Abstract
Obstructive sleep apnea–hypopnea syndrome (OSAHS) with episodic hypoxia–reoxygenation is associated with increased cardiovascular morbidity and mortality. Therefore, increased homocysteine, asymmetric dimethylarginine (ADMA), oxidative status, and decreased nitric oxide levels have been implicated as possible mechanisms for development of cardiovascular diseases. We aimed to investigate changes in the levels of these substances in patients with OSAHS in comparison with nonapneic controls. Thirty-four OSAHS patients and 15 healthy controls were included in this study. In the blood samples, oxidative status and nitric oxide levels were measured with spectrophotometric methods. Plasma ADMA and homocysteine levels were determined by using high-performance liquid chromatography with fluorescence detection. Nitric oxide levels were significantly low in OSAHS patients (p < 0.05) and correlated with mean SaO2 (r = 0.513, p < 0.002) and lowest SaO2 (r = 0.363, p < 0.03). Oxidative status, ADMA, and homocysteine levels were higher in OSAHS patients, but difference did not reach statistical significance. After dividing patients into moderate (AHI = 5–29) and severe (AHI ≥ 30) OSAHS groups, significantly increased homocysteine levels were observed in the severe OSAHS group (p < 0.05). Nitric oxide levels negatively correlated with oxidative status in total OSAHS patients (r = −0.415, p < 0.02) and also in severe OSAHS group (r = −0.641, p < 0.007). Hyperhomocysteinemia and diminished NO production may be causal factors in endothelial dysfunction seen in OSAHS and may explain the association between OSAHS and cardiovascular diseases. These modifiable factors should be monitored in patients suspected of having OSAHS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea–hypopnea syndrome (OSAHS) is characterized by repetitive apnea and hypopnea attacks followed by arousals and affects patients’ physical, emotional, and intellectual capacities and functional quality of life. OSAHS has been linked to increased prevalence of cardiovascular and cerebrovascular diseases, stroke, metabolic syndrome, cognitive impairment, and systemic or pulmonary hypertension [1, 2]. Therefore, recent research has focused on exact mechanisms for the elucidation of the relationships between these diseases and OSAHS.
Changes in ventilatory function and concomitant sleep disruption may result in a cascade of events including oxidative stress, inflammation, vascular endothelial dysfunction, and metabolic dysregulation. Repeated apnea-related hypoxic events in OSAHS are similar to hypoxia/reperfusion injury, which initiates oxidative stress. Supporting evidence was obtained from previous studies showing increased free radical production and increased lipid peroxidation accompanying with lower antioxidant levels in OSAHS patients [3–5]. Reactive oxygen and nitrogen species are the most important free radicals causing oxidative/nitrosative stress and tissue injury related to the several diseases, which are also seen in OSAHS patients [6–8]. Nitric oxide (NO) is synthesized in mammalian cells from l-arginine through enzymatic reactions catalyzed by a family of NO synthases (NOS). Besides acting as a key vasodilator in the vascular system, it is also regulates a great variety of physiological functions including the memory process and sleep–wake cycle and inflammatory process [6, 9]. Lower NO levels were shown in OSAHS patients compared with healthy controls in previous studies [10–12].
Asymmetric dimethylarginine (ADMA), which is an endogenous NOS inhibitor, is generated by catabolism of proteins containing methylated arginine residues and metabolized by the enzyme dimethylarginine dimethyl aminohydrolase (DDAH), the activity of which regulates ADMA concentration and indirectly modulates NOS activity. During the methylation of proteins, S-adenosylmethionine is utilized as a methyl donor, and S-adenosylhomocysteine is generated as a byproduct [13]. Homocysteine is accepted as an independent cardiovascular risk factor, and its altered levels lead to endothelial dysfunction, alterations in the antithrombotic function of the endothelium [14], increase in oxidative stress [15], and decrease in NO availability [16]. Furthermore, recent studies with animal and human cell cultures indicated that ADMA is metabolically linked to homocysteine and elevated ADMA levels and may be a unifying mechanism for endothelial dysfunction during hyperhomocysteinemia [17, 18].
In the view of these research results, we focused on the changes in the oxidative status, NO, ADMA, and homocysteine levels in OSAHS patients and aimed to clarify the effects of the repetitive hypoxia resulting from OSAHS on these parameters.
