0 kg/m2, ESS 10.0 ± 4.2) diagnosed with moderate to severe OSA who underwent a successful CPAP titration. Patients were randomised into the CPAP or the ABPR-PAP treatment group. We used the same device (BIPAP® Auto, Philips Respironics) for CPAP or ABPR-PAP. Apnea–hypopnea index (AHI) was determined using polysomnography before (AHI 40.6 ± 18.3 per hour) and after treatment.
Results
Eighteen patients received CPAP and the remaining 17 received APBR-PAP. Groups were similar in terms of demographics and OSA severity. There were no serious adverse events during the trial. CPAP was fixed by a sleep expert and ABPR-PAP varied (range 5–15 cmH2O). AHI decreased in the CPAP group to 6.4 ± 5.7 per hour and in the ABPR-PAP group to 4.8 ± 3.6 per hour in the first night (N = 35). After 3 months, the AHI decreased in the CPAP group to 4.4 ± 5.3 per hour and in the ABPR-PAP group to 2.6 ± 3.8 per hour (N = 32). Differences between the groups were not statistically significant. There were no differences in compliance.
Conclusions
ABPR-PAP is a promising new ventilation mode that enables effective treatment of OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasal continuous positive airway pressure (CPAP) is the gold standard treatment for patients with obstructive sleep apnea syndrome (OSAS). CPAP eliminates nocturnal breathing difficulties, enhances sleep quality, reduces self-reported sleepiness, improves quality of life and reduces cardiovascular risk [1, 2]. Despite the proven value of CPAP, compliance has been reported to be low [3, 4]. Predictors of poor compliance include the development of side effects such as pressure intolerance, mouth dryness or nasal congestion.
Technical advances in positive airway pressure (PAP) therapies have led to the development of new modes of therapy such as automatic and pressure relief technologies. Although these technologies have been shown to be safe and effective, no compliance or clinical benefits have been demonstrated in CPAP-naive patients [5–7].
Bi-level PAP has been shown to be an effective alternative to CPAP in patients with restrictive lung disease, Cheyne–Stokes respiration, nocturnal hypoventilation syndromes and in OSAS patients with pressure intolerance [8–10].
Automatic bi-level therapy with pressure relief (ABPR-PAP: BiPAP® auto with Bi-Flex®; Philips Respironics Inc. Murrysville, PA, USA) is a new automatic mode of bi-level therapy that reduces the inspiratory pressure at end inspiration and the expiratory pressure during early expiration in relation to flow. These pressure adjustments may further enhance comfort during the transition from the inspiratory to expiratory phase and reduce expiratory effort. An automatic, flexible mode of bi-level therapy is a combination of validated technologies already being used in routine clinical practice, and has been shown to be as effective as CPAP at treating OSA in non-compliant patients [11].
We hypothesised that ABPR-PAP would be as effective as standard CPAP in treating OSAS, and that CPAP-naive patients using ABPR-PAP chronically would demonstrate higher compliance than those receiving CPAP. In order to test this, we conducted a prospective randomised double-blind comparison study comparing the efficacy of the ABPR-PAP against standard CPAP and subjective and objective compliance over 90 days of use in CPAP-naive OSAS patients.
Methods
Subjects
Trial characteristics can be found in Fig. 1. This study was approved by the ethics committee from Charité University Hospital. Prior to their inclusion, patients with suspected sleep apnea referred to our outpatient department underwent an unattended ambulatory monitoring (not in hospital with no medical professional present) using a portable diagnostic system (Embletta®, Flaga hf, Reykjavik, Iceland). Those with an apnea–hypopnea index (AHI) of more than 10 per hour, with symptoms of excessive sleepiness and those with an AHI >20 per hour were subsequently referred for attended cardio-respiratory polysomnography (PSG) in the sleep laboratory.
Thirty-five patients (34 men and 1 woman; age 54.2 ± 11.7 years; mean BMI 30.9 ± 5.7 kg/m²) from the Center of Sleep Medicine, Charité-Universitätsmedizin Berlin aged 18 to 75 years with an AHI ≥15 per hour on PSG, BMI <45 kg/m², the ability to follow the study specific instructions and a willingness to return for follow-up were consented into the study. Patients were excluded if they had another sleep disorder such as narcolepsy, an acute or chronic cardiac or pulmonary disorder, acute or chronic psychiatric or neurological disorder or were abusing sleep-inducing agents, alcohol or drugs. Patients who had previously used an OSA treatment, an inability to wear a nasal mask due to claustrophobia or facial/anatomic abnormalities and patients with suspected or confirmed central sleep apnea syndrome were also excluded.
