Abstract
Purpose
Our study aims to compare the difference in clinical efficacy between auto-trilevel positive airway pressure (auto-trilevel PAP) ventilator and conventional fixed bilevel positive airway pressure (BiPAP) ventilator for obesity hypoventilation syndrome (OHS) patients with coexisting moderate or severe obstructive sleep apnea hypopnea syndrome (OSAHS).
Methods
Twenty-three OHS patients with moderate or severe OSAHS enrolled between January 2015 and September 2017 underwent ventilation by three different modes of positive airway pressure (PAP) for 8 h per night. A single variable mode was applied at the first night followed by two nights when no PAP therapy was carried out as a washout period between each mode. The inspiratory positive airway pressure (IPAP) decided by PaCO2 was consistently used for modes 1, 2, and 3. In mode 1, the expiratory positive airway pressure (EPAP) issued by BiPAP was decided by the minimal PAP levels for cessation of snoring. However, in mode 2, the EPAP was fixed at 3 cmH2O higher than this value. With the use of auto-trilevel PAP in mode 3, the EPAP was set to initially match that of mode 1 but the end of EPAP (EEPAP) was automatically regulated to be elevated according to upper airway patency condition. We also compared the following parameters including apnea hypopnea index (AHI), minimal SpO2 (miniSpO2), arousal index, and sleep efficiency during sleep; PaCO2 in the morning and Epword sleepiness score (ESS) at daytime were measured prior to and during PAP treatment as well as between three selected PAP modes.
Results
Compared with the parameters before ventilation therapies, all three variable modes of ventilation were associated with a higher nocturnal miniSpO2 and sleep efficiency (all P < 0.01). Among the three variable modes, mode 3 resulted in not only the lowest arousal index and daytime ESS but also the highest sleep efficiency. Compared to mode 1, mode 2 demonstrated a significantly reduced AHI and an elevated miniSpO2 and morning PaCO2 (all P < 0.05), while mode 3 was associated with a decreased AHI, an increased miniSpO2 (all P < 0.05), and no statistical change of PaCO2 following the end of PAP treatment (P > 0.05). Comparison between mode 2 and mode 3 revealed that mode 3 had a significantly lower PaCO2 (P < 0.05), but displayed no remarkable changes of AHI and miniSpO2 (all P > 0.05).
Conclusion
Compared to fixed BiPAP ventilation, auto-trilevel PAP ventilation could more effectively correct hypercapnia, achieve lower index of nocturnal apnea and hypopnea, more improved sleep quality, and lower daytime sleepiness score. Auto-trilevel PAP ventilation is therefore more efficacious than conventional BiPAP ventilation in non-invasive ventilation therapy for OHS patients with concurrent moderate or severe OSAHS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity hypoventilation syndrome (OHS) and obstructive sleep apnea hypopnea syndrome (OSAHS) are often comorbid conditions [1]. Compared to patients with either OHS or OSAHS alone, those with both diseases commonly experience more serious hypoxiemia, CO2 retention, and an accelerated onset of pulmonary artery hypertension and cor pulmonale [2, 3]. The current study was aimed to discover more efficacious non-invasive ventilation (NIV) treatment for those with concurrent OHS and OSAHS.
Continuous positive airway pressure (CPAP) treatment, as a conventional NIV treatment for OSAHS, has been used with caution for OSAHS patients with OHS due to its inability to solve hypercapnia [4]. Alternatively, bilevel positive airway pressure (bilevel PAP, BiPAP) ventilators with a relatively larger pressure difference (P difference) between inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) has been used to treat hypercapnic OHS patients [4]. However, because too high IPAP levels may cause lower compliance to NIV treatment while too low EPAP levels fail to eliminate residual obstructive sleep apnea events for OHS patients with moderate or severe OSAHS. Ventilator therapists often face a dilemma in attempting to overcome both CO2 retention and residual OSA events [5].