Materials and methods
Study subjects
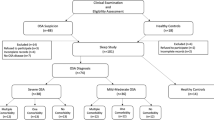
OSAHS patients and control groups involved in this study were selected from 93 consecutive subjects attended to Sleep Disorders Center in Department of Chest Diseases, Dışkapı Education and Research Hospital, for suspected sleep apnea. Before admission, all subjects were interviewed for the presence of sleep-related symptoms, snoring, witnessed apnea, and excessive daytime sleepiness. Epworth sleepiness test was used to determine the level of daytime sleepiness. After examination by an otorhinolaringologist, patients having anatomical nasal problems such as septal deviation were excluded from the study. Presence and severity of OSAHS were determined by standard overnight polysomnography (16 channels, Embla, Flaga). All data obtained from the electroencephalogram (electrodes at positions C3-A2, C4-A1, O1-A2, O2-A1), electrooculogram, and electromyogram (submental EMG and EMG tibialis), oronasal air flow measurements (cannula), breathing movements of the chest and abdomen, snoring detected by using a tracheal microphone, body position, and pulse oxymetry at the finger tip of the patients were recorded and evaluated by the Somnologica 3.2 version software program. Analysis of sleep stages were performed according to the Criteria of Rechtschaffen and Kales [19].
Apnea was defined as an absence of air flow for at least 10 s, and hypopnea was defined as a 50% reduction in airflow accompanied by a reduction in oxygen saturation by 4% from baseline or arousal. Desaturation was defined as a reduction in oxygen saturation by 4% from the baseline. Mean oxygen saturation (mean SaO2) was defined as average of oxygen saturation during the night. Lowest oxygen saturation (lowest SaO2) was defined as the lowest value of oxygen saturation during the night. The apnea–hypopnea index (AHI) was defined as the average number of apneic and hypopneic events per sleep hour. An AHI of more than 5/h was considered as diagnostic of OSAHS. Subject who had an AHI equal to or less than 5/h was placed in the control group. After excluding subjects having other diseases or taking medication, 34 OSAHS and 15 healthy control subjects were designated as the groups in this study. Altitude is 680 in Ankara, Turkey. All participants’ rights were protected, and informed consent was obtained according to the Helsinki Declaration. The study protocol was approved by the local Ethic Committee.
Blood sampling
Peripheral venous blood samples were obtained in the morning after the diagnostic study night. Serum and heparinized plasma samples were immediately stored at −70°C until analysis.
Measurement of NO levels
Serum NO levels were measured as sum of nitrate and nitrite levels. After serum samples were deproteinized with ethanol, 100 μL of the supernatant were loaded in the polystyrene microtiter plate, then 100 μL saturated VCl3 solution in 1 M HCl was added to convert nitrate to nitrite. After the addition of 100 μL Griess reagent, plates were incubated at 37°C for 45 min, and absorbance of the samples were measured by using a plate reader at 540 nm [20].
Measurement of ADMA levels
Plasma ADMA levels were determined by using high-performance liquid chromatography (HPLC) with fluorescence detection. After plasma proteins were separated with 5-sulfosalicylic acid, 10 μL of the supernatant was mixed with 100 μL o-phthaldialdehyde reagent and applied to the HPLC system. Fluorescence intensities were measured with excitation at 338 nm and emission at 425 nm [21].
Measurement of oxidative status
To determine the oxidative status of plasma samples, N,N-dimethyl-p-phenylene-diamine (DMPD) was used as an indicator for oxidative status. This indicator is based on the capability of DMPD to give a stable colored solution when it is transformed into its radical cation (DMPD+) originating from the oxidation of the DMPD itself by alkoxy and peroxy radicals derived from the iron-induced decomposition of hydroperoxide. Ten microliters of the plasma sample and 20 μL 1 mM DMPD solution were added to 2 mL acetate buffer (0.1 M, pH 4.8), and the formation of the colored DMPD+ radical cation was monitored by measuring the absorbance at 505 nm. H2O2 was used as a standard for oxidative status. The oxidative status of plasma was expressed as hydrogen peroxide equivalents. Intra- and interassay CV for oxidative status were 1.8 and 4.5%, respectively. [22].
Measurement of homocysteine levels
Total homocysteine levels were measured by the flourometric HPLC methods with some modification. The method is based on the derivatization of thiols with 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonic acid after reduction with tris(2-carboxyethyl)phosphine hydrochloride. Fluorescence intensities were measured with excitation at 385 nm and emission at 515 nm [23, 24].
Statistical methods
Statistical analyses were performed with SPSS Statistical Package Program (SPSS 8.0 version, USA). Values were expressed mean ± SD. Mann–Whithney U test, Kruskal–Wallis variance analysis, and Spearman Rank correlation analysis were applied to data. Statistical significance was set at p < 0.05.