Titration and polysomnography
After a single diagnostic night, patients underwent a daytime CPAP training session at a constant pressure of 5 cmH2O lasting 10 to 20 min. Several different masks and models of interface were applied to find the optimum. A manual titration was performed on the subsequent night, during which pressure was increased in increments of 1 cmH2O from a baseline level of 5 cmH2O in response to the occurrence of apneas, hypopnoeas, oxygen drops below 3% and respiratory-related arousals until the effective pressure was reached. Following a successful titration (AHI <15 per hour), patients were randomised to one of two treatments for a 3-month period, ABPR-PAP or manually titrated CPAP.
PSG was performed during the manual titration on therapy immediately after randomisation and after 12 weeks of therapy using the Embla® (Flaga hf, Reykjavik, Iceland) and Alice 5® (Philips Respironics, Inc Murrysville, PA, USA) systems. Sleep stages were scored according to Rechtschaffen and Kales by two experts to minimize intra-scorer variability [12]. Every recording was scored just by one scorer, randomly selected. The following PSG parameters were calculated: sleep latency, total sleep time (TST), sleep efficiency and the percent of TST in sleep stages NREM-I, NREM-II, SWS and REM. Arousals were scored with durations of 3 to 15 s according to the ASDA criteria [13]. Apneas and hypopneas were scored according to standard criteria [14]. Apnea was defined as a cessation of the oronasal airflow for ≥10 s and hypopnea as a reduction in oronasal airflow or thoraco-abdominal respiratory excursion by at least 50% for ≥10 s, if accompanied by a drop in oxygen of a minimum of 3%, and/or terminated by an arousal.
Expected primary and secondary outcomes
Primary outcome
AHI was measured at diagnosis, titration and 12 weeks of therapy.
Secondary outcomes
Daytime symptoms
Measured by the Epworth sleepiness scale (ESS) and the Pittsburgh sleep quality index (PSQI) before diagnostic PSG and at 1, 4 and 12 weeks of therapy.
Compliance and therapy data
Measured by assessment of hours of therapy and delivered pressure measured at 1 and 12 weeks of therapy.
Blinding
Patients were informed that they would receive one of two different modes of PAP therapy. Both therapy modes were provided by the same device (BiPAP® Auto with Bi-Flex®; Philips Respironics, Inc Murrysville, PA, USA). Devices were set by the study coordinators who de-activated the LCD display so that the patient and investigators did not become aware of device allocation. The study coordinators were also responsible for randomising the patients, training them on the use of the device, downloading the compliance data from the Encore Pro® Smartcard (Philips Respironics, Inc Murrysville, PA, USA) located in the side of the device and troubleshooting. The investigator making and analysing the PSG recordings on therapy and other outcome measures did not have access to information from the therapy device within their PSG montage.
There were no concrete information about characteristics of these different PAP types in the informed consent.
Device
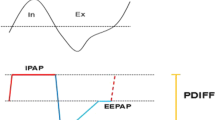
When delivering ABPR-PAP, the pressure regulates from the inspiratory positive airway pressure (IPAP) level to the expiratory positive airways pressure (EPAP) level at end inspiration (normal BiPAP® delivery) and an additional pressure reduction is delivered in relation to flow during early expiration (Bi-Flex®). The pressure reduction may be regulated at a flow-dependent comfort setting of 1, 2 and 3. When set to comfort setting 3, Bi-Flex®softens the pressure transition from inhalation to exhalation the most.
The automatic adjustments of EPAP and IPAPs are based on continuous pneumotachographical measurements and assessments of the respiratory-flow curve. The ABPR-PAP system detects flow limitations, snoring, apneas, hypopneas and leakage. The device adapts EPAP and IPAP therapeutic pressures according to the detected results.