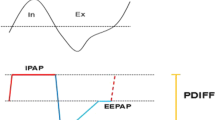
The newly established auto-trilevel PAP ventilation mode, differing from BiPAP in its EPAP setting, seems to be promising in solving both of these issues. Auto-trilevel PAP is equipped with two different EPAP levels during the expiratory period; i.e., at the first half of expiratory phase, the EPAP was relatively lower while at the second half of expiratory phase, the end EPAP (EEPAP) became adjustably higher [6] (Fig. 1). The lower initial EPAP is designed in favor of removal of CO2 at the early expiratory phase, while the elevated EEPAP level helps to prevent the residual events of obstructive sleep apnea (OSA) and hypopnea resulting from upper airway (UA) collapse or obstruction which is more likely to take place towards the end part of expiration if the EPAP is lower [7, 8]. Moreover, because both OHS and OSAHS can cause elevated critical pressure of the upper airway (UA), patients with concurrent OHS and moderate or severe OSAHS are susceptible to residual OSA and hypopnea events when the UA’s intrinsic pressure is too low, even under PAP treatment.
Characteristic of trilevel positive pressure support at a respiratory cycle. IPAP, inspiratory positive airway pressure; EPAP, expiratory positive airway pressure; EEPAP, end expiratory positive airway pressure; PDIFF, pressure difference between IPAP and EPAP. In auto-trilevel PAP mode, EPAP was displayed with two different parts, i.e., a relatively lower EPAP level at the beginning part of expiratory phase while an adjustably elevated EEPAP level at the end of expiratory phase
According to the periodic property of the UA patency and collapsibility (Fig. 2), the UA’s anti-collapsibility is stronger at the early expiratory phase because of higher intrinsic positive airway pressure (PAP) at this period. However, towards the end of the expiratory phase, the UA’s anti-collapsibility becomes weak probably as a result of reduced intrinsic positive airway pressure and increased visceral attraction force [8, 9]. That is why towards the end of expiratory phase such patients are susceptible to OSA and hypopnea [8, 9].
In this study, we compared the efficacy of the conventional BiPAP versus auto-trilevel PAP ventilation modes in treatment of the patients with concurrent OHS and moderate or severe OSAHS.
Patients and methods
Patients
A total of 23 OHS patients with moderate or severe OSAHS (15 males, 8 females) were finally recruited from our hospital’s outpatient or inpatient departments of Respiratory and Critical Care Medicine between January 2015 and September 2017. Our enrolled patients had a mean age of 37.4 ± 12.6 yrs. (range = 29~62 years) and an average body mass index (BMI) of 35.3 ± 3.2 (range = 31.7~38.9). Patients with a history or evidence of renal dysfunction, stroke, diabetes, infectious disease, or surgery or trauma < 6 months prior to enrollment, or their unwilling to complete our NIV treatment were excluded from this investigation. During our recruitment, 7 patients were excluded because 4 cases felt they could not tolerate the higher IPAP during sleep and another 3 cases felt discomfort with the facial mask on their face during sleep.
All procedures conducted in our current study associated with human participants were in consistent with the ethical criteria of Patient Ethics and Research Committee of the First Affiliated Hospital with Nanjing Medical University and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in this investigation.
Polysomnography protocol
Full polysomnography (PSG) examination was carried out using the Compumedics E-series Sleep System (Compumedics Sleep, Abbotsford, Australia) to monitor overnight PSG parameters such as electro-encephalogram(EEG), electro-cardiogram (ECG), electrooculogram, chin and bilateral anterior tibials electromyogram, chest and abdominal respiratory movements measured with respiratory inductance plethysmography (RIP) (Protech, Murrysville Pennsylvania, USA), and pulse oxyhemoglubin saturation (SpO2). The criteria of all tracing scoring techniques and dianosis for OSAHS were based on international standard [10] and our previous study [6].
Diagnosis of OHS
OHS is defined by a resting daytime PaCO2 > 45 mmHg, a body mass index (BMI) > 30 kg/m2, and the absence of an alternative cause for alveolar hypoventilation. OHS is associated with worsened OSAHS and nocturnal hypercapnia and hypoxiemia [3].
NIV treatment design
Standard nasal masks were used for patients underwent treatment of NIV ventilators (Prisma25ST, Lowenstein Medical Inc., Germany) which can deliver both BiPAP and auto-trilevel ventilation modes. The three variable PAP modes (auto-trilevel and two types of BiPAP modes) were then compared. Prior to our PAP therapy, we performed EPAP and IPAP’s titration beforehand for our recruited patients. An end tidal CO2 (ETCO2) analyzer (compact airway module M-COVX, Datex-Ohmeda, Helsinki, Finland) was connected to the nasal cannula which passed through the gastric port of the mask of a ventilator; the IPAP was always set as the lowest pressure to keep ETCO2 ≤ 45 cmH2O for all three modes.
