Abstract
Objective
To evaluate the effect of continuous positive airway pressure (CPAP) therapy on pro-brain natriuretic peptide (BNP) and cardiac markers in patients with obstructive sleep apnea syndrome and normal cardiac function.
Methods
Thirty-three consecutive patients with sleep apnea syndrome were analysed for serum pro-BNP and cardiac markers prior to and after 6 months of CPAP therapy.
Results
Twenty five patients had normal (83.3%) while remaining five (16.7%) revealed high pro-BNP values. We did not detect any significant difference between severity of obstructive sleep apnea syndrome and serum pro-BNP levels (p = 0.534). A statistically significant difference was not observed between basal and sixth-month creatine kinase (CK), creatine kinase-MB (CK-MB), troponin I, pro-BNP, aspartate transaminase (AST), and CK levels in patients with sleep apnea syndrome (p > 0.05).
Conclusion
Obstructive sleep apnea syndrome does not induce myocardial damage enough to increase serum pro-BNP, CK, CK-MB, troponin I, and AST levels. Markers sensitive to ischemia could be preferred to evaluate effect of obstructive sleep apnea syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activated sympathetic nerve system activity due to apnea and hypoxia causes an increase in systemic blood pressure, and endothelial dysfunction causes systemic inflammation in obstructive sleep apnea syndrome (OSAS). Therefore, cardiovascular diseases such as congestive heart failure, hypertension, arytmia, and coronary artery diseases are common in these patients [1]. Continuous positive airway pressure (CPAP) is the mainstay of therapy which eliminates obstructive events and hence, prevents daytime somnolence and cardiovascular complications [2]. Brain natriuretic peptide (BNP) is a cardiac neurohormone which is secreted by myosites after myocardial hypoxemia and ventricular volume expansion [3]. Studies have revealed that secretion of N-terminal (NT)-pro-BNP is increased in cardiac problems such as heart failure, coronary artery disease, and cardiac hypertrophy and related with poor prognosis [1, 4, 5]. Ventricular load during apneic period results in ventricular strain and elevated level of BNP is known as prognostic marker in obstructive sleep apneic patients [5, 6]. Elevation in serum levels of cardiac biomarkers are expected after ischemia or damage/necrosis of myosites. While some cardiac biomarkers, such as ischemia-modified albumin, are sensitive to ischemia, elevation in the levels of creatine kinase (CK), CK-MB, and troponin I can be detected after minor cardiac damage occurs [7].
In this study, we evaluated the effect of CPAP therapy on pro-BNP and cardiac markers that are sensitive to cardiac damage in patients with OSAS and normal cardiac function. We aimed to evaluate whether repeated apneas and hypoxia influence the levels of cardiac biomarkers in OSAS patients and if these effects could be reversed by treatment.
Methods
The study was conducted between August 2007 and June 2009. Thirty consecutive patients referred to Gaziantep University Sleep Disorders Center were included in this prospective study. The study was approved by the university ethics committee, and informed consent was obtained from each patient.
Inclusion criteria were as follows: being older than 18 years, accepting to give consent for the study, having an apnea–hypopnea index (AHI) > 5 in full-night polysomnography, exclusion of cardiac failure via clinical and echocardiographic evaluation, having normal lung function tests, and being suitable for CPAP therapy.
Exclusion criteria were as follows: having AHI > 5 under CPAP treatment in the sixth month of therapy, having lung function test abnormalities, and having history of recent facial trauma/operation.
Clinical data were obtained with initial visit. In addition to physical examination, neck length, weight, and height were measured. Body mass index was calculated as kilogram per square meter. Posteroanterior chest scan, electrocardiography, and lung function tests were obtained. Ejection fraction >%55 was used to exclude cardiac failure [8]. Complete blood count, routine biochemical analysis, and thyroid hormone levels were studied.
Recommendations including avoiding of caffeinated drinks or foods, alcohol consumption, and drugs which could interfere with sleep architecture were made prior to the sleep study. Recordings were obtained with Viass Sleep Screen (Viasy Healtcare, Germany) device. Sleep scoring was performed by one doctor according to the guidelines of American Academy of Sleep Medicine as 30-s epochs. Apnea was defined as cessation of the airflow for at least 10 s while airflow limitation of at least 50% accompanying arousal and/or at least 3% desaturation was defined as hypopnea [9]. Patients were classified as having mild, moderate, and severe OSAS by AHI 5–15, 15–30, ≥30, respectively. CPAP titration was conducted automatically with full-night polysomnographic measurement. Detailed explanations about CPAP use were achieved, and control visits were conducted 1 and 3 months after CPAP initiation. During control visits, data about CPAP use was obtained, and problems, if any, were evaluated. Subjective reporting of device usage at least 5 h or 70% of night without any problem were accepted as good compliance. At the sixth-month visit, control polysomnography including patients own devices were conducted, and blood samples were obtained from patients who had AHI < 5 in this studies in order to avoid device-based treatment failure. Blood samples were collected using standard venipuncture technique between 9:30 a.m. to 11:00 a.m. after 12 h fast. Serum samples were separated immediately after centrifugation at 4°C, 2,000×g for 10 min and stored at −80°C until analysis. After 6 months of CPAP treatment, samples were obtained from patients who were compliant with the therapy. CK and aspartate transaminase (AST) were determined in Abbott Aeroset device (Abbott, USA) with enzymatic (N-acetyl-l-cysteine) and NADH (without p-5′P) procedure, respectively. CK-MB and troponin I were studied in Abbott Axsym device (Abbott, USA) with electrochemiluminescence method, and pro-BNP was studied in Minividas device (Biomerieux, France) with enzyme-linked fluorescent assay method.