Device setup
Manually titrated CPAP was set at the effective pressure from the titration night. ABPR-PAP devices were set to deliver a minimum EPAP of 5 cmH2O, a maximum IPAP of 15 cmH2O and a minimum delta of 3 cmH2O. The resulting IPAP and EPAP windows were 8–15 and 5–12 cmH2O, respectively. Bi-Flex® pressure relief was set to its maximum level (level 3). Patients were given verbal and written instructions on how to use their equipment before returning home.
Daytime symptoms
The ESS and PSQI were completed before the diagnostic PSG and at 1, 4 and 12 weeks of therapy. The PSQI is a standardized self-administered questionnaire for the assessment of subjective sleep quality. The PSQI showed adequate reliability and good validity [15].
Compliance and therapy data
Assessment of hours of therapy use and the delivered pressure was obtained at 2 and 12 weeks of therapy from the Encore Pro® Smartcard located in the side of the machine.
Follow-up
Subjects were examined at 2 weeks. Twelve patients who had not used their therapy for greater than 4 h per night on at least 70% of nights received standard clinical interventions to improve compliance (education, counseling, change of mask and if not already present, heated humidification). Medications were prescribed to treat allergic or nasal symptoms as appropriate. Patients were also encouraged to call the study coordinator if further difficulties were encountered. Heated humidification was routinely offered during each contact with the patient. Fifteen patients needed heated humidification (8 in the CPAP group, 7 in the ABPR-PAP group).
At the end of the 12-week trial, all subjects returned to have their compliance and pressure data downloaded, complete the ESS and PSQI and undergo a PSG control night unless lost to follow-up. Subjects were then transferred back into clinical practice for the routine management of their OSAS.
Statistical analysis
This study was designed to collect pilot data; therefore, no power calculation was performed. Data was checked for normality and presented as appropriate. We compared PSG parameters at baseline and the two follow-up PSG recordings, and therapy effectiveness (compliance and questionnaires) using paired t tests in SPSS 15.0 (SPSS Inc.).
Results
Thirty-five patients participated in the study: 18 patients received manually titrated CPAP and 17 patients ABPR-PAP. The patients were middle-aged, obese and suffered from severe OSAS. There were no differences between the groups on these variables or their sleep characteristics at baseline (Table 1).
Efficacy
At the first follow-up PSG recording (night 1), the mean AHI decreased to 6.4 ± 5.7 per hour and 4.8 ± 3.6 per hour in the manually titrated CPAP and ABPR-PAP groups, respectively. The difference in AHI between the groups was not statistically significant (p = 0.352) and sleep characteristics were similar. Thirty-two patients completed the 90-day trial, 17 of which received manually titrated CPAP and 15 patients ABPR-PAP. There were three dropouts whom declined to use their PAP device further and did not return for follow-up. Mean follow-up occurred at 92.9 and 89.4 days in the CPAP and ABPR-PAP groups, respectively. Mean therapeutic pressure, in the ABPR-PAP group mean of the 90th percentile, over the study period was 9.5 ± 1.3 cmH2O in the manually titrated CPAP group and 11.7 ± 2.2 cmH2O (IPAP)/8.7 ± 2.2 cmH2O (EPAP) in the ABPR-PAP group. At the second follow-up, PSG recording (week 12), the mean AHI had decreased further in both groups to 4.3 ± 5.3 per hour and 2.5 ± 3.8 per hour. The difference in AHI between the groups was not statistically significant (p = 0.256) and sleep characteristics were similar (Table 2).
Compliance and daytime symptoms
Compliance over the 90-day treatment period was similar between the two groups. Ninety-four percent of patients used their device on at least 80% of days. Compliance was essentially determined within the first 2 weeks of treatment. There were no group mean differences between compliance at 2 weeks versus 90 days for CPAP versus ABPR-PAP, respectively—no differences in percentage of days used (96.4% vs. 95.2%), percentage of days with more than 4 h of use per night (79% vs. 80.5%) and average hours of use per night (5.6 vs. 5.3 h). Seventeen patients required added humidification. The ESS demonstrated statistically significant improvements from baseline to follow-up (p = 0.001) for both groups without in-group differences (Table 3).