While the IPAP was kept the same across all three modes, the EPAP was varied with different modes. In mode 1, EPAP was chosen as the lowest pressure for stop of snoring. In mode 2, EPAP was fixed as the level of 3 cmH2O higher than that in mode 1 for each patient. In mode 3 (auto-trilevel PAP therapy), the EPAP levels varied from the early period (beginning stage) to the end EPAP (EEPAP) of treatment. Although the beginning EPAP stage was kept unchanged from mode 1, the EEPAP automatically rised based on the alteration in ventilation volume. Mode 3 belongs to auto-trilevel NIV mode. Each single NIV mode was applied per night for 8 h with an interval of two nights when no NIV therapy was conducted at this washout duration.
Blood gas analysis
Arterial blood gas analysis was performed at 9:00 P.M. just prior to PAP therapy and at 6:00 A.M. just after nocturnal NIV treatment. When IPAP was titrated, ETCO2 was constantly monitored through a nasal mask as previously reported [6].
Anthropometric tests
Following each night of NIV treatment, all patients were measured for their body height (BH), body weight (BW), body mass index (BMI), and morning blood pressure at 6:00 A.M.
Judgment of drowsiness during the day
All patients’ extent of sleepiness at the day just prior to and after NIV treatment was judged by the questionnaire of Epworth sleepiness scale (ESS) [11].
Statistical analysis
Values were displayed as mean ± standard deviation (SD) for continuous variables. ANOVA of repeated measures data was done to reveal any statistical difference among the 3 modes (p < 0.05). If Mauchly’s test of sphericity was not satisfied, the Greenhouse-Geisser adjustment result was performed. Bonferroni adjustment was applied for multiple comparisons. Statistical software, SPSS version of 13.0 for Windows (SPSS, Chicago, IL), was used for data analysis.
Results
Basement condition of recruited patients and PAP levels
The general condition of recruited patients and ventilator PAP levels were shown in Table 1. As our enrolled patients demonstrated various extent of CO2 retention, the average IPAP in NIV treatment was kept relatively higher. Both the sleepiness during the day judged with ESS and PaCO2 at 6:00 A.M. showed no statistical difference prior to each NIV mode therapy (all P > 0.05) (Table 1).
Alteration in sleep respiratory parameters by NIV treatment
The AHI prior to NIV therapy was remarkably decreased at each mode of NIV therapy (all P < 0.01). Comparison among three NIV modes revealed a significant difference in AHI from mode 1 to mode 2 NIV treatment and from mode 1 to mode 3 NIV treatment (all P < 0.05). However, no significant difference in AHI was demonstrated between mode 2 and mode 3 NIV treatment (P > 0.05) (Table 2). Further dividing AHI into obstructive apnea hypopnea index (OAHI) and central apnea hypopnea index (CAHI), we found that both OAHI and CAHI were significantly lower during three modes of NIV therapy than those before NIV therapy (all P < 0.01). Comparison among three NIV treatment modes demonstrated that the OAHI in mode 2 and mode 3 showed no significant difference (P > 0.05), but was statistically lower than that in mode 1 (all P < 0.05). The CAHI displayed no significant difference among three NIV modes (all P > 0.05) (Table 2).
The miniSpO2 levels before NIV treatment were all obviously elevated by each NIV treatment mode (all P < 0.01). The miniSpO2 during mode 1 NIV treatment was remarkably lower than that at mode 2 and mode 3 NIV treatment (P < 0.01), but no significant difference in miniSpO2 was detected between mode 2 and mode 3 NIV treatment (P > 0.05) (Table 2).
The PaCO2 at 6:00 A.M. following nocturnal NIV therapy were invariably decreased in all three modes of NIV treatment, compared with that before NIV treatment (all P < 0.01). Evaluation of post-NIV PaCO2 levels showed a significant difference not only between mode 1 and mode 2 but also between mode 2 and mode 3 NIV treatment (all P < 0.05), even though no significant changes was found between mode 1 and mode 3 NIV treatment (P > 0.05) (Table 2).