Results
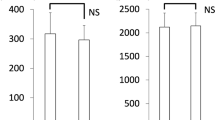
Baseline demographics characteristics and sleep study results of OSAHS patients and control group are given in Table 1. As shown, OSAHS patients and healthy controls had similar values of biochemical parameters.
The serum levels of NO in OSAHS patients were significantly lower than that in control subjects (p < 0.05). Plasma oxidative status and serum ADMA levels did not show any significant difference between these groups. Serum homocysteine levels were higher in OSAHS patients than in control subjects, but the difference between the two groups bordered on statistical significance (Table 2). In OSAHS patients, NO levels showed positive correlations with mean SaO2 (r = 0.513, p < 0.002) and lowest SaO2 (r = 0.363, p < 0.03). NO levels were also negatively correlated with oxidative status in this group (r = -0.415, p < 0.02). No statistically significant correlation was observed between homocysteine, NO, and ADMA levels.
After OSAHS patients were divided into moderate (AHI 5–29) and severe (AHI ≥ 30) OSAHS groups; increased oxidative status and homocysteine levels and decreased NO levels were observed in the severe OSAHS group; however, only decreased homocysteine levels reached statistical significance (p < 0.05; Table 3). There was also significant negative correlation between NO levels and oxidative status in the severe OSAHS group (r = −0.641, p < 0.007).
There was no significant difference between OSAHS patients and controls in terms of Epworth sleepiness Scale Score. Only the severe OSAHS group had a significantly higher score than control group (p < 0.04). There was no significant correlation between sleepiness score and measured parameters. Furthermore, there was no significant correlation between individual characteristics (age, BMI, SBP, and DBP) and measured parameters.
In total study group, homocysteine positively correlated with AHI (r = 0.797, p < 0.002) and with lowest SaO2 (r = 0.673, p < 0.02). NO levels negatively correlated with desaturation number (r = −0.415, p < 0.003) and positively correlated with mean SaO2 (r = 0.556, p < 0.0001) and lowest SaO2 (r = 0.431, p < 0.002).
Discussion
Although previous epidemiological studies have shown an association between OSAHS and coronary heart disease, heart failure, and pulmonary hypertension [25–27], exact causes of this association is still unknown. Hypercoagulability [28], polymorphonuclear neutrophil activation [29], increased inflammatory markers [30], and impaired endothelium dependent vasodilatation [31], were reported in OSAHS patients. Because the repetitive episode of nocturnal apnea leading to intermittent hypoxia and recurrent reoxygenation secondary to reperfusion are similar to ischemia/reperfusion injury, OSAHS is also considered to have links to oxidative stress and endothelial dysfunction.
Previous studies showed increased oxidative stress in OSAHS patients and in some of these studies, decreases in oxidative stress markers were observed after treatment [3–5, 32, 33]. Consistent with these studies, we observed increased oxidative status in patients, especially with severe OSAHS. Probable free radical sources in OSAHS have been suggested as hypoxia/reoxygenation insult, mitochondria, inflammatory leukocytes, and oxidation of small molecules such as homocysteine and additional sources [7]. Increased oxidative stress is one of the factors leading to endothelial dysfunction and also decreasing NO levels, which are characteristics of atherosclerotic process. In the present study, decreased NO levels and negative correlation between NO and oxidative status, especially in severe OSAHS patients, indicate that oxidative stress depending on the severity of hypoxia might have an important role in the cardiovascular abnormalities seen in OSAHS patients. In addition, because oxygen is a cosubstrate of NOS, OSAHS-related nocturnal desaturation might result in depressed synthesis of NO. Results of our study show significant positive correlations between NO and with mean SaO2 and negative correlation with lowest SaO2 in OSAHS patients. These results are also supportive of adverse cardiovascular effects.
Several research have indicated relationship between high ADMA, homocysteine and endothelial dysfunction [34, 35, 36]. In addition to as oxidative stress, elevated ADMA and homocysteine levels are important factors leading to impairment of NO availability and diminished endothelium-dependent vasodilatation, and their increased levels have been reported in cardiovascular disease [37, 38]. There is only one study reporting ADMA levels in obstructive sleep apnea patients [11]. In that study, Ohike et al. showed that plasma ADMA levels of OSAHS patients were decreased inversely to the improvement of flow-mediated vasodilatation after CPAP therapy, but the difference in ADMA levels before and after treatments did not reach statistical significance. In addition, increased homocysteine levels have been reported in OSAHS patients [39] and declined with CPAP therapy [40]. Kokturk et al. [41] showed that OSAHS patients with and without cardiovascular disease had significantly higher homocysteine levels, and its levels were independently associated with severity of OSAHS. In agreement with these previous studies, we observed elevated homocysteine levels even higher than the normal fasting range (5–15 μmol/l) especially in severe OSAHS patients. However, other studies reported similar mean homocysteine values in OSAHS and non-OSAHS groups [42, 43].