Discussion
Improving tolerance and compliance to PAP therapy to reduce symptoms and mortality [16] is one of the main driving forces for the development of new technologies in this field. An automatic, flexible mode of bi-level therapy is a combination of validated technologies already being used in routine clinical practice; however, the efficacy and clinical benefits of these combined technologies has not been previously demonstrated. The data presented shows that in uncomplicated CPAP-naive OSAS patients, ABPR-PAP is as effective as standard CPAP but does not improve compliance or daytime symptoms over a 90-day period.
ABPR-PAP therapy was as effective as manually titrated CPAP on the first night (4.8 ± 3.6 per hour and 6.4 ± 5.7 per hour, respectively) and after 12 weeks of treatment (2.5 ± 3.8 per hour and 4.3 ± 5.3 per hour, respectively). The residual AHI on ABPR-PAP is also similar to that reported on APAP [17, 18] and a Bi-Flex® precursor in a traditional bi-level device [19].
ABPR-PAP did not improve compliance or daytime symptoms over the 90-day treatment period; however, the compliance levels in the CPAP group were already high (>5 h) making it unlikely that the additional comfort of ABPR-PAP would lead to higher compliance and clinical benefits. Further research is required to determine which sub-groups of OSAS patients will benefit most from this therapy. Candidates include those with low compliance due to a high therapeutic pressure (>15 cmH2O) and/or side effects.
It may well be that pressure-related complaints do not occur with great enough frequency to show an overall benefit for a study group of patients that are unselected for this attribute. Complaints of nasal and pharyngeal symptoms and lack of subjective perceived benefit from treatment tend to represent more common reasons for non-compliance [20]. Proponents of PR-CPAP cite the considerable face validity of their hypothesis (patients should tolerate CPAP better if the pressure being exhaled against is lower), but the literature thus far does not consistently support this theory.
A recent meta-analysis [5] demonstrated that APAP treatment does not result in higher compliance than CPAP. By contrast, there are only a limited number of studies investigating the effects of flexible PAP modes. In his pioneering study, Aloia and co-workers showed higher compliance in CPAP-naive OSAS patients using C-Flex® over a 3-month period that equated to 1.7 h greater use per night [21]; however, this was not a randomised study as patients received the mode of therapy that was available at the time of therapy allocation. By contrast, Nilius et al. in the first randomised controlled trial investigating the effects of C-Flex® against CPAP in PAP-naive patients did not find any differences in objective or subjective compliance over 7 weeks of usage, although patients in the C-flex group reported an improvement in oral dryness [6]. A third study was recently published examining auto-titrating CPAP with and without pressure relief in experienced CPAP users [22] There was a subjective preference for the pressure relief mode in this group of patients but only a non-significant trend in terms of greater subjective comfort with the pressure relief modality. Other outcome measures showed no significant differences (e.g., AHI, sleep efficiency, mean oxyhemoglobin saturation). Gentina et al. found over a 10-week period in non-compliant CPAP patients the auto device improved compliance and clinical outcomes; however, even though they showed a good AHI reduction during BiPAP auto treatment, they did not validate how effective the device was in reducing AHI against a control [11].
Our study had a number of limitations. First, CPAP-naive OSAS patients may not have been the correct patient population to target an improvement in compliance with ABPR-PAP; however, the primary outcome of our study was to demonstrate the efficacy of this new technology, and from this perspective, the selected patient population was adequate. Patients were informed that they would receive one of two different modes of PAP therapy, and it could be suggested that some subjects might notice a difference between CPAP and ABPR-PAP treatment. The 90-day follow-up may have been insufficient to determine long-term compliance benefits, however, in line with the data from other studies [23, 24]. Compliance was established within the first weeks of therapy for both treatments making it unlikely that a longer-term follow-up would uncover any differences in compliance.
The incidence of known and serious adverse device effects was zero during the trial.
Conclusion
ABPR-PAP is as good as standard CPAP in CPAP-naive OSAS patients but does not improve compliance or daytime symptoms over a 90-day period. Further research is required to determine which sub-groups of OSAS patients will benefit most from this therapy.