Influence of NIV mode variation to sleep quality
The arousal index (AI) levels were all obviously lower during mode 1, mode 2, and mode 3 NIV treatment than AI prior to NIV treatment (all P < 0.05). Evaluation of AI during NIV treatment at various ventilation modes indicated the AI in mode 2 was the highest while in mode 3 was the lowest, with a statistical difference among various NIV treatment modes(all P < 0.05) (Table 2).
The N1+N2/TST% prior to NIV treatment noticeably declined during all three modes of NIV treatment (all P < 0.01). Evaluation of N1+N2/TST% between various modes of NIV treatment demonstrated a significant change not only from mode 1 to mode 2 but also from mode 1 to mode 3 NIV treatment (all P < 0.05)with no significant difference between mode 2 and mode 3 (P > 0.05) (Table 2).
The N3/TST% during all three modes of NIV treatment became obviously higher than that prior to NIV treatment (all P < 0.01). Comparison among various NIV modes revealed that there was a statistical change of N3/TST% from mode 1 to mode 2 and from mode 1 to mode 3 NIV treatment (all P < 0.05) with no significant difference between mode 2 and mode 3 (P > 0.05) (Table 2).
Although no significant difference in REM/TST% was found between mode 1 and before NIV treatment (P > 0.05). The REM/TST% was significantly higher in mode 2 and mode 3 NIV treatment than that before NIV treatment (all P < 0.05). By comparison of REM/TST% among three modes of NIV treatment, it was detected that there was no significant difference among various modes of NIV treatment (all P > 0.05) (Table 2).
Sleep efficiency (TST/TRT%) became remarkably higher at each mode of NIV treatment than that before NIV treatment (all P < 0.01). Comparison of sleep efficacy among various modes of NIV therapy revealed the TST/TRT% was the highest in mode 3 but the lowest in mode 1 with mode 2 in between. A statistical difference existed among three NIV modes (all P < 0.05) (Table 2).
Alteration in ESS during the day
ESS at the day following each mode of nocturnal NIV treatment declined remarkably, compared to that prior to NIV treatment (all P < 0.05). Analysis among various NIV modes demonstrated that the ESS level reduced to the lowest following mode 3 NIV treatment but to the highest following mode 2 NIV treatment with the daytime ESS following mode 1 NIV treatment in between. No statistic changes of ESS during the day were detected between mode 1 and 2 NIV treatment. However, a significant change in ESS during the day was found not only from mode 3 to mode 1 but also from mode 3 to mode 2 NIV treatment (all P < 0.05) (Table 2).
Discussion
OHS often coexists with OSAHS. OHS, as a special manifestation of pathological obesity, is characterized by extremely excessive obesity, daytime sleepiness, hypoxia and hypercapnia, dyspnea, secondary erythrocytosis, systemic and pulmonary hypertension, etc. [3]. OHS is sometimes misdiagnosed as chronic obstructive pulmonary diseases or cardiac diseases. However, OHS patients usually do not have chronic respiratory diseases or pulmonary emphysema detectable by a chest X-ray. The pathogenesis of OHS consists mainly of (a) excessive obesity causing such a huge accumulation of adipose tissue in thoracic and abdominal cavities that the ventilation is limited by elevated diaphragm [12, 13] or (b) a reduction in the sensitivity of the respiratory center to PaCO2 change [14].
Hypoventilation is caused by UA’s narrowness or obstruction in OSAS but by pulmonary ventilation limitation in OHS. Therefore, ventilation for OHS with coexisting OSAHS should ideally keep the UA patent to prevent UA obstruction or narrowness and enlarge tidal volume to prevent CO2 retention.
BiPAP ventilation has been served as an effective treatment for OHS patients with OSAHS. However, for such patients, their need for EPAP levels is varied from awake to sleep since in patients with OSAHS their OSA and hypopnea events selectively occur only when they are asleep. Although a relatively higher EPAP is needed during sleep, CO2 retention is also easier to occur if IPAP keeps unchanged because of reduced P difference. However, if IPAP level is set too high, patients can feel discomfort followed by a poorer compliance with NIV treatment. Auto-trilevel PAP promotes CO2 discharge by a lower EPAP at the early expiratory phase and eliminates residual OSA events with an adjustable higher EEPAP towards the end expiratory phase under the precondition that IPAP was kept unchanged during inspiration.