Statistics
Data were expressed as mean ± standard deviation. Results were presented with a 95% confidence interval, and p < 0.05 was accepted as statistically significant. Paired samples t test, Mann–Whitney U, and Fisher's exact test were used for statistical evaluation. SPSS for Windows 13.0 was used for statistical analysis (SPSS Inc., Chicago, IL, USA).
Results
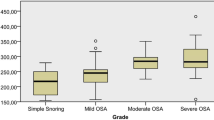
Three patients who had AHI >5 after 6-month treatment were excluded. The baseline demographic and clinical characteristics of the study participants are presented in Table 1. Twenty of thirty participants were male, and mean age was 49.97 ± 10.3. Initial pulmonary artery pressure (PAB) was measurable in six of 30 patients by echocardiography. Mean PAB prior to treatment was measured as 32.8 ± 3.1 mm Hg (30–38) while it was detected as 31.5 ± 4.1 (25–38) after CPAP therapy (p = 0.18). Forty percent of patients had hypertension while 16.7% had diabetes mellitus. Thirty percent of the study population had moderate OSAS while 70% had severe OSAS. There was no patient with mild OSAS. Mean CPAP pressure was 9.83 ± 1.46 cmH2O. Mean duration of CPAP treatment was 10.27 ± 5.07 months(6–24) . Mean AHI before and after CPAP therapy were 55.9 ± 26.1 and 3.38 ± 2.84, respectively (p = 0.000). Twenty five patients had normal (83.3%) while five patients (16.7%) had high pro-BNP values. Pro-BNP levels were not inflenced by current smoking status (p = 1). Smoking status and pro-BNP levels were shown in Table 2. All patients with high pro-BNP levels had severe OSAS. Pro-BNP levels in respect to OSAS classification were shown in Table 3. Mean basal oxygen saturation was (mean; min–max) 90.97 ± 3.43 (79–96), while mean minimum oxygen saturation was 74.73 ± 12.11 (40–89; p > 0.05). Cardiac biomarkers before and after CPAP therapy were included in Table 4. We did not find statistically significant difference between the levels of serum pro-BNP and cardiac biomarkers in patients with OSAS (p > 0.05).
Discussion
It is well known that there is a linear relationship between the severity of OSAS and cardiovascular morbidity and mortality [9]. It is assumed that main factors influencing cardiac damage in OSAS depend on sympathetic system activation due to hypoxia and intrathoracic pressure fluctuations and inhibition of inflammatory pathways selectively due to intermittent reoxygenation perfusion damage and therefore, increasing oxidative stress [10, 11]. Pathogenetic mechanism is inducing arrhythmia, left ventricular systolic and diastolic dysfunctions, and congestive heart failure as end organ damage. There is limited data about predicting this OSAS-related cardiac damage before manifestation of clinical and echocardiographic findings. Maeder et al. claimed that high level of NT-pro-BNP is a convenient marker in detecting the damage in cardiovascular system in OSAS patients independent of low peak oxygen consumption [12]. Another study evaluating the snoring children found that sleep apneic subjects had higher serum BNP values compared to simple snorers [13]. A study revealed that decreases in serum pro-BNP levels were detected in congestive heart failure patients having OSAS concomitantly after CPAP therapy [14]. Kita et al. found that plasma BNP levels were increased in subjects with OSAS during sleep and decreased by CPAP therapy but levels had no correlation with the severity of OSAS and polysomnographic parameters [15]. Hubnera et al. also did not detect difference between serum BNP levels and AHI, BMI, mean, and minimal saturation, left ventricular ejection fraction, and left ventricular mass index [16]. We did not find any correlation between serum pro-BNP levels and OSAS severity, AHI, and oxygen desaturation as compatible with the literature. We also found that there was no correlation between OSAS classification, AHI, oxygen desaturation, and markers of CK, CK-MB, AST, and troponin I which predicts cardiac damage. Kragelund et al. have demonstrated that dietary factors and exercise influence BNP levels while smoking status were similar between different levels of BNP, as our study [17]. Our findings do not support the hypothesis of nocturnal hypoxia inducing myosite damage and increasing blood troponin levels [18]. We did not detect any difference in the levels of pro-BNP and cardiac biomarkers after CPAP treatment. This result is incompatible with the study of Zhao et al. But this study was conducted in OSAS patients with congestive heart failure [14]. We did not include the patients with congestive heart failure, confirmed either clinically or echocardiographically, in our study.