There are metabolic relationships between ADMA and homocysteine. ADMA levels and ADMA/l-arginine ratios were increased under hypoxic conditions [44]. Shear stress to the vessel occurs in response to repetitive apnea, and shear stress enhances the gene expression of arginine methyltransferase and ADMA release. Homocysteine produced during the synthesis of ADMA can alter ADMA metabolism by inhibiting DDAH. In addition, homocysteine was shown to decrease endothelial NOS and reduce DDAH expression in microvascular endothelial cells [45]. Despite these relationships, we did not observe any correlation between homocysteine and ADMA levels, and in contrast to our expectation, we did not find any difference between OSAHS patients and controls in terms of ADMA levels.
OSAHS patients have a high incidence of cardiovascular disease and mortality rate increases with severity of OSAHS. Although we think that a larger study group are necessary to clarify the exact interactions among NO, ADMA, homocysteine, and oxidative status, hyperhomocysteinemia and diminished NO production may be a causal factors in endothelial dysfunction seeing in OSAHS and may explain the association between OSAHS and cardiovascular diseases. These modifiable factors should be monitored in patients suspected of having OSAHS.
References
Baldwin CM, Bootzin RR, Schwenke DC, Quan SF (2005) Antioxidant nutrient intake and supplements as potential moderators of cognitive decline and cardiovascular disease in obstructive sleep apnea. Sleep Med Rev 9:459–476
Jordan AS, McEvoy RD (2003) Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev 7:377–389
Barcelo A, Barbe F, de la Pena M, Vila M, Perez G, Pierola J, Duran J, Agusti AGN (2006) Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J 27:756–760
Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI (2003) Reactive oxygen metabolites (ROS) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 7:105–109
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27:123–128
Gautier-Sauvigné S, Colas D, Parmantier P, Clement P, Gharib A, Sarda N, Cespuglio R (2005) Nitric oxide and sleep. Sleep Med Rev 9:101–113
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Moncada S, Palmer RMJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Haight JSJ, Djupesland PG (2003) Nitric oxide (NO) and obstructive sleep apnea (OSA). Sleep Breath 7:53–61
Ip MSM, Lam B, Chan LY, Zheng L, Tsang KWT, Fung PCW, Fung PCW, Lam WK (2000) Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Repir Crit Care Med 162:2166–2171
Ohike Y, Kozaki K, Iijima K, Eto M, Kojima T, Ohga E, Santa T, Imai K, Hashimoto M, Yoshizumi M, Ouchi Y (2005) Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure—possible involvement of nitric oxide and asymmetric NG,NG-dimethylarginine. Cir J 69:221–226
Schulz R, Schmidt D, Blum A, Lopes-Ribeiro X, Lucke C, Mayer K, Olschewski H, Seeger W, Grimminger F (2000) Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax 55:1046–1051
Leiper JM, Vallance P (2006) The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur J Clin Pharmacol 62:33–38
Guldener CV, Stehouwer CDA (2000) Hyperhomocysteinemia, vascular pathology, and endothelial dysfunction. Semin Thromb Hemost 26:281–289
Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocysteinemia and its impact on endothelial function. Curr Drug Metab 6:27–36
Bagi Z, Ungvari Z, Szollár L, Koller A (2001) Flow-induced constriction in arterioles of hyperhomocysteinemic rats is due to impaired nitric oxide and enhanced thromboxane A2 mediation. Arterioscler Thromb Vasc Biol 21:233–237
Dayal S, Lentz SR (2005) ADMA and hyperhomocysteinemia. Vasc Med 10:S27–33
Stühlinger MC, Stanger O (2005) Asymetric dimethyl-L-arginine (ADMA): a possible link between homocyst(e)ine and endothelial dysfunction. Curr Drug Matab 6:3–14
Rechtschaffen A, Kales A (1968) A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NIH publication no. 204. National Institute of Neurological Disease and Blindness, Bethesda, MA
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Chen BM, Xia LW, Zhao RQ (1997) Determination of NG, NG-dimethylarginine in human plasma by high-performance liquid chromatography. J Chromatogr B 692:467–471
Verde V, Fogliano V, Ritieni A, Maiani G, Morisco F, Caporaso N (2002) Use of N,N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free Radic Res 36:869–873