References
Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ (2006) Continuous positive airways pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev CD001106
Kushida CA, Nichols DA, Quan SF, Goodwin JL, White DP, Gottlieb DJ, Walsh JK, Schweitzer PK, Guilleminault C, Simon RD, Leary EB, Hyde PR, Holmes TH, Bloch DA, Green S, McEvoy LK, Gevins A, Dement WC (2006) The apnea positive pressure long-term efficacy study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med 2:288–300
Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF (1993) Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 147:887–895
Reeves-Hoche MK, Meck R, Zwillich CW (1994) Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med 149:149–154
Ayas NT, Patel SR, Malhotra A, Schulzer M, Malhotra M, Jung D, Fleetham J, White DP (2004) Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep 27:249–253
Nilius G, Happel A, Domanski U, Ruhle KH (2006) Pressure-relief continuous positive airway pressure vs. constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest 130:1018–1024
Bakker J, Campbell A, Neill A (2010) Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep 33:523–529
Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, Boehlecke B, Brown TM, Coleman J Jr, Friedman L, Kapen S, Kapur VK, Kramer M, Lee-Chiong T, Owens J, Pancer JP, Swick TJ, Wise MS (2006) Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 29:375–380
Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR (2008) Randomised trial of CPAP vs. bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 63:395–401
Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T (2008) Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne–Stokes respiration. Sleep Med 6:652–659
Gentina T, Fortin F, Douay B, Dernis JM, Herengt F, Bout JC, Lamblin C (2010) Auto bi-level with pressure relief during exhalation as a rescue therapy for optimally treated obstructive sleep apnea patients with poor compliance to continuous positive airways pressure therapy—a pilot study. Sleep Breath. doi:10.1007/s11325-009-0322-y
Danker-Hopfe H, Kunz D, Gruber G, Klosch G, Lorenzo JL, Himanen SL, Kemp B, Penzel T, Roschke J, Dorn H, Schlogl A, Trenker E, Dorffner G (2004) Interrater reliability between scorers from eight European sleep laboratories in subjects with different sleep disorders. J Sleep Res 13:63–69
Anonymous (1992) EEG arousals: scoring rules and examples a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15:173–184
Anonymous (1999) Sleep-related breathing disorders in adults recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, De la Cruz-Moron I, Dela V, Fernandez-Palacin A (2005) Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 128:624–633
Abdenbi F, Chambille B, Escourrou P (2004) Bench testing of auto-adjusting positive airway pressure devices. Eur Respir J 24:649–658
Fietze I, Glos M, Moebus I, Witt C, Penzel T, Baumann G (2007) Automatic pressure titration with APAP is as effective as manual titration with CPAP in patients with obstructive sleep apnea. Respiration 74:279–286
Gay PC, Herold DL, Olson EJ (2003) A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 26:864–869
Janson C, Noges E, Svedberg-Randt S, Lindberg E (2000) What characterizes patients who are unable to tolerate continuous positive airway pressure (CPAP) treatment? Respir Med 94:145–149
Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP (2005) Treatment adherence and outcomes in flexible vs. standard continuous positive airway pressure therapy. Chest 127:2085–2093
Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT (2007) Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs. standard CPAP: a two-night randomized crossover trial. Sleep Breath 11:31–37
Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Smith PL, Schwartz AR, Schubert NM, Gillen KA, Dinges DF (1997) Night-to-night variability in CPAP use over the first three months of treatment. Sleep 20:278–283
Aloia MS, Arnedt JT, Stanchina M, Millman RP (2007) How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behav Sleep Med 5:229–240
Acknowledgements
This study was supported by an unrestricted grant from Philips Respironics, Inc. 1001 Murry Ridge Lane, Murrysville, PA, 15668, USA. Alexander Blau and Mihaela Minx recruited patients and performed outcome measures and statistical analysis. Jan Giso Peter, Martin Glos and Thomas Penzel were responsible for performing unblinded activities such as randomisation, device set-up and troubleshooting. Gert Baumann and Ingo Fietze oversaw the study and advised on statistical analysis.
Conflict of interest
Dr. Fietze, Prof. Penzel, Dr. Peter and Dr. Blau have received travel grants and honorariums for lecturing from Philips Respironics. The other authors have no significant conflicts of interest with any companies/organizations whose products or services may be discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Registered at www.controlled-trials.com under ISRCTN53816305.
Rights and permissions
About this article
Cite this article
Blau, A., Minx, M., Peter, J.G. et al. Auto bi-level pressure relief–PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath 16, 773–779 (2012). https://doi.org/10.1007/s11325-011-0574-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0574-1