In the current study, two BiPAP modes were applied in order to compare with auto-trilevel NIV mode. In mode 1, with a high enough P difference, CO2 retention previously existed was effectively eliminated. However, this resulted in a relatively higher occurrence of residual OSA events. Although in mode 2, the residual OSA events were noticeably removed with a higher EPAP level, a higher PaCO2 was resulted in. Auto-trilevel PAP looks like a product of combination of BliPAP and auto-EPAP models. The three PAP levels delivered by the auto-trilevel PAP ventilator, (IPAP, EPAP, and EEPAP) have unique functions. A relatively high IPAP with a lower EPAP corrects CO2 retention and a relatively elevated EEPAP reduces residual OSA because UA is easier to collapse at the end stage of expiration when intrinsic airway pressure tends to be quite low. By auto-regulating EPAP based on patients’ real-time ventilation volume, auto-trilevel PAP ventilation is available for synchronized removal of both CO2 retention and residual OSA events [6].
By enrollment of 23 OHS patients with moderate or severe OSAHS and comparison before and during two different assistant ventilation models, the current study indicated that the auto-trilevel PAP model was more effective in controlling sleep breathing disorder, respiratory effort related arousal and CO2 retention and achieving better sleep quality and compliance with PAP treatment.
In conclusion, our preliminary clinical observation demonstrated that auto-trilevel NIV treatment was more efficacious than conventional BiPAP NIV treatment for OHS patients with concurrent moderate or severe OSAHS, since such a novel NIV mode could result in lower nocturnal apnea and hypopnea index, better sleep quality, less daytime drowsiness, and simultaneously removal of CO2 retention.
References
Raveendran R, Wong J, Singh M, Wong DT, Chung F (2017) Obesity hypoventilation syndrome, sleep apnea, overlap syndrome: perioperative management to prevent complications. Curr Opin Anaesthesiol 30:146–155
Shetty S, Parthasarathy S (2015) Obesity hypoventilation syndrome. Curr Pulmonol Rep 4:42–55
Pierce AM, Brown LK (2015) Obesity hypoventilation syndrome: current theories of pathogenesis. Curr Opin Pulm Med Curr Opin Pulm Med 21:557–622
Nicolini A, Banfi P, Grecchi B, Lax A, Walterspacher S (2014) Non-invasive ventilation in the treatment of sleep-related breathing disorders: a review and update. Rev Port Pneumol 20:324–335
Piper AJ, BaHammam AS, Javaheri S (2017) Obesity hypoventilation syndrome choosing the appropriate treatment of a heterogeneous disorder. Sleep Med Clin 12:587–596
Su M, Huai D, Cao J, Ning D, Xue R, Xu M, Huang M, Zhang X (2018) Auto-trilevel versus bilevel positive airway pressure ventilation for hypercapnic overlap syndrome patients. Sleep Breath 22:65–70
Heinzer R, White DP, Malhotra A, Lo YL, Dover L, Stevenson KE, Jordan AS (2008) Effect of expiratory positive airway pressure on sleep disordered breathing. Sleep 31:429–432
Braga CW, Chen Q, Burschtin OE, Rapoport DM, Ayappa I (2011) Changes in lung volume and upper airway using MRI during application of nasal expiratory positive airway pressure in patients with sleep-disordered breathing. J Appl Physiol 111:1400–1409
Morrell MJ, Arabi Y, Zahn B, Badr MS (1998) Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 158:1974–1981
American Academy of Sleep Medicine Task Force (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G (1993) Effects of obesity on respiratory resistance. Chest 103:1470–1476
Pankow W, Podszus T, Gutheil T, Penzel T, Peter J et al (1998) Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol 85:1236–1243
Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J (2009) Neural respiratory drive in obesity. Thorax 64:719–725
Acknowledgments
The authors are grateful for language editing during revision of the manuscript provided by Sabrina Saeed at Brown University, Providence, RI, USA02912.
Funding
The 333 high level personnel training program in Jiangsu Province (BRA-2017239) provided financial support in the form of ventilator training funding and the key research and development program of Huai’an (social development HAS201611) provided financial support in the form of equipment funding for this study. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zou, C., Sheng, W., Huai, D. et al. Comparison between auto-trilevel and bilevel positive airway pressure ventilation for treatment of patients with concurrent obesity hypoventilation syndrome and obstructive sleep apnea syndrome. Sleep Breath 23, 735–740 (2019). https://doi.org/10.1007/s11325-018-1750-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1750-3