Krıjt J, Vackova M, Kozıch V (2001) Measurement of homocysteine and other aminothiols in plasma: advantages of using tris(2-carboxyethyl)phosphine as reductant compared with tri-n-buthylphosphine. Clin Chem 47:1821–1828
Vester B, Rasmussen K (1991) High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem 29:549–554
Lavie P, Herer P, Hoffstein V (2000) Obstructive sleep apnoea syndrome as a risk factor for hypertention: population study. BMJ 320:479–482
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM (2001) Sleep-disordered breathing and cardiovascular disease: croos-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 163:19–25
Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD (1999) Risk factors for central and obstructive apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 160:1101–1106
Von Kanel R, Le DT, Nelesen RA, Mills PJ, Ancoli-Israel S, Dimsdale JE (2001) The hypercoagulable state in sleep apnea is related to comorbid hypertention. J Hypertens 19:1445–1451
Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F (2000) Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Am J Respir Crit Care Med 162:566–570
Çiftçi TU, Kokturk O, Bukan N, Bilgihan A (2004) The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 28:87–91
Carlson JT, Rangemark C, Hedner JA (1996) Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens 14:577–584
Hernández C, Abreu J, Abreu P, Colino R, Jimenéz A (2006) Effects of nasal positive airway pressure treatment on oxidative stress in patients with sleep apnea-hypopnea syndrome. Arch Bronconeumol 42:125–129
Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Rüther E, Kornhuber J, Bleich S (2006) Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm 113:239–254
Doshi S, McDowell I, Goodfellow J, Stabler S, Boger R, Allen R, Newcombe R, Lewis M (2005) Relationship between S-adenosylmethionine, S-adenosylhomocysteine, asymmetric dimethylarginine, and endothelial function in healthy human subjects during experimental hyper- and hypohomocysteinemia. Metabolism 54:351–360
Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG (1999) Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 100:1161–1168
Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C (2005) Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol 46:518–523
Özkan Y, Özkan E, Şimşek B (2002) Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int J Cardiol 82:269–277
Valkonen VP, Paiva H, Salonen JT, Lakka TA, Lehtimaki T, Laakso J, Laaksonen R (2001) Risk of acute coronary events and serum concentration of asymmetric dimethylarginine. Lancet 358:2127–2128
Lavie L, Perelman A, Lavie P (2001) Plasma homocysteine levels in obstructive sleep apnea. Association with cardiovascular morbidity. Chest 120:900–908
Jordan W, Berger C, Cohrs S, Rodenbeck A, Mayer G, Niedmann PD, von Ahsen N, Rüther E, Kornhuber J, Bleich S (2004) CPAP-therapy effectively lowers serum homocysteine in obstrüctive sleep apnea syndrome. J Neural Transm 111:683–689
Kokturk O, Ulukavak-Ciftci T, Mollarecep E, Ciftci B (2006) Serum homocysteine levels and cardiovascular morbidity in obstructive sleep apnea syndrome. Respir Med 100:536–541
Cheng CH, Huang MC, Liu SC, Lin KL, Huang YC (2006) Traditional cardiovascular risk factors but not homocysteine are associated with obstructive sleep apnea. Nutr Res 26:59–64
Robinson GV, Pepperell JCT, Segal HC, Davies RJO, Stradling JR (2004) Circulating cardiovascular risk factors in obstructive sleep apnea: data from randomized controlled trials. Thorax 59:777–782
Yildirim AO, Bulau P, Zakrzewicz D, Kitowska KE, Weissmann N, Grimminger F, Morty RE, Eickelberg O (2006) Increased protein arginine methylation in chronic hypoxia: role of protein arginine methyltransferases. Am J Respir Cell Mol Biol 35:436–443
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC (2005) Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289:H2649–2656
Acknowledgment
We thank Prof. Kemal Erbil and Asist. Prof. Erdinç Çakır for their helpful assistance in ADMA measurements. This study was supported by The Scientific and Technological Research Council of Turkey (SBAG-AYD-457, 104S066).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozkan, Y., Fırat, H., Şimşek, B. et al. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea–hypopnea syndrome (OSAHS). Sleep Breath 12, 149–154 (2008). https://doi.org/10.1007/s11325-007-0148-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-007-0148-4