Limitations of this study were the small number of the study group, subjective evaluation of the CPAP compliance, lack of a healthy control group, and mild OSAS patients.
Based on the findings of this study, we concluded that pro-BNP, CK, CK-MB, AST, and troponin I levels were not changed in patients with sleep apnea disorder after 6 months of treatment with CPAP. This can be explained by normal basal levels of the markers prior to the therapy and/or insufficient sensitivity of these markers to ischemia as troponin and CK-MB assays are sensitive to myosite damage or necrosis. Therefore, markers sensitive to ischemia could be preferred to evaluate effect of sleep apnea syndrome (and its treatment) on myocardial integrity. According to the present findings, it is also possible to assume that hypoxia induced by sleep apnea syndrome does not affect basal levels of the cardiac markers studied. In conclusion, this study revealed that pro-BNP and cardiac markers are insufficient to predict cardiac effects of CPAP therapy in earlier stages of the disease than in OSAS patients without heart failure. We suggest that biomarkers that are sensitive to ishemia are more prefable in determining minor prolonged effects on myosites in OSAS.
References
Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS (2004) Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 350:655–663
Hukins CA (2006) Obstructive sleep apnea—management update. Neuropsychiatr Dis Treat 3:309–326
Goetze JP, Friis-Hansen L, Rehfeld JF, Nilsson B, Svendsen JH (2006) Atrial secretion of B-type natriuretic peptide. Eur Heart J 27:1648–1650
Grabowski M, Filipiak KJ, Karpinski G, Wretowski D, Rdzanek A, Huczek Z, Horszczaruk GJ, Kochman J, Ruduwski R, Opolski G (2004) Serum B-type natriuretic peptide levels on admission predict not only short term death but also angiographic success of procedure in patients with acute ST-elevation myocardial infarction treated with primary angioplasty. Am Heart J 148:655–662
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Trouqhton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG (2003) B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 107:2786–2792
Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD (2003) Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 348:1233–1241
Anwaruddin S, Januzzi JL Jr, Baggish AL, Lewandrowski EL, Lewandrowski KB (2005) Ischemia-modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 123:140–145
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W (2006) American society of echocardiography's nomenclature and standards committee; task force on chamber quantification; American college of cardiology echocardiography committee; American heart association; European association of echocardiography. European society of cardiology. Recommendations for chamber quantification. Eur J Echocardiog 7:79–108
American Academy of Sleep Medicine (1999) Sleep related breathing disorders in adults: recommendations for syndrome definition techniques in clinical research. The report of an American Academy of Sleep Medicine Task force. Sleep 22:667–689
Butt M, Dwivedi G, Khair O, Lip GY (2009) Obstructive sleep apnea and cardiovascular disease. Int J Cardiol (epub ahead of print)
McNicholas WT, Bonsigore MR, Management Committee of EU COST ACTION B26 (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178
Maeder MT, Ammann P, Rickli H, Schoch OD, Korte W, Hürny C, Myers J, Münzer T (2008) N-terminal pro-B-type natriuretic peptide and functional capacity in patients with obstructive sleep apnea. Sleep Breath 12:7–16
Kaditis AG, Alexopoulos EI, Hatzi F, Kostadima E, Kiaffas M, Zakynthinos E (2006) Overnight change in brain natriuretic peptide levels in children with sleep-disordered breathing. Chest 130:1377–1384
Zhao ZH, Liu ZH, Luo Q, Xiong CM, Ni XH, Zhang J, Zhang S, Yang YJ (2006) Positive pressure ventilation treatment reduces plasma levels of amino terminal-probrain natriuretic peptide in congestive heart failure patients with sleep apnea. Circ J 70:572–574
Kita H, Ohi M, Chin K, Noquchi T, Otsuka N, Tsuboi T, Itoh H, Nakao K, Kuno K (1998) The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res 7:199–207
Hubnera RH, El Mokhtarib NE, Freitag S, Rauscheb T, Goderd R, Tirokeb A (2008) NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med 102:134–142
Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R (2005) N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 352:666–675
Gami AS, Svatikova A, Wolk R, Olson EJ, Duenwald CJ, Jaffe AS, Somers VK (2004) Cardiac troponin T in obstructive sleep apnea. Chest 125:2097–2100
Acknowledgment
We would like to thank to Meltem Akın and Eda İkbal for statistical evaluation of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çifçi, N., Uyar, M., Elbek, O. et al. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath 14, 241–244 (2010). https://doi.org/10.1007/s11325-009-0306-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-009-0306-y